Manuscript accepted on : 06 February 2016
Published online on: 25-02-2016
Preparation of Maltotriose syrup from microbial Pullulan by using Pullulanase Enzyme
Bishwambhar Mishra*, Akula Manikanta and Deveeka Zamare
Department of Biotechnology, Sreenidhi Institute of Science and Technology, Ghatkesar, Hyderabad – 501 301
DOI : http://dx.doi.org/10.13005/bbra/2058
ABSTRACT: The present work deals with the preparation and single point optimization of maltotriose syrup prepared from microbial pullulan. The enzyme pullulanase was used in this process for hydrolysis of pullulan. Production of pullulan was carried out in a shake flask fermenter with isopropanol precipitation. Various parameters like time, temperature, pH, amount of ethanol and effect of amount of enzyme on the hydrolysis of pullulan were optimized by single point optimization studies. The optimized values for the process parameters were 8h, 46°C, 5.5, 6 (v/v) and 10 ASPU/g for time of hydrolysis, temperature, pH , amount of ethanol and amount of pullulanase enzyme respectively. The results obtained from HPLC, confirmed that major component present in the hydrolysate were composed of maltotriose. Results of experiments indicated that this was a promising way of preparation of maltotriose.
KEYWORDS: Pullulan; Pullulanase; Maltotriose; Hydrolysis; HPLC; Optimization
Download this article as:| Copy the following to cite this article: Mishra B, Manikanta A, Zamare D. Preparation of Maltotriose syrup from microbial Pullulan by using Pullulanase Enzyme. Biosci Biotech Res Asia 2016;13(1) |
| Copy the following to cite this URL: Mishra B, Manikanta A, Zamare D. Preparation of Maltotriose syrup from microbial Pullulan by using Pullulanase Enzyme. Biosci Biotech Res Asia 2016;13(1). Available from: https://www.biotech-asia.org/?p=6769 |
Introduction
Maltotriose syrup is a type of sweetener which is produced from starch. It is a part of liquid glucose (a commercial sweetener composed of maltotriose, glucose, maltose, and maltotetrose) and brown rice syrup1. It can be used in breads, cakes and other baked goods due to its water reserving properties. Maltotriose rich syrups are being produced from maltose syrup by cation exchange resin chromatography and maltose solution of 98% purity is obtained as a by-product2,3. Pullulanase can be used for the production of maltotriose by the enzymatic hydrolysis of pullulan. Generally, pullulanase is a de-branching enzyme, which cleaves the glycoside linkages present in the pullulan4.
This syrup possess many excellent properties as low freezing point depression, mild sweetness, keeps in moisture, prevention of retrogradation of starch in foodstuffs, less color formation compared with maltose syrups, glucose syrups or sucrose, good heat stability, low solution viscosity, high fermentability and favoring glassy states. These properties are useful in food and pharmaceutical industries5 . High maltotriose syrup may be applied in the food industry for the manufacturing of desserts, baking and brewing, as well as in the pharmaceutical industry for replacing glucose in intravenous feeding6.
Maltotriose syrup has so many applications in food industries. It possesses excellent properties like low freezing point depression, mild sweetness, less colour formation, good heat stability etc., as shown in Figure 1. Although maltotriose producing amylase enzyme can make the syrup, but its purity and yield is not satisfactory7 . Hence an alternative way for production of high maltotriose syrup content is to be investigated.
 |
Figure 1: Various application of maltotriose syrup |
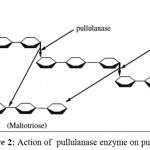 |
Figure 2: Action of pullulanase enzyme on pullulan |
In this paper, we have tried to preare the maltotriose syrup from the microbial pullulan produced from Aurebasidium pullulan-SB-1 with the help of pullulanase enzyme through hydrolytic breakdown. In addition to this, different process parameters were optimized for the production of maltotriose.
