How to Cite | Publication History | PlumX Article Matrix
Novel Bioactive Molecules from Marine Actinomycetes
Naiyf S. Alharbi
Department of Botany and Microbiology, College of Science, King Saud University, Riyadh-11451, Saudi Arabia.
Corresponding Author E-mail: nalharbi1@ksu.edu.sa
DOI : http://dx.doi.org/10.13005/bbra/2346
ABSTRACT: Actinomycetes are virtually unlimited sources of novel compounds with many therapeutic applications and hold a prominent position due to their diversity and proven ability to produce novel bioactive compounds. There are more than 25,000 known microbial secondary metabolites, 75% of which are produced by actinomycetes, 15% from fungi, 7% from Bacillus spp. and 1–2% by other bacteria. Among the actinomycetes, streptomycetes group are considered economically important because out of the approximately more than 10,000 known antibiotics, 50–55% are produced by this genus. The ecological role of actinomycetes in the marine ecosystem is largely neglected and various assumptions meant there was little incentive to isolate marine strains for search and discovery of new drugs. The search for and discovery of rare and new actinomycetes is of significant interest to drug discovery due to a growing need for the development of new and potent therapeutic agents. Modern molecular technologies are adding strength to the target-directed search for detection and isolation of bioactive actinomycetes, and continued development of improved cultivation methods and molecular technologies for accessing the marine environment promises to provide access to this significant new source of chemical diversity with novel/rare actinomycetes including new species of previously reported actinomycetes.
KEYWORDS: Novel Actinomycetes; Bioactive compounds; Marine environment; Secondary metabolites
Download this article as:| Copy the following to cite this article: Alharbi N. S. Novel Bioactive Molecules from Marine Actinomycetes. Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Alharbi N. S. Novel Bioactive Molecules from Marine Actinomycetes. Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=17257 |
Introduction
The sea, covering more than 70% of the surface of planet Earth, contains an exceptional biological diversity, accounting for more than 95% of the whole biosphere. Microbial diversity constitutes an infinite pool of novel chemistry, making up a valuable source for innovative biotechnology1,2. So far only the surface has been scratched. The recent estimates suggest that the culturability of microorganisms in marine sediments (0.25%), and especially seawater (0.001–0.10%) is considerably lower compared to soil (0.30%). A number of valuable antibiotics and metabolites have been derived from terrestrial microorganisms (99% of the known microbial compounds) although efforts in this area have diminished since the late 1980s because of the feeling that this resource has been exhaustively studied1,3. In this respect, researchers switched over to new environments for novel pharmaceutical compounds and for combating human pathogens
The ocean floor has been recently demonstrated as an ecosystem with many unique forms of actinomycetes. It appears that they are widely distributed throughout the ocean and found in intertidal zones, seawater, animal, plants, sponges4, 5, and in ocean sediments. Some of the rare marine actinomycetes require seawater for their growth6. This kind of unique adaptation characteristic of actinomycetes 7 in the marine environment is a source of interesting research for new species and a promising source of pharmaceutically important compounds. The oceans are a rich and relatively available source of new microbial natural products (MNPs) in comparison with the terrestrial environment. Till date, over 23,000 bioactive compounds produced by microorganisms have been reported and more than 10,000 among these compounds were isolated from actinomycetes8. It is worth mentioning that of 10,000 compounds; about 80% have been obtained from Streptomyces which is the most productive genus in the microbial world9. For instance, 20 marine antitumor compounds under clinical trial, 16 are derived from microbial sources10. Till date, five phyla Actinobacteria, Bacteroidetes, Cyanobacteria, Firmicutes, and Proteobacteria that represent 95 % of all cultivated and published species produce bioactive molecules11. Despite this promise, relatively little work has been done on marine actinomycetes and only a small fraction of that has been directed at examining metabolite profiles12, 13. Since environmental conditions of the sea are extremely different from terrestrial conditions, it is felt that marine actinomycetes may have different characteristics from terrestrial actinomycetes and therefore might produce novel bioactive compounds and new antibiotics. The research to date supports this hypothesis and it has been shown that marine actinomycetes produce novel types of new secondary metabolites2, 14. Many of these metabolites possess novel biological activities and have the potential to be developed as therapeutic agents. Additionally, sequencing marine actinomycete genomes may provide insights useful in the discovery of novel agents7,15. This review article highlights the study of marine actinomycetes for the discovery of novel/new secondary metabolites.
Biodiversity of marine actionmycetes
Of the total sea surface, only 7–8% is coastal area and the rest is deep sea, of which 60% is covered by water more than 2000 m deep. The deep sea is a unique and extreme environment characterized by high pressure, low temperature, lack of light and variable salinity and oxygen concentration. Though the deep sea area is geographically vast, scientific knowledge and research on deep sea microbial diversity is meager. However, it has been shown to be a good source of novel microorganisms for the discovery of new antibiotics. Actinobacteria isolated from deep sea sediments in earlier studies however poorly characterized. More recently, culture independent studies have shown that indigenous marine actinomycetes certainly exist in the oceans. These include members of the genera Dietzia, Rhodococcus 16,17,18 Streptomyces19,20, the newly described genera Salinispora,21,22,23 and Marinispor 21, 24, 25 both of which require seawater for growth and have marine chemotype signatures; and Aeromicrobium marinum which also has an obligate requirement for salt. Another recently characterized genus, Salinibacterium, can tolerate up to 10% NaCl but does not have a salt requirement for growth and his colleagues reported that a wide range of biologically active halophilic Actinomycetes was evaluated using a polyphasic approach which showed the presence of a new genus and many new species of the Actinopolyspora, Nocardiopsis, Saccharomonospora, Streptomonospora and Saccharopolyspora genera. Some of these species were found to produce unique compounds, such as salinosporamides, that are now in clinical trials as potent anticancer agents. The recently reported Verrucosispora strain AB-18-032 26 also might qualify as an indigenous marine actinobacterium. According to Fenical and Jensen (2006)2, representatives of at least 13 new genera of indigenous marine actinomycetes, including Salinispora and Marinispora, have been isolated and identified. Goodfellow and Fiedler (2010)27 mention members of 50 actinomycetes genera isolated from marine sources, although only Aeromicrobium, Dietzia, Rhodococcus, Salinibacterium, Salinispora, Sciscionella and Serinicoccus genera contain or are composed of indigenous marine isolates. The recent phylogenetic diversity of marine actinobacteria isolated from the Chukchi Shelf Marine sediments in the Arctic Ocean shown that 14 different genera were isolated and identified as Agrococcus, Arsenicicoccus, Arthrobacter, Brevibacterium, Citricoccus, Janibacter, Kocuria, Microbacterium, Microlunatus, Nocardioides, Nocardiopsis, Saccharopolyspora, Salinibacterium and Streptomyces28. Recently, Cheng et al. (2015)29 reported 23 different genera and including four putatively new species belonging to genera Geodermatophilus, Microlunatus, Rhodococcus and Actinomycetospora in marine sponges.
