Manuscript accepted on : 05 July 2015
Published online on: 14-12-2015
Influence of freezing and drying on phytochemical properties of various fruit
Gennadiy Vyacheslavovich Semenov, Irina Stanislavovna Krasnova*, Oleg Aleksandrovich Suvorov, Irina Dmitrievna Shuvalova and Nikita Dmitrievich Posokhov
Moscow State University of Food Production, Talalikhina Street 33, Moscow 109316, Russia
DOI : http://dx.doi.org/10.13005/bbra/1786
ABSTRACT: Initially the total antioxidant activity, the total flavonoid contents, the total phenolic contents and the ascorbic acid were measured in fresh fruits. Then samples for freezing and vacuum freeze-drying were frozen what led to a decrease in total antioxidant activity by 11% (persimmon) - 28% (kiwi fruit), a reduction of total phenolic and total flavonoid contents for about 10% and the content of ascorbic acid decreases by 8% (kiwi fruit) - 30% (apple). The remaining frozen purees were subjected to vacuum freeze-drying at the temperature plus 25-27 ºC and a chamber pressure of 80 Pa in the primary drying and the temperature plus 38-40 ºC and a chamber pressure of 80 Pa at the secondary drying. The total antioxidant activity of freeze dried samples in comparison with fresh fruit decreased by 20% (persimmon) - 50-55% (apple), the total content of flavonoids and phenolic compounds decreased by 20-30%, reduction of the ascorbic acid ranged from 23-29% (apple and persimmon) to 55% (pear). The other part was subjected to convective drying and as a result the total antioxidant activity decreased by 46% (persimmon) to 68% (pear and apple), the total content of flavonoid contents compounds decreased by 42% (apple, kiwi, persimmon) - 56% (pear). The total content of phenolic compounds in all samples decreased by 40-45% and the content of ascorbic acid reduced by 45-55%. The study results demonstrated that the antioxidant properties of the fruits studied in the following order: fresh fruit> frozen fruit> freeze-dried fruit> convective dried fruit.
KEYWORDS: Total antioxidant activity; total phenolic contents; total flavonoid contents; sensorial analyses; apples; pears; kiwi; persimmon; coulometry; freeze-drying
Download this article as:| Copy the following to cite this article: Semenov G. V, Krasnova I. S, Suvorov O. A, Shuvalova I. D, Posokhov N. D.Influence of freezing and drying on phytochemical properties of various fruit. Biosci Biotech Res Asia 2015;12(2) |
| Copy the following to cite this URL: Semenov G. V, Krasnova I. S, Suvorov O. A, Shuvalova I. D, Posokhov N. D. Influence of freezing and drying on phytochemical properties of various fruit. Biosci Biotech Res Asia 2015;12(2). Available from: https://www.biotech-asia.org/?p=2177 |
Introduction
Plant foods are becoming more important in the diet of populations worldwide. Studies have demonstrated that fruits and vegetables are a rich source of natural antioxidants (AO), which prevent the development of cardiovascular diseases, cancer and other diseases (Albarracin et al., 2012; Kabir et al., 2015; Fiedor et al., 2014; Kundu et al., 2014; Parashar et al., 2014; Zhao, 2009). Antioxidants have significant health benefits hence there has been tremendous interest in the quantitative content of AO in foods. The AO levels in foods vary greatly and depend mainly on the storage technology used for fruits and vegetables from field to fork (Oancea et al., 2014). One important aspect of storage technology is the need to preserve foods so as to extend their shelf-life (Allaith et al., 2012; Adamczak et al., 2009; Quintero Ruiz et al., 2014; Shofian et al. 2011; Wiset et al., 2012). Convective hot-air drying is a traditional and widely used method for conserving plant materials. Goncalves et al. (2005) studied the levels of phenol compounds and antioxidant activity of two varieties of fresh and sun-dried pears (S. Bartolomeu and S. Amendoa) and showed that both pear varieties had lower AO levels after drying in the sun compared with the fresh fruits.