Materials And Methods
Pullulan Production : The production and characterization of pullulan was performed in our previous study8 from the isolate of Aurebasidium pullulan-SB-1. In this process, pullulan was produced in the 250mL Erlenmeyer flask The composition of the medium for the production of pullulan was as follows: Sucrose 60.0 g, K2HPO4 7.5 g, NaCl 1.5 g, MgSO4.7H2O 0.4 g, Yeast Extract 0.4 g and distilled water 1,000 mL. The pH of the medium was adjusted to 6.5 by adding 0.1 M NaOH. After preparation and sterilization of the medium, 1 mL of the prepared inoculum (5×106spores) was inoculated to 100 mL of the production medium and was incubated for 6 days under agitation (150 rpm) at 25°C.
Hydrolysis Of Microbial Pullulan In The Fermentation Broth
After the 6 days of fermentation, the culture was centrifuged at 7,000 rpm for 20 min at 4°C in order to separate the biomass from the broth. The pullulanase enzyme (Source: Bacillus acidopullulyticus, Sigma, USA) was added to the Erlenmeyer flask containing 100mL of the centrifuged broth. Different amount of pullulanase enzyme ( 2, 4, 6, 8, 10, 12 and 14 ASPU/g) was added in order to find optimum enzyme concentration for maximum maltotriose syrup production. The reaction mixture was kept in a thermostatic water bath in different range of temperature (40°C, 42°C, 44°C, 46°C, 48°C, 50°C and 52 °C ) for different time durations (2 h, 4 h, 6 h, 8 h, 10h and 12 h). Aliquot have taken from the reaction mixture and was added with 0.1M of trichloroacetic acid in order to terminate the reaction. The Dextrose Equivalent (DE) value of the reaction mixture was calculated in order to find the degree of hydrolysis, which is the function of the production of maltotriose syrup in the broth. The experiment was performed in triplicate.
Precipitaion Of The Maltotriose Syrup
The reaction mixture was filtrated through a filtration membrane (1000 Da interception membrane) and made it concentrated. To the filtrate, different volume of ethanol (2, 4, 6, 8, 10 and 12) was added for its precipitation. The precipitate was dried at 80°C in a hot air oven up to attainment of constant weight. The optimum amount of ethanol required was calculated according to the highest recovery of the maltotriose. The experiment was performed in triplicate.
HPLC Analysis Of Maltotriose Syrup
In order to find the purity of the product, the HPLC analysis was performed with a Water 1525 binary HPLC pump equipped with C 18 column (150 mm × 4.5 µm) and Waters 2487 dual λ absorbance detector having rheodyne manual injector. The sample for the study was prepared with de-ionized water (1mg/mL). The chromatogram was developed using the mobile phase consisting of HPLC grade water with the constant flow rate of 0.7 mL per minute in the isocratic mode at the room temperature. The retention time for each signal was recorded at the wavelength of 210 nm. The data was processed with the Empower software. The commercial pullulan (Sigma, USA) was used as a substrate for the pullulanase enzyme and the released maltotriose was recovered with ethanol precipitation and was used as the standard for the analysis.
Results And Discussion
Hydrolysis Of Microbial Pullulan In The Fermentation Broth And Its Optimization
Various parameters like time, temperature, pH, amount of ethanol and effect of amount of enzyme on the hydrolysis of pullulan were optimized by single point optimization studies.
Effect Of Time On Hydrolysis
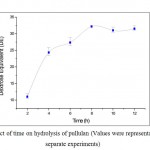 |
Figure 3: Effect of time on hydrolysis of pullulan (Values were representative of three separate experiments) |
The time course of hydrolysis of pullulan with pullulanase enzyme was made for a period of 12 h. There was an obvious increase within 4 h in Dextrose Equivalent (DE) production, which is directly proportional to the extent of the hydrolysis. Maximum amount of DE was achieved after 8 h. Hence optimum hydrolyzing time was considered to be 8 h (Figure 3).
Effect Of Temperature On Hydrolysis
The temperature of the reaction mixture plays an important role in the activity of enzyme kinetics, that directly affect the activity of pullulanase enzyme for hydrolysis. The effect of different temperature varying from 40°C to 52°C for the pullulanase activity in terms of the production of DE is shown in Figure 4. Maximum DE of the hydrolysate was obtained at 46°C. Conversely, different temperatures like 50°C, 75°C, 90°C for the hydrolysis of pullulan with pullulanase were reported by various researchers9,10,11,12 . The variation in the optimum temperature for the maltotriose production by pullulanase enzyme may be due to differences in the source of the enzymes13,14.