The actinomycetes are active components of marine microbial communities21 and form stable, persistent populations in various marine ecosystems. The discovery of several new marine actinomycete taxa with unique metabolic activity in their natural environments,2 and their ability to form stable populations in different habitats and produce novel compounds with various biological activities 7,21,30,31,32, 33) clearly illustrate that indigenous marine actinomycetes indeed exist in the oceans and are an important source of novel secondary metabolites.
Role of actinomycetes in marine environment
Actinomycetes have a profound role in the marine environment apart from antibiotic production 34, 35. The degradation and turnover of various materials are a continuous process mediated by the action of a variety of microorganisms 21. There is a speculation that the increase or decrease of a particular enzyme-producing microorganism may indicate the concentration of natural substrate and conditions of the environment. Metagenomic analysis of sea water column has also identified considerable number of actinomycete taxa36. In addition, they play a significant role in mineralization of organic matter, immobilization of mineral nutrients, fixation of nitrogen, phosphate solubilization, improvement of physical parameters and environmental protection37.
Marine streptomycetes – a boundary microorganism
Streptomycetes are an economically important group of organisms among actinomycetes and they are the pivotal source for wide range of biologically active compounds1. About three quarters of all the known commercially and medicinally useful antibiotics38 and several agriculturally important compounds were obtained from the streptomycetes. Moreover, approximately 60% of the antibiotics discovered in the year 1990 and most of the antibiotics used in agriculture are from the genus Streptomyces. Streptomycetes have been shown to have the ability to synthesize antibacterial1,39, 40,41, insecticidal, antitumo1,42, anti-inflammatory 43, anti-parasitic, antiviral, antifouling 44, anti-infective, nematicidal activity 45 and herbicidal and plant growth promoting compounds45 as well as many other agents such as enzyme inhibitors and vitamins and hence, they are widely recognized as industrially important microorganisms. Further, they are well known for their capability of producing various extracellular hydrolytic enzymes including ribonucleases. These characteristics make this genus an important research subject from the academic and industrial point of view.
Actinomycetes as producers of secondary metabolites
Microbial secondary metabolites have been in the frontier in the discovery of novel antimicrobial agents for pharmaceutical industry and today all evidence suggests that novel compounds with potential therapeutic applications are still waiting to be discovered from secondary metabolites especially those produced by actinomycetes. Actinomycetes are prolific producers of secondary metabolites with biological activities46. Secondary metabolites are metabolic products that are not essential for vegetative growth of the producing organisms but they are considered differentiation compounds conferring adaptive roles, for example, by functioning as defense compounds or signaling molecules in ecological interactions. They are produced at the end of the exponential growth phase and their syntheses greatly depend on the growth conditions. Production is usually when growth is limited by the exhaustion of one key nutrient such as carbon or nitrogen47,48. They are structurally diverse and most of them are endowed with biological activities, such as antimicrobial agents, toxins, pesticides, ionophores, bioregulators, and quorum signalling. These bioactive metabolites are profoundly used as antimicrobial agents for the treatment of diverse ailments49. Three diketopiperazine dimers including a new compound iso-naseseazine B and two known compounds naseseazine B and aspergilazine A were isolated from marine actinomycets 11. Recently, Wu et al. (2016)50 identified the secondary metabolites like isocoumarins, undecylprodiginine, streptorubin B, 1H-pyrrole-2-carboxamide, acetyltryptamine and fervenulin were produced in Streptomyces sp. MBT76. Another two prominent examples of studies into the biosynthesis of secondary metabolites from marine actinomycetes include those for cytotoxic compounds thiocoraline and BE-14106. Lombó et al. (2006)51 have cloned and analyzed thiocoraline biosynthesis gene cluster from marine Micromonospora isolate.
Secondary metabolites usually comprise various chemical moieties, such as polyketide backbones, amino acid derivatives and sugars. Biosynthesis of secondary metabolite is catalysed by a number of enzymes, usually encoded by genes. These genes occur adjacent to one another in cluster. The gene cluster contains all the necessary genes for the synthesis of a particular secondary metabolite. This includes: the genes that encode the biosynthetic enzymes, regulatory proteins, genes for resistance to the toxic action of secondary metabolites and genes for secretion of the metabolites. Enzymes such as synthase (PKS) and non-ribosomal peptide synthetase (NRPS) are involved in the synthesis of secondary metabolites52. Other enzymes responsible for the synthesis of other constitutive compounds, such as sugars, are often encoded by genes adjacent to the gene cluster. Through processes such as elongation, synthesis, glycosylation, alkylation and oxidation, structurally diverse and complex metabolites are produced. The whole process of production and transportation of secondary metabolites are strictly regulated by transcriptional regulators and transporters53. The genes encoding for tailoring enzymes, transcriptional regulators and transporters are often located adjacent to PKS and NRPS genes. The size of the gene cluster responsible for the synthesis of each secondary metabolite is usually between 10 -100 kb.