At the present frozen storage at temperatures below zero degrees or freezing followed by frozen storage at low temperatures are competing options (Mullen et al., 2002; Sikora et al, 2012). Mullen et al. (2002) investigated the effects of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries and concluded that the antioxidant capacity of the fresh fruit and the levels of vitamin C and phenolic were not affected by freezing.
Recently vacuum freeze-drying has become more widely used (Marques et al., 2010; Beh et al., 2012; Semenov, 2012). This technology may ensure the preservation of the native properties of fruits and vegetables for 2-3 years in unregulated temperature conditions when combined with high quality packaging. Beh et al. (2012) conducted a comparative analysis of the antioxidant activity and total phenol compound levels of freeze-dried commercial and fresh fruit juices (apple, lime, guava, mango, orange) and demonstrated the potential value of commercial fruit juice as a replacement for fresh fruit. The authors noted that only commercial mango and orange juices were able to retain antioxidant activity and total phenolic content similar to that of the fresh fruit. Marques et al. (2010) studied the hydrophilic and lipophilic antioxidant activity in fresh, frozen and freeze-dried strawberries and showed that the mean TAA based on dry weight was significantly higher for fresh strawberries than for freeze-dried, frozen and jam.
The aim of this work was to compare the effects of several traditional and modern preservation methods (freezing, vacuum freeze-drying and convective drying) on the antioxidant activity of the most popular different kinds of fruit (apples, pears, persimmons and kiwis).
Materials and methods
Sample preparation
The samples were fresh, frozen, freeze-dried and convective dried fruits – apple fruit (Malus pumila “Antonovka”), pear fruit (Pyrus communis), kiwi fruit (Actinidia chinensis) and persimmon fruit (Diospyros kaki). All fruits were rinsed under running tap water and blotted dry with paper tissue. Samples of each fruit type were then peeled and pureed and divided into four equal portions which were prepared as fresh, frozen, convective dried, freeze dried fruit samples and used as described below.
Convective drying
Fruit purees were put on trays in layers; thickness 10 ± 2 mm. Convective drying was achieved by blowing a stream of hot air with air velocity 1.5-2 m/s at atmospheric pressure across the fruit purees. The temperature of the air was initially 70-850С and 50-550С at the final. Temperature was controlled using thermocouples located close to the dried samples so that the temperature of fruit purees did not exceed 50°C at any time during drying. On an industrial scale, this process usually takes place in tunnel drying equipment. The accuracy of the temperature gauge was 0.1°C. This allowed the product temperature to be controlled within ±1°C. The total duration of the drying process was 5-6 h. The final moisture content of the dried fruit was 18-20%.
Freezing
Fruits purees were frozen at -20°C under conditions of forced convection in a freezer for 10-12 h. At the end of the freezing stage, frozen samples were taken and the AOA levels determined. The remaining frozen purees were subjected to vacuum freeze-drying.
Vacuum freeze-drying
For vacuum freeze-drying, trays with frozen purees were placed in a lab scale freeze dryer (Semenov, 2012), and treated as follows. Primary drying was carried out at a temperature of 25-27°C and a chamber pressure of 80 Pa. Secondary drying was carried out at a temperature of 38-40°C and a chamber pressure of 80 Pa. The total duration of the drying cycle was 12 – 14 h. The final moisture content of the freeze dried fruit purees was 1.5-2.5%.
Preparation of fruit extracts
Fresh, frozen and dried samples of fruit purees were prepared in order to determine their respective antioxidant activities, total flavonoid contents and total phenolic compounds. Fruit puree samples (0.2g) were placed in conical flasks (50mL), 35mL ethanol was added to each flask, and the mixture was centrifuged for 1h at 3600g. All solutions were then filtered through 0.45microns filters into separate volumetric flasks (50mL), the filters were washed three times, each time with 3.0 ml ethanol, and each filtrate plus respective 9 ml of washings were combined in fresh volumetric flasks (50mL). The volume was adjusted to the mark with ethanol. Prepared extracts (aliquots – 1ml from each preparation) were used for the measurement of the antioxidant activity and total flavonoid contents. Prepaired extracts (aliquots – 0.25ml from each preparation) were used for the measurement of the total phenolic compounds.