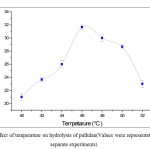 |
Figure 4: Effect of temperature on hydrolysis of pullulan(Values were representative of three separate experiments) |
Effect Of Ph On Hydrolysis Of Pullulan
The pH of reaction mixture generally influence the activity of the pullulanase enzyme and hence for the production of maltotriose from pullulan. Therefore, effect of different pH values ranging from 4.0 to 6.5 on pullulan hydrolysis in term of DE were investigated. From Figure 5, it was found that, maximum DE of the hydrolysate was achieved at pH of 5.5. Different values of optimum pH values like 5.5, 5.9, 6.0 and 7.0 for the hydrolysis of pullulan with pullulanase have been reported by some researchers15, 16, 17 . The variation in the optimum pH values for the maltotriose production by pullulanase enzyme may be due to differences in the source of the enzymes.
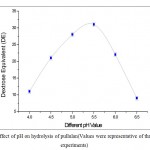 |
Figure 5: Effect of pH on hydrolysis of pullulan(Values were representative of three separate experiments) |
Effect Of Amount Of Ethanol On Recovery Of Maltotriose
The hydrolysates were filtered through a membrane filter with the help of vacuum pump and was concentrated about 20% (w/v) h the help of rotary evaporator before ethanol precipitation. Different volumes of ethanol ranging from 2 to 12 (v/v) were taken in to consideration for the recovery of the maltotriose. From the Figure 6, it showed that, maltotriose recovery was increased with increasing the amount of ethanol. However, when the volumes of ethanol was increased more than 6, the recovery of maltotriose was unaffected. Hence, optimal volume of ethanol required for the precipitation of maltotiose was found to be 6 (v/v).
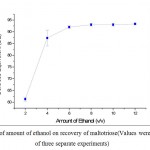 |
Figure 6: Effect of amount of ethanol on recovery of maltotriose(Values were representative of three separate experiments) |
Effect Of Pullulanase Enzyme
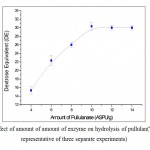 |
Figure 7: Effect of amount of amount of enzyme on hydrolysis of pullulan(Values were representative of three separate experiments) |
Figure 7 shows the effect of amount of pullulanase on the production of maltotriose. The DE values increased with increasing amount of pullulanase up to 10 ASPU/g and beyond which, the DE value was found to be constant. Hence maximum DE was observed at 10 ASPU/g of pullulanase, and at this level pullulan was saturated with pullulanase enzyme.
Hplc Analysis Of Maltotriose Syrup
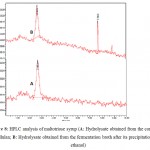 |
Figure 8: HPLC analysis of maltotriose syrup (A: Hydrolysate obtained from the commercial pullulan; B: Hydrolysate obtained from the fermentation broth after its precipitation with ethanol) |
The HPLC analysis was performed in order to find the purity of the maltotriose syrup obtained from both commercial pullulan (Sigma, USA) and microbial pullulan respectively under the same experimental conditions. The hydrolysate obtained from the commercial pullulan after its hydrolysis with pullulanase was used as the standard for this analysis. Figure 8 shows that the significant peaks found in both standard (A) and test (B) are nearly superimposed with each other. Hence it confirms that major component present in the hydrolysate are composed of maltotriose.
Conclusion
Maltotriose can be prepared by hydrolyzing of pullulan with pullulanase. The optimum hydrolyzing conditions were as follows: time, 6 h; pH, 5.0; temperature, 45 °C; amount of pullulanase, 10 ASPU/g. Under these conditions, the maximum DE was obtained. The hydrolysates were subject to Wltrating through a Wlter membrane that could intercept any particle whose molecular weight was more than 1,000 Da in order to remove any polymers, concen-trated to about 20% (w/w) and precipitated with 8 volume of ethanol. The precipitates obtained were dried at 80 °C for 2 h. The content of maltotriose in the product and the yield of maltotriose were 93.5% (w/w) and 87.3% (w/w), respectively. All samples were white powder and soluble in water.