Previous studies showed that the gene cluster responsible for the production of secondary metabolites is not found in all bacteria and even in those present it is not uniformly distributed among them. For example Streptomyces coelicolor possesses more than 20 gene clusters while S. avermitilis possesses 30 gene clusters for the synthesis of secondary metabolites54. Genome mining for new candidate secondary metabolic pathways based on clustering and coexpression has proved to be a highly successful approach in microbes 55. This helps to predict the types of antibiotic one might expect to find after extraction and purification. With the growing number of genome nucleotide sequence information in the GenBank and the advent of next generation sequencing it will be possible to search for candidate secondary metabolite gene cluster in a wide range of actinomycetes species. The evolution of microbial natural product collections and development of high-throughput screening methods have attracted researchers to the use of natural product libraries in drug discoveries. Ecopia Biosciences Inc used high-throughput genome scanning to detect secondary metabolite gene clusters, then used the signature sequences to predict the structures56. Actinomycetes continue to be a productive and successful focus for natural products research, with many novel compounds been of eminent pharmacological valuable.
Antibiotics compound produced by actinomycetes
Streptomyces, Micromonospora, Actinomadura, Amycolatopsis, Streptoverticillium, Actinoplanes, Nocardia, Saccharopolyspora Streptosporangium, Streptoalloteichus, Dactylosporangium, Frankia and Streptosporangium spp. are increasingly playing a significant role in the production of a wide range of antimicrobial metabolites of enormous important to the pharmaceutical industries. Important classes of antibiotics produced by actinomycetes include: β lactams, aminoglycosides, lipopeptides, glycopeptides, asamycins, anthracyclines, nucleosides, peptides, polyenes, polyethers, tetracyclines and macrolides.
Aminoglycosides
Streptomycin (Fig.1) is an antibiotic drug, the first of a class of drugs called aminoglycosides to de discovered, and it was the first antibiotic remedy of tuberculosis. It is derived from the actinobacterium Streptomyces griseus. Streptomycin is a bactericidal antibiotic. Generally, aminoglycosides are protein synthesis inhibitors. Tobramycin, kanamycin, paromomycin and spectinomycin (Fig. 1) were an aminoglycoside antibiotic derived from Streptomyces tenebrarius, Streptomyces kanamyceticus, Streptomyces krestomuceticus and Streptomyces spectabilis and used to treat various types of bacterial infections. Neomycin (Fig. 1) is an aminoglycoside antibiotic derived from Streptomyces fradiae and it was in many topical medications such as creams, ointments and eyedrops.
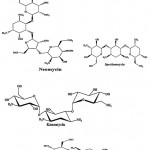 |
Figure 1: Chemical structure of aminoglycoside
|
β-lactams, Glycopeptides, polyenes and actionomycins
Cephamycins (Cephalosporins) (Fig. 2) are a group of [1]β-lactam antibiotics from Streptomyces indicated for use against many bacterial infections. Vancomycin (Fig. 2) is a glycopeptides antibiotic used in the prophylaxis and treatment of infection caused by Gram-positive bacteria. It is a naturally occurring antibiotic made by the soil actinobacterium Amycolatopsis orientalis. Nystatin (Fungicidin) (Fig. 2) is a polyene antifungal medication to which many molds and yeast infections are sensitive, including Candida. These polyene antimycotics are typically obtained from some species of Streptomyces. Actinomycin (Fig. 2) is the most significant
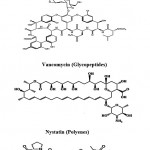 |
Figure 2: Chemical structure of β-lactams, Glycopeptides, polyenes and actionomycins Peptides.
|
Peptides
Peptides are short chains of amino acids. Most of the actinobacterial peptides are cyclic and contain further rare structural elements such as chromophores or uncommon amino acids. Peptides include mechercharmycins which are the new cytotoxic substance obtained from marine-derived Thermoactinomyces sp. YM3-251. Cyclic nature of mechercharmycin A (Fig. 3) exhibited relatively strong antitumor activity and the related compound, mechercharmycin B (Fig. 3) exhibited no such activity57. Thiocoraline (Fig. 3) is a novel cyclic thiodepsipeptide isolated from Micromonospora sp. L-13-ACM2-092 and it showed a potent antitumor activity against P388, A549, and MEL288. There was also a strong antimicrobial activity against Gram-positive microorganisms58,59. Cyclomarin A (Fig. 3) is a new cyclic heptapeptide produced by a Streptomyces sp. It showed significant antiinflammatory activity in both in vivo and in vitro assays43. Piperazimycins (Fig. 3) are cytotoxic hexadepsipeptides obtained from the fermentation broth of a Streptomyces sp. strain CNQ-593, isolated from the marine sediments at a depth of approximately 20 m near the island of Guam. Piperazimycin A exhibited potent in vitro cytotoxic activity against the human colon carcinoma HCT-116 cell line60.
Salinamides A and B (Fig. 3) are bicyclic depsipeptides, produced by Streptomyces sp. CNB-091, isolated from the jelly fish Cassiopeia xamachana. These metabolites are valuable as antibiotic and anti-inflammatory agents 61. Arenamides A–C (Fig. 3) are three new cyclohexadepsipeptides isolated from the fermentation broth of a marine actinobacterial strain identified as Salinipora arenicola CNT-088 which was obtained from the marine sediment sample collected at a depth of 20 m off the Great Astrolab Reef, in the Kandavu Island chain, Fiji, in 2006. Arenamides A and B showed weak in vitro cytotoxicity against human colon carcinoma HCT-116 with IC50 values of 13.2 and 19.2 g/ml, respectively. The effect of arenamides A and B on NFκB activity was studied with stably transfected 293/NFκB Luc human embryonic kidney cells induced by treatment with tumor necrosis factor (TNF). Lucentamycins A–D (Fig. 3) are four new 3-methyl- 4-ethylideneproline-containing peptides isolated from Nocardiopsis lucentensis strain CNR-712, obtained from the sediments of a shallow saline pond from the island of Little San Salvador, the Bahamas. Lucentamycins A and B exhibited significant in vitro cytotoxicity against human colon carcinoma cell line HCT-116 with IC50 values of 0.20 and 11 M, respectively. However, lucentamycins C and D were not cytotoxic in the same assay suggesting that the presence of an aromatic ring is essential for the biological activity of this class of compounds62. Proximicins are novel aminofuran antibiotics produced by Verrucosispora strain MG-37 isolated from the sediments collected in the Raune Fjord, Norway at a depth of 250 m and the Sea of Japan at a depth of 289 m26,63. The characteristic structural element of proximicins is 4-amino-furan-2-carboxylic acid, a hitherto unknown γ-amino acid. Fig. 3 has shown the structure of Proximicins A (a), B (b) and C (c). It has an inhibitory effect against the human gastric adenocarcinoma AGS and hepatocellular carcinoma Hep G264.