Measurement of antioxidant activity
Evaluation of the antioxidant activity was performed by coulometric titration with electrogenerated bromine using an EKSPERT 006 coulometric analyzer (EkoniksEkspert, Moscow) with glassy carbon electrodes (Chukicheva et al., 2012). The reference (anode of was 2.3 cm2 in area) and the auxiliary (cathode) electrodes were glassy carbon rods of 3mm in diameter; needle-like platinum electrodes were the indicators; 0.2M potassium bromide in 0.1M sulphuric acid was the supporting electrolyte; the operating current was 5.27mA, the auxiliary current was 0.79mA; the level of measurement was 300mV, the level of reduction was 500mV. The cathode and anode compartments were separated by a semi permeable membrane.
The supporting electrolyte (30mL) was placed into a coulometric cell, then reference, auxiliary, and indicator electrodes were dipped into the electrolyte. The cell was placed on a magnetic stirrer to maintain constant stirring throughout the experiment. The coulometric analyzer was operated as per the manufacturer’s instructions. The antioxidant content of the samples was calculated according to Faraday’s law using the built-in software.
Each sample was analyzed five times and the average value calculated.
Total phenolic contents
A colorimetric assay using the Folin–Ciocalteu reagent was employed (Zin et al., 2006). A 0.25ml aliquot of the extract solution was mixed with 0.25 ml of Folin–Ciocalteu reagent (previously diluted with water 1:1 v/v) 0.5 ml of saturated sodium carbonate (Na2CO3) solution and 4 ml of water. The mixture was then allowed to stand at room temperature for 25 min, followed by centrifugation at 5000 rpm for 10 min. Absorbance of the supernatant was then measured at 725 nm. With the calculation from gallic acid standard curve, the results were expressed in milligram of gallic acid per 1 gram dry weight (mg GAE/g DW).
Total flavonoid contents
Total flavonoids were determined by the method of Zhishen et al. (1999). One-milliliter aliquot of each extract solution were placed in 10mL volumetric flasks containing 5mL of distilled water. Then, 0.3mL of 5% sodium nitrite was added; after 5 min, 0.3mL of 10% aluminum chloride was added. After 6 min, 2mL of 1 m NaOH was added and diluted to the final volume with distilled water. Immediately, absorbance was measured at 510 nm using a spectrophotometer (Model Spekord М40; Carl Zeiss Industrielle Messtechnik GmbH”, Germany). Total flavonoids were calculated from a catechin standard curve, the results were expressed in milligram of catechin per 1 gram dry weight (mg CA/g DW).
Ascorbic acid analysis
Vitamin C concentration was determined in the samples using methods of AOAC (2000) (Method 967.21)
Sensorial analyses
SSensory acceptances acceptability of fruit purees were done determined usingwith a panel of 9 non-experts panelists (5 males and 4 females from Moscow State University of Ffood Pproduction). The panel consisted of It was requested the participation of healthy adult volunteers (from 18 to 50 years old) who were declared consumers of apples’s consumers, pear’s consumers, persimmon’s consumers and, kiwi’s consumers and, had that did not present any reaction concerning the consumption of the same and the same ones manifested its consent no allergies to these fruits.
Fresh purees were used as standards. The sensorial analysis included colour; smell; flavour; texture and overall acceptance. Panellists evaluated each attribute using a five-point scale compared with fresh purees. Sample of frozen puree were lift the freeze, sample of drying puree were recovered. About 50 ml of each puree type were given individually to the panellists. A three digit code was used to identify the individual samples. Mineral water was used to wash the oral cavity between tastings.
Statistical analysis
The results are presented as the values ± standard deviation (SD). Tukey′s test (P < 0.05) (Bower, 2009) was used to detect significant differences between treatments. P-values below 0.05 were considered significant.