Acknowledgement
The authors are very much thankful to the Sreenidhi Institute of Science and Technology, Ghatkesar, Hyderabad for providing the smart laboratory facilities and good infrastructure to carry out this valuable research.
References
- Bender, H., Wallenfels K. Investigations on pullulan. II. Specific degradation by means of a bacterial enzyme. Biochem. Z., 1961; 334:79–95.
- Cheng, K. C., Demirci A., Catchmark J. M . Continuous pullulan fermentation in a biofilm reactor. Appl Microbiol Biotechnol., 2011;90(3): 921–927.
- Finkelman, M.A.J., Vardanis A. Simplified Microassay for Pullulan Synthesis. Appl. Environ. Microbiol., 1982; 43(2):483-485.
- Ghina, A., Rihouey C., Le Cerf D., Picton L. Effect of carboxymethyl groups on degradation of modified pullulan by pullulanase from Klebsiella pneumoniae. Carbohydr. Polym., 2013; 93 (1):109–115.
- Singh, R.S., Saini G.K., Kennedy J.F. Maltotriose syrup preparation from pullulan using pullulanase. Carbohydr. Polym, 2010;80(2):401–407.
- Shengjun, W., Hanqing C., Qunyi T., Xueming X., Zhengyu J. Preparation of maltotriose by hydrolyzing of pullulan with pullulanase. Eur. Food Res. Technol., 2009; 229(5):821–824.
- Dharmendra, K. K., Bhattacharjee P., Singhal R. S. (2003). Studies on downstream processing of pullulan. Carbohydr. Polym., 2003;52(1):25–28.
- Bishwambhar, M. , Suneetha, V. Characterization of exopolysaccharide a pullulan produced by a novel strain of Aureobasidium pullulans-SB-1 isolated from the phylloplane of Brassica oleracea cultivated in Orissa State, Asian J Microbiol Biotechnol Env Sci , 2012; 14 (3):369-374
- Badal, C., Saha J., Zeikus G. Novel highly thermostable pullulanase from thermophiles . Trends Biotechnol., 1989;7(9):234–239.
- Kuriki, T., Park J.H., Imanaka T. Characteristics of thermostable pullulanase from Bacillus stearothermophilus and the nucleotide sequence of the gene. J Ferment Bioeng, 1990; 69(4):204–210.
- Swamy, M.V. and G. Seenayya .Thermostable pullulanase and α-amylase activity from Clostridium thermosulfurogenes SV9-Optmization of culture conditions for enzyme production. Process Biochem, 1996; 31(2):157–162.
- Messaoud, E.B., Ammar Y.B. , Mellouli L., Bejar S. Thermostable pullulanase type I from new isolated Bacillus thermoleovorans US105: cloning, sequencing and expression of the gene in E. coli . Enzyme Microb Tech., 2002; 31(6):827–832.
- Kuroiwa, T., Shoda H., Ichikawa S., Sato S., Mukataka S. Immobilization and stabilization of pullulanase from Klebsiella pneumoniae by a multipoint attachment method using activated agar gel supports. Process Biochem, 2005;40(8): 2637–2642.
- Kim, C.H., Nashiru O., Ko J.H. Purification and biochemical characterization of pullulanase type I from Thermus caldophilus GK-24. FEMS Microbiol Lett , 1996; 138(2-3):147–152.
- Kriegshauser, G., Liebl W. Pullulanase from the hyperthermophilic bacterium Thermotoga maritima: purification by cyclodextrin affinity chromatography. J Chromatogr B , 2000; 737(1):245–251.
- Roy, A., Messaoud E.B., Bejar S. Isolation and purification of an acidic pullulanase type II from newly isolated Bacillus sp. US149. Enzyme Microb Tech , 2003;33(5):720–724.
- Choudhury, A.R., Puja S., Prasad G.S . Pullulan production by an osmotolerant Aureobasidium pullulans RBF-4A3 isolated from flowers of Caesulia axillaris. Carbohydr Polym., 2011; 83(4): 1547–1552.

This work is licensed under a Creative Commons Attribution 4.0 International License.