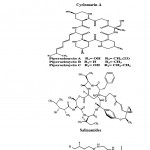 |
Figure 3: Chemical structure of Peptides
|
Chloramphenicol, tetracyclines, anthracyclines and nucleosides
Chloramphenicol (Fig. 4) is an antibiotic derived from Streptomyces venezuelae and it was in the treatment of typhoid65. Tetracycline (Fig. 4) is a borad-specturm antibiotic produced by the Streptomyces sp. indicated for use against many bacterial infections66. Anthrocyclines are a class drugs used in cancer chemotherapy derived from Streptomyces peucetius. The first anthracycline discovered was daunorubicin and doxorubicin (Fig. 4) which is produced naturally by S. peucetius, a species of actinobacteria67. Oxanosine (Fig. 4) was isolated as a novel nucleoside antibiotic from Streptomyces capreolus MG265-CF368.
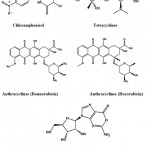 |
Figure 4: Chemical structure of Chloramphenicol, tetracyclines, anthracyclines and nucleosides
|
Macrolides
Macrolides are a cluster of drugs whose activity stems from the presence of a macrolide ring, a large macrocyclic lactone ring to which one or more deoxy sugars may be attached. Generally, macrolides are protein synthesis inhibitors. Chalcomycin A (Fig. 5) is a macrolide antibiotic obtained from the crude extract of the marine Streptomyces sp. M491 from the Qingdao coast (China). It exhibited activity against E. coli, B. subtilis, and S. aureus and a weak inhibition of S. viridochromogenes in biological screening69. Chalcomycin B (Fig. 5) is a new macrolide antibiotic obtained from the culture broth of a marine Streptomyces sp. B7064, isolated from the mangrove sediments near Pohoiki, Hawaii (Pacific Ocean)70.
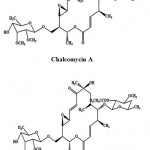 |
Figure 5: Chemical structure of Macrolides
|
Antimalarial compound produced by actinomycetes
Malaria is a highly infectious disease caused by a protozoan parasite of the genus Plasmodium. The transmission of this parasite is by the bite of infectious female Anopheles sp mosquitoes. Five species of Plasmodium are associated with malarial fever viz, P. falciparum, P. vivax, P. Malariae, P. ovale and P. knowlesi. Among which, P. falciparum is extremely virulent. While, P. vivax is relatively less virulent and is more common in all over the world and remain three species are linked to the slight outbreak in several parts of the world. For the control of more virulent malarial parasite Plasmodium falciparum, three approaches were considered. It includes the step up of effective vaccines, vector control and development of new drugs. Due to the exhibition of multiple antigenicity, the development of a vaccine becomes difficult. The vector control shows limited success. On the other hand, malarial parasites show increasing resistance to the existing drug hence, there is an urgent need for new antimalarial agents71,72. There is a need of new drug that ideally directed against new targets such as heme and malarial proteases. However, the isolation of novel enzyme inhibitor from terrestrial sources is uncommon hence marine actinobacteria will provide new potential inhibitors.
Trioxacarcins are the complex compounds which showed higher anti-malarial activity against the malarial pathogens and some of them possess high antitumor and antibacterial activities. Trioxacarcin A, B and C (Fig. 6) obtained from Streptomyces ochraceus and Streptomyces bottropensis73. Some of these compounds possess extremely high antiplasmodial activity, which is comparable to that shown by artemisinin, the most active compound against the pathogen of malaria.
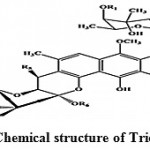 |
Figure 6: Chemical structure of Trioxacarcin
|
New secondary metabolites salinos poramide A (Fig. 7) screened from marine actinomycetes tropica, is a highly potent inhibitor of the human malaria parasite in vitro and in vivo. Moderately conserved sequences of the proteasome subunits across species and exponential growth of the parasite in red blood cells, suggests proteasome as the probable target of salinosporamide A in the malaria parasite. The inhibitory effect of salinosporamide with an IC50 of 11.4 nM is as competent as the majority of conventional antimalarials such as artemisinin or chloroquine. The pure compound, salinosporamide A, was tested for its inhibitory effect against parasite development in vitro (P. falciparum) and in vivo (P.yoelii). The biochemical and structural-based analyses are reliable with the parasite 20S proteasome being the molecular target. The divergence at the structural level facilitates the discovery of an increased specificity of Plasmodium proteasome inhibitors30.
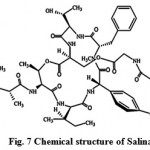 |
Figure 7: Chemical structure of Salinamides
|
Today, there is an urgent need for discovering the new small and inexpensive antimalarials molecules. Using a [3H] hypoxanthine incorporation assay on the parasite’s erythrocytic stages, it was determined that secondary metabolites produced by marine actinomycetes have significant antimalarial activities. These findings displays that the natural products are the most important sources of medicines against the parasite. The crude extract was obtained from marine actinomycetes isolated from the mangrove and sea grass sediments of Dar es Salaam, Tanzania. The hypoxanthine incorporation assay was used to determine parasite inhibition profiles and 50% inhibitory concentration values of the Actinomycetes crude extracts. The three crude extracts showed strong growth inhibition with an IC50 in the low microgram range (0.44 – 7.98 μg/ml)74.