Results and discussion
Drying conditions
Typical kinetic curves of changes in weight loss during convective drying and freeze-drying is presented in Fig. 1. As can be seen from the figure the total duration of the process for convective drying was 5-6 hours to achieve final moisture content in puree 18-20%. (Ирин, как правильно по-английски написать, что в сублимационной установке, встроены датчики, которые определяют изменение массовой доли влаги в продукте? Англичанин про это говорит) During convective drying the main part of moisture migrated quickly for 3-4 hours, and the rest of the process was necessary to achieve the optimum moisture content of the order of 15-20%.
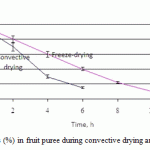 |
Figure 1: Weight loss (%) in fruit puree during convective drying and freeze – drying |
Freeze drying is characterized by a much lower intensity rate of removal water (по-русски, мы говорим «более низкая скорость удаления влаги», наверно так правильнее) then convective drying. During primary drying, the sample dries as a discrete boundary (the sublimation interface), which recedes through the sample from surface to base as drying progresses. The freeze-drying front can be observed as a discrete boundary that moves through the frozen sample to form an increasingly deeper layer of dried sample above the frozen sample. Heat is conducted from the shelf through the sample base and the frozen sample layer to the freeze-drying front where ice is converted into water vapour. By the end of the drying process the material has a porous structure formed by the removal of ice from micro- and macro capillaries. The dehydrated, freeze-dried samples prepared for eating in 5-10 minutes (rehydration time) and freeze dried products can be stored for 1-2 years without deterioration due to their low final moisture
Antioxidant activity
The antioxidant contents of various fruit purees measured by coulometry are shown in Fig. 2.
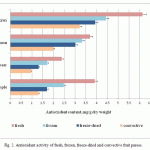 |
Figure 2: Antioxidant activity of fresh, frozen, freeze-dried and convective fruit purees |
These results show that the antioxidant content of all the samples was between 1 and 6.2 mg/g dry weight. Data from the coulometric titrations showed that the antioxidant activity of frozen fruit purees decreased in all cases compared to the fresh control samples (Fig 2.) (Англичанин имел ввиду, что номер рисунка не тот вставили. Может здесь убрать номер рисунка вообще, поскольку в начале раздела написали, что данные представлены на рисунке 2)The difference in antioxidant content between the samples treated using the various processes showed the same results for each fruit type. Simply the antioxidant levels decreased in the order fresh fruit>frozen>freeze-dried>convective dried.
The lowest decrease in antioxidant content was shown to occur after freezing. Freezing is associated with decreasing the temperature of a sample to its cryoscopic temperature. The characteristic cryoscopic temperatures of plant products lie between -0.5 and -2.5°C. The isothermal process of converting the liquid phase into ice occurs after the cryoscopic temperature has been reached. There are irreversible changes in plant cell membrane structures during freezing and, consequently, there is a decrease in the water holding capacity, especially in pulpy plant products (Semenov, 2013). At first, the crystallization process takes place in the intercellular spaces: the liquid phase with a low binding energy freezes most readily, this process leads to an increase in the content of electrolytes with low freezing points. As a result, the difference in the concentration of electrolytes inside and outside the cell decreases sharply releasing enzymes, acids and other cell substances. The local changes in pH and conductivity appear at different points throughout the frozen object, destroying vitamins and other active components (Walkowiak-Tomczak, 2007).
The level of damage caused by the formation of ice crystals during freezing depends on the freezing rate, and the final temperature of the frozen fruit, their variety and size. Decreases in the AOA of fruit purees after freezing are related to the processes described above.
The greatest decrease in antioxidant activity in fruit purees was observed after convective drying. Convective drying is associated with prolonged contact with air, especially oxygen, which leads to damage to, and oxidation of, thermolabile components (polyphenols, bioflavonoids, volatiles substances, enzymes). This damage is exacerbated by the effects of temperature. Furthermore, the evaporation of moisture leads to the destruction of water-soluble vitamins, which provide a large proportion of the antioxidant activity hence the antioxidant activity decreases after this process. This decrease in antioxidant activity is consistent with findings of Asami et al., (2003), who compared the effects of three common processing treatments (freezing, freeze-drying, and air-drying) on the total phenol and vitamin C content of samples of marionberry (blackberry and raspberry hybrid), strawberries and corn and reported the highest levels of total phenol compounds were consistently found in the extracts of frozen samples, followed by freeze-dried and then air-dried. Alfaro et al. (2014) found that freeze dried murtilla retained a higher total polyphenol and anthocyanin content than fruit dried by convective hot-air drying.
Results from this study are in agreement with the results of other studies. Tsai et al., (2007) compared the antioxidant activity of methanolic extracts of fresh juice, rind and freeze-dried flesh of Citrus maxima (“Pomelo” or “pummelo”), and found that the extracts obtained after freeze-drying retained only 20-40% of the AOA compared with fresh fruit. Orak et al., (2012) compared the antioxidant activity of strawberries and grapes freeze-dried and heat-dried and showed that freeze-drying preserves antioxidant activity better than thermal drying. Vinson et al., (2005) in experiments, in vitro and in vivo, showed that freeze-dried fruits such as apricots, cranberries, dates, figs, grapes and plums had lower total polyphenolic levels on a dry weight basis compared to fresh fruit. Jung et al., (2005) reported if fresh fruits are not available, freeze-dried persimmon can be successfully used because fresh and dried persimmon possess high levels of bioactive compounds and have a high antioxidant potential.
Novaković et al., (2011) investigated the influence of different drying treatments (convective drying (air-drying), osmotic drying and freeze-drying) on the antioxidant activity and phenol content of raspberries. The results showed the superiority of freeze-drying; convective drying caused slight changes whilst osmotic dehydration caused a significant decrease in phenolic compounds and antioxidant activity.
Total phenolic contents
The initial value for the fruit puree before drying was 5.2mg of GAE per 1 g dry weight (apple puree), 0.8mg of GAE per 1 g dry weight (pear puree), 2.6mg of GAE per 1 g dry weight (persimmon puree) and 6.5mg of GAE per 1 g dry weight (kiwi puree). The contents of total phenolic after freezing, vacuum freeze-drying and convective drying are presented in Fig. 3. Freezing leads to the highest retention of phenolic compounds (89-92%). Total phenolic compounds reduced to 18 – 25% of that of the fresh fruit in all fruit purees after vacuum freeze-drying. Reductions after convective drying were substantial (40-45%).
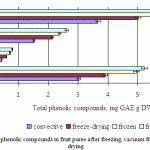 |
Figure 3: Average content of phenolic compounds in fruit puree after freezing, vacuum freeze-drying and convective drying |
Total flavonoid contents
Figure 4 shows the effect of freezing and drying methods on the total flavonoids content. The initial value for the fruit puree before drying was 2.5mg of CE/g dry weight (apple), 0.74mg of CE/g dry weight (pear), 7.5mg of CE/g dry weight (persimmon) and 2.6mg of CE/g dry weight (kiwi). Reductions in the content of total flavonoids compounds showed a similar pattern to the reductions of the average content of total phenolic compounds. The content of flavonoid compounds in the samples dried by vacuum freeze-drying amounted to 5.6 mg CE/g dry weight (persimmon), 1.8-1.9 mg CE/g dry weight (apple and kiwi) and 0.6 mg CE/g dry weight (pear), which was higher than in the convective-dried samples, but lower than in the samples subjected to freezing. Compared to fresh fruit, the greatest change in total flavonoids content was found in the convective fruit puree.
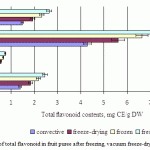 |
Figure 4: Average contents of total flavonoid in fruit puree after freezing, vacuum freeze-drying and convective drying |
Ascorbic acid analysis
Figure 5 presents the ascorbic acid content determined for fresh, frozen, freeze-dried and convective fruit puree. The fresh kiwi puree showed higher ascorbic acid content (3.27mg/g dry weight) than fresh persimmon puree (0.7mg/g dry weight), fresh apple puree (0.34mg/g dry weight) and fresh pear puree (0.1mg/g dry weight). Frozen kiwi puree was found to have a significantly (p < 0.05) higher ascorbic acid content (2.78 mg/g dry weight) compared to persimmon (0.61mg/g dry weight), apple (0.28mg/g dry weight) and pear (0.08mg/g dry weight).