Anti-tumor compounds produced by actinomycetes
Although, recent advances have been done in the field of the molecular biology of cancer stimulation and progression in its different forms, it is still one of the most threatening illnesses which affect human health and quality of life75. One of foremost problem in treatment of various infectious diseases and cancer is the multidrug resistance. There is a need to develop a drug with high great novelty, potency and less toxicity. Marine microbes especially Streptomyces sp., is the major source of the antitumor antibiotics. When these antitumor antibiotics interact with DNA it results to cause cell death76. Anthracyclines are a special class of antitumor antibiotics which act via topoisomerase II inhibition77. An extracellular alkaloid, Pimprinine has (Fig. 8a) been isolated from the culture filtrate of Streptomyces CDRIL-31278. The novel aporphine alkaloid SSV isolated from bioactive streptomyces sp. KS1908, is an antitumor antibiotic obtained from fraction of fermented broth and chemically characterized with advanced spectroscopic data. Hence, aporphine alkaloid SSV has been act as a promising agent for treatment of cancer and microbial infections79. Seven new benzoxazole derivatives, nocarbenzoxazoles A−G (Fig. 8b) were isolated from the halophilic strain Nocardiopsis lucentensis DSM 44048, act as an antitumor activity and structure was elucidated by using NMR spectrometric analysis5.
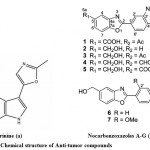 |
Figure 8: Chemical structure of Anti-tumor compounds
|
Antitubercular compounds produced by actinomycetes
Tuberculosis (TB) is a highly infectious disease caused by Mycobacterium tuberculosis, is one among the leading cause of infectious disease worldwide. One of the world population suffered with M. tuberculosis and hence at a risk of active TB80. In the late of 1940s and 1950s, a number of effective antitubercular agents were discovered, with rifampicin (Fig. 9a) getting introduced in 1960s 81. In 1940s, Streptomycin was the first drug to be introduced for the treatment of TB but immediately after its introduction many of the patients started showing resistance against this antibiotic82. Multidrug resistance (combined isoniazid and rifampicin) strongly impacts on control program of TB. However, there is an urgent to discover novel antibiotics against drug resistance M. tuberculosis.
The two natural antibiotics, Lasalocid and Monensin (Fig. 9b and 9c) isolated mainly from fungal mycelium of Streptomyces cinnamonensis (Actinomycetes). These antibiotics belong to the family of the polycyclic carboxylic polyethers 83. Monensin and lasalocid and their metal complexes with Tl (I), La (III) and Gd (III) are active against Mycobacterium tuberculosis. These ionophores could be considered as potential antitubercular agents for the future discovery of new drug design84. Recently, Streptomyces mutabilis isolated from soil samples in Saudi Arabia produced an anti-tuberculous polyketide macrolide identified as treponemycin (Fig. 9d)85. Nybomycin (Fig. 9e) isolated from the culture broth of a marine derived Streptomyces sp. showed potent anti-microbial activity against Mycobacterium sp. in both actively growing aerobic conditions and dormancy induced hypoxic conditions. In addition, compound was also effective to pathogenic strains of M. tuberculosis including clinically isolated strains86.
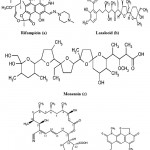 |
Figure 9: Chemical strucutre of Antitubercular compounds
|
Plant endophytes represent the rare and previously undiscovered microbial taxa to reduce the rediscovery of known compounds. 50 strains and 300 crude extracts in total were isolated from traditional Chinese medicines (TCMs) for growth inhibitory activity against Bacillus Calmette- Guérin (BCG)87, an attenuated strain of the bovine tuberculosis bacillus Mycobacterium bovis. The crude extract of Streptomyces sp. strain Y3111 associated with the stems of Heracleum souliei, showed good anti-BCG activity with an MIC value of 12.5μg/mL. The Bioassay-guided fractionations yield the structurally diverse heraclemycins A–D. The structures were determined by different spectroscopic techniques including HRMSESI, 1D NMR, and 2D NMR. Heraclemycin C showed strong anti-BCG activity with a MIC value of 6.25μg/mL. This inferred that the endophytic microbes associated with TCMs can facilitate the discovery of new natural products and potentially new and novel molecular scaffolds for drug discovery88. Nybomycin isolated from the culture broth of a marine derived Streptomyces sp. showed potent anti-microbial activity against Mycobacterium sp. in both actively growing aerobic conditions and dormancy induced hypoxic conditions. In addition, compound 1 was also effective to pathogenic strains of M. tuberculosis including clinically isolated strains.
Anti-diabetic compound produced by actinomycetes
Diabetes mellitus is a metabolic disorder characterized by hyperglycaemia, negative nitrogen balance sometime ketonemia and glycosuria. Resulting either from inadequate secretion of insulin, an inadequate response of target cells to insulin, or combination of these factors. Insulin deficiency is due to functional disorder of the pancreas.
Diabetes mellitus is the highest cause of death among other chronic diseases. More than 95% of diabetes is type 2 diabetes or often called non-insulin dependent diabetes 89. This cannot be cured, but can be controlled. Hence major efforts have been directed towards the development of oral hypoglycaemic drugs, to identify compounds able to enhance insulin action in target tissues. Attention has been focused on searching from terrestrial microorganism to look for new sources of drug and many new families of antibiotics are found from these microorganism.
For the treatment of type-2 diabetes; Voglibose, Acarbose, valienamine, adiposin-1, and trestatin-B were reported from Streptomyces hygroscopicus-limoneus 90,91, S. calvus92,93, and S. dimorphogenes84 respectively. Voglibose (Fig. 10) is an alpha-glucosidase inhibitor that lowers the post prandial blood glucose levels in people with diabetes mellitus. It is produced and marketed in India by trade name Volix® and Vocarb® as90. Acarbose (Fig. 10) is an oral alpha-glucosidase and alpha-amylase inhibitor, first launched by Bayer in Switzerland in 1989 for the oral treatment of type-2 diabetes mellitus95. Valielamine (Fig. 10), a precursor of voglibose and a new aminocyclitol, isolated from the fermentation broth of Streptomyces hygroscopicus subspecies limoneus. It has more potent a-glucosidase inhibitory activity against porcine intestinal sucrase, maltase and isomaltase than valienamine, validamine and hydroxylvalidamine which were reported as building blocks of validamycins and microbial oligosaccharide a-glucosidase inhibitors 96.