In freeze-dried fruit puree the content of ascorbic acid amounted to 2.1mg/g dry weight (kiwi), 0.5mg/g dry weight (persimmon), 0.2mg/g dry weight (apple) and 0.07 mg/g dry weight (pear), which also was higher than in the convective-dried samples, but lower than in the freezing samples.
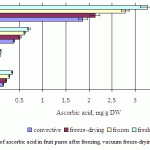 |
Figure 5: Average contents of ascorbic acid in fruit puree after freezing, vacuum freeze-drying and convective drying |
Sensorial analyses
Sensorial analyses are shown in Table 1. Rehydrated fruit purees after freezing and freeze-drying had highest quality. It is noted that panellists evaluated fruit purees after freezing and freeze-drying approximately the same. As expected, samples after convective drying had significantly change attributes. Thus, convective fruit puree has lowest points.
Table 1: Sensorial analysis; colour, smell, flavour, texture and acceptance.
| Fruit puree | Attribute | ||||
| Colour | Smell | Flavour | Texture | Overall acceptance | |
| Kiwi | |||||
| Fresh | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Frozen | 4.8 | 4.2 | 4.5 | 4.6 | 4.4 |
| Freeze-dried | 4.8 | 4.7 | 4.0 | 4.5 | 4.0 |
| Convective dried | 3.2 | 3.9 | 3.5 | 3.0 | 3.8 |
| Persimmon | |||||
| Fresh | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Frozen | 4.5 | 4.4 | 4.5 | 4.3 | 4.4 |
| Freeze-dried | 4.6 | 4.2 | 4.1 | 4.0 | 4.1 |
| Convective dried | 3.1 | 2.8 | 3.6 | 3.9 | 3.5 |
| Pear | |||||
| Fresh | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Frozen | 4.3 | 4.5 | 4.1 | 4.6 | 4.5 |
| Freeze-dried | 4.0 | 4.1 | 3.9 | 4.5 | 4.3 |
| Convective dried | 3.9 | 3.9 | 3.6 | 4.1 | 3.9 |
| Apple | |||||
| Fresh | 5.0 | 5.0 | 5.0 | 5.0 | 5.0 |
| Frozen | 4.6 | 4.4 | 4.5 | 4.4 | 4.5 |
| Freeze-dried | 4.4 | 4.0 | 4.1 | 4.3 | 4.4 |
| Convective dried | 4.0 | 3.4 | 3.1 | 3.2 | 3.8
|
Conclusion
This study has shown that all the storage technologies tested influence the antioxidant activity of fruit and revealed that the level of antioxidant decreased in the order: fresh fruit > frozen fruit > freeze-dried fruit > fruit convective drying.
The storage technologies examined in this study are now used everywhere to different extents. Althoughconvectivedrying causes the greatest decrease in antioxidant activity, it is the most commonly used technique in the food industries because its use requires less energy for the evaporation of moistureand the equipment is relatively simple. For this reasonthismethod is preferablefor the large-scale productionof dried fruits which are then used in the production ofbeveragesandvariousfood products.
Freezing causes the lowest decrease in antioxidant content, but this storage technology is connected with high- energy use and expensive equipment.
Equipment for freeze-drying is more difficult to use and more expensive than other storage technologies. The level of energy used is higher but freeze-dried products are high quality and preserve their useful properties in unregulated temperature conditions for a long time. Therefore, for higher cost raw materials, the preferred system for extended storage is freeze-drying
Vacuum freeze-drying is becoming more commonly used to obtain high-quality dry fruits. There is a tendency to increase the consumption of high quality products, so these are all reasons to forecast an increase in the scale of production of this type of product.