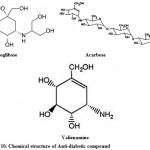 |
Figure 10: Chemical structure of Anti-diabetic compound
|
Insecticidal activity of actinomycetes
As the environmental contamination increases by toxic chemicals, for this alternative approaches for controlling pest populations have become research priorities. For limiting the destructive impacts of pest populations, ecological or biological control methods has evolved 97,98.
Actinomycetes play a significant role in the biological control against insect together with the house fly Musca domestica99, Culex quinquefasciatus100, cotton leaf worm spodopetra liltoralis101, Drosophila melanogaster102, Helicoverpa armigera103, Anopheles mosquito larvae104 and Culex pippins105.
The biological activity of secondary metabolites of some actinomycetes isolates had been investigated against the last instar larvae of the cotton leaf worm Spodoptera littoralis through the food plant (Castor leaves). Streptomyces and Streptoverticillum were the most potent actinomycetes, which cause larval and pupal mortality106. The biological activities of actinomycetes secondary metabolites against some phytopathogenic fungi as well as cotton leaf worm (S. littoralis) were investigated. They showed that the pellets of some Streptomyces isolates were more active against cotton leaf worm than culture filtrates. Generally, isolates S05, S08, S10 and S15 showed 80, 100, 70 and 80% mortality against cotton leaf worm, respectively. The result demonstrated the ability to use such Streptomyces isolates as effective biopesticide agents107.The purified compound, aminoglycosidic antibiotic produced from Streptomyces bikiniensis is also effective against 2nd instar larvae of cotton leaf worm Spodoptera littoralis108.
Antivirial activity of actinomycetes
Marine antiviral agents (MAVAs) represent a significantly unique natural marine resource whose multi-potential uses include the following applications: The biological control of human enteropathogenic virus contamination and disease transmission in sewage-polluted waters. This application would be particularly important to communities that utilize their coastal waters for recreational activities and for food industries (e.g. fish, shellfish), as well as to those regions of the country, such as Hawaii, where the loss of these marine resources would have a devastating effect on the lifestyle and economy of the people and the chemotherapy of viral diseases of humans and lower animals109. Wei et al. (2014)110 isolated a compound Pimprinethine (Fig. 11) and its derivatives from Streptomyces sp, exhibited inhibitory activity against EV71 and ADV-7, slightly activity against CVB3, HSV-1 and H1N1, with a few exceptions, in vitro. These compounds mainly act at the early stage of the EV71 replication period.
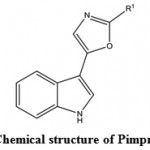 |
Figure 11: Chemical structure of Pimprinethine
|
Enzyme inhibitors from actinomycetes
Enzyme inhibitors have been obtained increasing attention as useful tools, not only for the study of enzyme structures and reaction mechanisms but also for potential utilization in pharmacology111. Imada (2005)112 reported different types of enzyme inhibitors viz. β-glucosidase, N-acetyl-β-D-glucosaminidase, pyroglutamyl peptidase, α-amylase inhibitors from marine actinobacteria and act as the potential source for production of enzyme inhibitors. Pyrizinostatin (Fig. 12) is an inhibitor of pyroglutamyl peptidase, isolated from culture of Streptomyces sp. SA-2289113. Pyrostatin A and B are inhibitors of N-acetyl-β-glucosaminidase, produced by Streptomyces sp. SA-3501111, 112. Raja et al. (2010)114 isolated 17 marine actinobacteria strains from the rhizosphere sediments of mangroves and reported the amylase inhibitor. Ganesan et al. (2011)115 studied three marine actinobacteria from Parangipettai coast, east coast of India and reported the α-glucosidases inhibitory activity
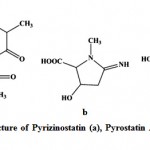 |
Figure 12: Chemical structure of Pyrizinostatin (a), Pyrostatin A (b) and Pyrostatin B (c).
|
Vaccines from actinomycetes
A common problem associated with infectious diseases is chronification of the process. This might be due to an inadequate immune response of the patient or to decreased sensitivity to anti-infective drugs used to treat the disease causing micro-organism. Chronic infections can lead to severe tissue damage and chronically infected individuals can act as a permanent source for the transmission of clinically important bacteria or viruses into the wider population. In addition, the treatment of chronic or recurrent infection is often prolonged and complicated. An early attempt at treating chronic, recurrent and acute infections was the use of the so-called autovaccine116. Autovaccination was regularly performed in human medicine at the beginning of the 20th century. However, since that time, with the exception of some eastern European countries, the use of autovaccination therapy steadily decreased. Today, autogenous vaccines or autovaccines are commonly used in veterinary medicine to treat chronic infectious diseases117. Jost et al. (1999) 118report that insertional inactivation of the A. pyogenes plo gene results in decreased virulence of the plo mutant and that PLO, like other TACYs, is cytotoxic for phagocytic cells. In addition, we provide evidence that recombinant His-tagged PLO (His-PLO) is efficacious as a vaccine in mice.
Strain improvement of actinomycetes
Methods for efficient chemical mutagenesis and recombination by protoplast fusion have been available for strain improvement in actinomycetes for over a quarter of acentury119, 120. More recently, mutagenesis and protoplast fusion have been combined for whole genome shuffling to improve the production of antibiotics4,44 ,121. In addition, a number of molecular engineering strategies, often focused on optimizing precursor levels or expression of rate-limiting enzymes involved in secondary metabolite biosynthesis, have been summarized in other reviews31,122,123, 124. The mutagenesis, recombination, and molecular engineering approaches continue to be very useful tools for industrial-scale strain improvement. Chemical mutagenesis [e.g., by N-methyl-N’-nitro-N-nitrosoguanidine (MNNG), ethyl methanesulfonate (EMS), or nitrous acid (NA)] and physical mutagenesis [e.g., by ultraviolet (UV) light or X-rays)] remain the single most robust methods to generate very high-producing strains for SM production over campaigns spanning many years125. Li et al. (2013)126 reported that the effect of pleuromutilin resistance on antibiotic production in Streptomyces. The results showed that Pler mutants could increase the production of daptomycin by approximately 30%. For genomics applications, the cloning and heterologous expression of silent or poorly expressed secondary metabolite gene clusters provides another approach to produce sufficient yields for isolation and characterization of novel compounds. This approach may be particularly useful for the expression of cryptic pathways from rare actinomycetes that grow slowly or are not amenable to industrial-scale fermentation for other reasons. There are a number of laboratory strains that have been used to express heterologous secondary metabolite gene clusters127, 128.