Acknowledgment
This research was financially supported by the Grant of The Ministry of education and science of the Russian Federation (grant No 14.577.21.0044, identification number RFMEFI57714X0044).
The authors also thank to head of department of Chemical Enzymology M.V. Lomonosov Moscow State University.
References
- Allaith, A.A., Ahmed, S.H., Jafer, F. (2012). Effect of different thermal treatments and freezing on the antioxidant constituents and activity of two Bahraini date cultivars (Phoenix dactylifera L.). International Journal of Food Science and Technology, 47, 783-792.
- Adamczak, A., Buchwald, W., Kozlowski, J., Mielcarek, S. (2009). The effect of thermal and freeze drying on the content of organic acids and flavonoids in fruit of European cranberry (Oxycoccus palustris Pers.). Herba polonica, 55, 94-102.
- Albarracin, S.L, Stab, B, Casas Z, Sutachan, J.J, Samudio, I, Gonzalez, J, Gonzalo, L, Capani, F, Morales, L, Barreto, G.E. (2012). Effects of natural antioxidants in neurodegenerative disease. Nutrition Neuroscience, 15, 1-9.
- Alfaro, S., Mutis, A, Quiroz, A., Seguel, I., Scheuermann, E. (2014). Effects of Drying Techniques on Murtilla Fruit Polyphenols and Antioxidant Activity. Journal of Food Research, 3, 73-82.
- Asami, D.K., Hong, Y.-J., Barrett, D.M., Mitchel, A.E. (2003).Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn grown using conventional, organic, and sustainable agricultural practices. Journal of Agricultural and Food Chemistry, 51, 1237-1241.
- Beh, L.K., Zuraini, Z., Beh, K.B., Ho, W.Y., Yeap, S.K. and Alitheen, N.B.M. (2012). Comparison of total phenolic content and antioxidant activities of freeze-dried commercial and fresh fruit juices. Journal of Medicinal Plants Research, 6, 5857-5862.
- Bower, J. (2009). Statistical Methods for food Science. Edinburgh: Wiley-Blackwell.
- Chukicheva, I.Yu., Buravlev, E.V., Fedorova, I.V., Borisenkov, M.F., Kutchina, A.V. (2010). Antioxidant properties of terpene-substituted phenols. Russian Chemical Bulletin, International Edition, 12, 2276-2280.
- Fiedor, J., Burda, K. (2014). Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients, 6, 466-488.
- Goncalves, F., Batista, S., Guine, R., Barroca, M.J., Perez, M.D., San Jose, M.L., Ferreira, D. (2005). Sun-dried pears: phenolic compounds and antioxidant activity. Encontro de Quimica dos Alimentos, 7, 589-592.
- Jung, S.T., Park, Y.S, Zachwieja, Z., Folta, M., Barton, H., Piotrowicz, J., Katrich, E., Trakhtenberg, S., Gorinstein, S. (2005). Some essential phytochemicals and the antioxidant potential in fresh and dried persimmon. International Journal of Food Sciences and Nutrition, 56, 105-113.
- Kabir, F., Tow, W.W., Hamauzu, Y., Katayama, S., Tanaka, S., Nakamura, S., (2015). Antioxidant and cytoprotective activities of extracts prepared from fruit and vegetable wastes and by-product. Food Chemistry, 167, 358-362.
- Kundu, J.K., Chun, K.S. (2014). The promise of dried fruits in cancer chemoprevention. Asian Pacific Journal of Cancer Prevention, 15, 3343-3352.
- Marques, K.K., Renfroe, M.H., Brevard, P.B., Lee, R.E., Gloeckner, J.W. (2010). Differences in antioxidant levels of fresh, frozen and freeze-dried strawberries and strawberry jam. International Journal of Food Sciences and Nutrition., 61, 759-769.
- Mullen, W., Stewart, A.J., Lean, M.E., Gardner, P., Duthie, G.G., Crozier, A. (2002). Effect of freezing and storage on the phenolics, ellagitannins, flavonoids, and antioxidant capacity of red raspberries. Journal of Agricultural and Food Chemistry, 50, 197-201.