Novel/New metabolites from marine actinomycetes
Although more than 30,000 diseases have been clinically described, less than one third of these can be treated symptomatically and fewer can be cured. New therapeutic agents are urgently needed to fulfill the medical needs that are currently unmet. Natural products once played a major role in drug discovery. Although the exploitation of marine actinomycetes as a source for discovery of novel secondary metabolites is at an early stage, numerous novel metabolites have been isolated in the past few years. Table 1 shows some examples of new secondary metabolites isolated from marine actinomycetes from 2010 to 2015. This is by no means an exhaustive search of all novel secondary metabolites produced by marine actinomycetes during this 5-year period, nevertheless this list is impressive and illustrate the many different diverse structures with biological activities reported. Among them, a few compounds such as staurosporinone, salinosporamide A, lodopyridone, arenimycin, marinomycins and proximicins from Table 1 are of particular interest due to their rarity and potent and diverse bioactivity. Salinosporamide A (Fig. 13a) is a novel rare bicyclic beta-lactone gamma-lactam isolated from an obligate marine actinomycete, Salinispora tropica7. Salinosporamide A is an orally active proteasome inhibitor that induces apoptosis in multiple myeloma cells with mechanisms distinct from the commercial proteasome inhibitor anticancer drug Bortezomib. It is being developed by Nereus Pharmaceuticals, Inc. (as NPI-0052) and was scheduled to enter clinical studies for treatment of cancer in humans in 2006. NPI-0052 is currently being evaluated in multiple phase I trials for solid tumors, lymphoma and multiple myeloma.
Lodopyridone (Fig. 13b) is a unique alkaloid produced by a marine Saccharomonospora sp. isolated from marine sediments collected at the mouth of the La Jolla Submarine Canyon129. Lodopyridone possess activity against the human colon adenocarcinoma cell line HCT-116 with an IC50 of 3.6 µM. Arenimycin (Fig. 13c) is a new antibiotic belonging to the benzo (β) naphthacene quinone class produced by the obligate marine actinomycete, Salinispora arenicola32. This new structural derivative is the first report of this class of antibiotics from this strain S. arenicola. Arenimycin has effective antibacterial activity against rifampin- and methicillin-resistant Staphylococcus aureus and it exhibits potent antimicrobial activities against drug resistant Staphylococci and other Gram-positive human pathogens. In addition, the Salinispora tropica strain also produces four unprecedented compounds, Sporolide A, Arenicolide A, Cyanosporaside A and Salinispyrone A7. These highlighted structures demonstrate the tremendous potential of marine actinomycetes for the production of novel secondary metabolites. Lipoxazolidinones A–C is a novel 2-alkylidene-5- alkyl-4-oxazolidinones isolated from novel and rare genus Marinispora. The biological activity of lipoxazolidinones exhibited broad spectrum antimicrobial activity similar to that of the commercial antibiotic linezolid (Zyvox), a 2-oxazolidinone130. Lynamicins A–E is a chlorinated bisindole pyrroles isolated from the rare Actinomycete Marinispora sp131.
Wang et al. (2013) 132reported an array of new and novel abyssomicins, a new class of unique polycyclic natural products with potent antibacterial, antitubercular, antitumor and anti-Bacille Calmette Guerin activity by Verrucosispora spp. Gifhornenolones A and B are new terpenoids isolated from the marine ascidian-associated Verrucosispora gifhornensis. The biological activity of gifhornenolone A showed potent inhibitory activity to the androgen receptor133. Thiochondrillines analogs of thiocoraline, are potent cytotoxic thiodepsipeptides isolated from the spongeassociated Verrucosispora sp134.
Table 1: Secondary metabolites isolated from marine actinomycetes from 2010 to 2015
| Organism | Compound | Activity | Reference |
| Dermacoccus sp. | Dermacozines | Cytotoxic, radical
scavenging |
Abdel-Mageed et al. 2010135 |
| Nocardiopsis sp. | TP-1161 | Antibacterial | Engelhardt et al. 2010136 |
| Streptomyces sp. | 2-Allyloxyphenol | Antimicrobial, antioxidant | Arumugam et al.
2010137 |
| Streptomyces sp. | ML-449 | Cytotoxic | Jørgensen et al.
2010138 |
| Micromonospora haikouensi | menaquinones | Antitumor | Xie et al. 2012139 |
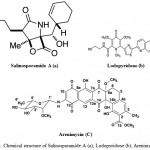 |
Figure 13: Chemical structure of Salinosporamide A (a); Lodopyridone (b); Arenimycin (C)
|
Conclusion
As the scope of naturally found bioactive compounds increases day by day, search for novel active biomolecules increases. From the last many decades research on bioactive molecules produced from marine microorganism has geared up. Among all actinomycetes has proved to be the best. These are diverse in nature having various chemical entities responsible for the activity against many other disease causing microorganisms. Hence these bioactive compounds are considered to be as potent therapeutic agents. However, actual potential of actinomycetes is yet to be discovered. In future we will discover the new activities of microbial metabolites and expand their practical utilization.
References
- Berdy J. Bioactive microbial metabolites. J Antibiot. 2005;58:1–26.
CrossRef - Fenical W., Jensen P. R., Developing a new resource for drug discovery: marine actinomycete bacteria. Nat. Chem. Biol. 2006;2:666–673.
CrossRef - Xiong Z. Q., Wang J. F., Hao Y. Y., Wang Y. Recent advances in the discovery and development of marine microbial natural products. Mar Drugs. 2013;11:700–717.
CrossRef - Zhang Y. X., Perry K., Vinci V. A., Powell K., Stemmer W. P. C., del Cardayre, S.B. Genome shuffling leads to rapid phenotypic improvement on bacteria. Nature. 2002;415:644–646.