- Novaković, M.M., Stevanović, S.M., Gorjanović, S.Ž., Jovanovic, P.M., Tešević, V.V., Janković, M.A., Sužnjević, D.Ž. (2011). Changes of hydrogen peroxide and radical-scavenging activity of raspberry during osmotic, convective, and freeze-drying. Journal of Agricultural and Food Chemistry, 76, 3-8.
- Oancea, S., Moiseenco, F., Ketney, O., Traldi, P. (2014). Effects of freezing and oven drying of Romanian wild and cultivated red raspberries on total anthocyanins and total antioxidant capacity. Romanian Biotechnological Letters, 19, 9240-9247.
- Orak, H.H., Aktas, T., Yagar, H., Isbilir, S.S., Ekinci, N., Sahin, F.H. (2012). Effects of hot air and freeze drying methods on antioxidant activity, colour and some nutritional characteristics of strawberry tree (Arbutus unedo L.) fruit. Food Science Technology International, 18, 391-402.
- Parashar, S., Sharma, H., Garg, M. (2014). Antimicrobial and Antioxidant activities of fruits and vegetable peels: A review. Journal of Pharmacognosy and Phytochemistry; 3, 160-164.
- Quintero Ruiz, N.A., Demarchi, S.M., Giner, S.A. (2014). Effect of hot air, vacuum and infrared drying methods on quality of rose hip (Rosa rubiginosa) leathers. International Journal of Food Science and Technology, 49, 1799-1804.
- Semenov, G.V. (2012). Vakuumnaya sublimacionnaya sushka [Vacuum freeze-drying], 264 pp., Moscow, Russia, Deli plus.
- Shofian, N.M., Hamid, A. A., Osman, A., Saari, N., Anwar, F., Dek, M.S.P., Hairuddin, M.R. (2011). Effect of Freeze-Drying on the Antioxidant Compounds and Antioxidant Activity of Selected Tropical Fruits. International Journal of Molecular Sciences, 12, 4678–4692.
- Sikora, E., Bieniek, M.I., Borczak, B. (2013). Composition and antioxidant properties of fresh and frozen stored blackthorn fruits (Prunus spinosa L.). Acta Scientiarum Polonorum, Technologia Alimentaria, 12, 365-372.
- Tsai, H.L., Chang, S.K., Chang, S.J. (2007). Antioxidant Content and Free Radical Scavenging Ability of Fresh Red Pummelo [Citrus grandis (L.) Osbeck]. Juice and Freeze-Dried Products. Journal of Agricultural and Food Chemistry, 55, 2867-2872.
- Vinson, J.A., Zubik, L., Bose, P., Samman, N., Proch, J. (2005). Dried Fruits: Excellent in Vitro and in Vivo Antioxidants. Journal of the American College of Nutrition, 24, 44-50.
- Wiset, L., Poomsa-ad, N., Srilaong, V. (2012). Comparisons of Antioxidant Activity and Bioactive Compounds of Dragon Fruit Peel from Various Drying Methods. World Academy of Science, Engineering and Technology, 6, 992-995.
- Walkowiak-Tomczak, D. (2007). Changes in antioxidant activity of black chokeberry juice concentrate solutions during storage. Acta Scientiarum Polonorum, Technologia Alimentaria, 6, 49-55.
- Zhao, B. (2009). Natural antioxidants protect neurons in Alzheimer’s disease and Parkinson’s disease. Neurochemical Research, 34, 630-638.
- Zhishen, J., Mengcheng, T., Jianming, W. (1999). The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chemistry, 64, 555–559.
- Zin, Z.M, Hamid, A.A., Osman, A., Saari, N. (2006). Antioxidative activities of chromatographic fractions obtained from root, fruit and leaf of Mengkudu (Morinda citrifolia L.). Food Chemistry, 94, 169-178.

This work is licensed under a Creative Commons Attribution 4.0 International License.