CrossRef - Sun M., Zhang X., Hao H., Li W., Lu, C. Nocarbenzoxazoles A–G, Benzoxazoles Produced by Halophilic Nocardiopsis lucentensis DSM 44048. J. of natur. Produt., 2015; 78(8): 2123-2127.
- Ian, E., Malko, D.B., Sekurova, O.N., Bredholt, H., Rückert, C., Borisova, M.E., Albersmeier, A., Kalinowski, J., Gelfand, M.S., Zotchev, S.B. Genomics of sponge-associated Streptomyces spp. closely related to Streptomyces albus J1074: insights into marine adaptation and secondary metabolite biosynthesis potential. PloS one, 2014; 9(5): e96719.
CrossRef - Jensen, P.R., Williams, P.G., Oh, D.C., Zeigler, L., Fenical, W. Species-specific secondary metabolite production in marine actinomycetes of the genus Salinispora. Appl. Environ. Microbiol., 2007; 73:1146–52.
CrossRef - Xu, D.B., Ye, W.W., Han, Y., Deng, Z.X., Hong, K. Natural products from mangrove actinomycetes. Marine drugs., 2014); 12(5): 2590-2613.
CrossRef - Watve, M.G., Tickoo, R., Jog, M.M., Bhole, B.D. How many antibiotics are produced by the genus Streptomyces? Arch. Microbiol., 2001; 176: 386–390.
CrossRef - Simmons, T.L., Coates, R.C., Clark, B.R., Engene, N., Gonzalez, D., Esquenazi, E., Dorrestein, P.C., Gerwick, W.H. Biosynthetic origin of natural products isolated from marine microorganism–invertebrate assemblages. Proc. Natl. Acad. Sci .,USA, 2008; 105:4587–4594.
CrossRef - Xiong, Z. Q., Liu, Q. X., Pan, Z. L., Zhao, N., Feng, Z. X.,Wang, Y. Diversity and bioprospecting of culturable actinomycetes from marine sediment of the Yellow Sea, China. Arch. Microbiol., 2015; 197(2), 299-309
CrossRef - Lane, A.L., Moore, B.S. A sea of biosynthesis: marine natural products meet the molecular age. Nat. Prod. Rep., 2011; 28:411–428.
CrossRef - Zotchev, S.B. Marine actinomycetes as an emerging resource for the drug development pipelines. J. Biotechnol., 2012; 158:168–175.
CrossRef - Dharmaraj, S. Marine Streptomyces as a novel source of bioactive substances. World J Microbiol. Biotechnol., 2010; 26:2123–2139.
CrossRef - Gomez-Escribano, J.P., Alt, S., Bibb, M.J. Next Generation Sequencing of Actinobacteria for the Discovery of Novel Natural Products. Marine Drugs., 2016; 14(4): 78.
CrossRef - Teng, H., Tang, H., Xiao, H., Shu, Y., Li, H. Isolation and identification of actinobacteria from Rhizospheric soil in the Arctic Yellow river station. Advanc. Polar. Sci., 2009; 21(1):33–42.
- Qin, S., Li, J., Chen, H.H., Zhao, G.Z., Zhu, W.Y., Jiang, C.L., Xu, L.H., Li, W.J. Isolation, diversity, and antimicrobial activity of rare Actinobacteria from medicinal plants of tropical rain forests in Xishuangbanna, China. Appl. Environ. Microbiol., 2009; 75:6176–6186.
CrossRef - Mehbub, M.F., Amin, A.K.M.R. Isolation and identification of Actinobacteria from two south Australian marine sponges Aplysilla rosea and Aplysina sp. Bangladesh Res. Publ J, 2012; 7:345–354.
- Bredholt, H.; Fjaervik, E.; Johnsen, G.; Zotchev, S.B. Actinomycetes from sediments in the Trondheim Fjord, Norway: Diversity and biological activity. Mar. Drugs, 2008; 6:12–24.
CrossRef - Duncan, K., Haltli, B., Gill, K.A., Kerr, R.G. Bioprospecting from marine sediments of New Brunswick, Canada: exploring the relationship between total bacterial diversity and actinobacteria diversity. Marine drugs., 2014; 12(2): 899-925.
CrossRef - Jensen, P.R., Moore, B.S., Fenical, W. The marine actinomycete genus Salinispora: A model organism for secondary metabolite discovery. Nat. Prod. Rep., 2015; 32:738–751.
CrossRef - Freel, K.C.; Edlund, A.; Jensen, P.R. Microdiversity and evidence for high dispersal rates in the marine actinomycete ‘Salinispora pacifica’. Environ. Microbiol., 2012; 14:480–493.
CrossRef - Steinert, G., Taylor, M. W., & Schupp, P. J. Diversity of Actinobacteria Associated with the Marine Ascidian Eudistoma toealensis. Marine Biotechnol.,, 2015; 17(4):377-385.
CrossRef - McArthur, K.A., Mitchell, S.S., Tsueng, G., Rheingold, A., White, D.J., Grodberg, J., Lam, K.S., Potts., B.C. Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marine Actinomycete. J .Nat. Prod., 2008; 71:1732–1737.
CrossRef - Subramani, R., Aalbersberg, W. Marine Actinomycetes: an ongoing source of novel bioactive metabolites. Microbiol. Res., 2012; 167:571–580.
CrossRef - Riedlinger, J., Reicke, A., Zähner, H., Krismer, B., Bull, A.T., Maldonado, L.A., Ward, A.C., Goodfellow, M., Bister, B., Bischoff, D. Abyssomicins, inhibitors of the para-aminobenzoic acid pathway produced by the marine Verrucosispora strain AB-18-032. J. Antibiot., 2004; 57:271–279.
CrossRef - Goodfellow, M., Fiedler, H.P., A guide to successful bioprospecting: informed by actinobacterial systematics. Antonie Van Leeuwenhoek, 2010; 98:119–142.
CrossRef - Yuan, M., Yu, Y., Li, H.R., Dong, N., Zhang, X.H. Phylogenetic diversity and biological activity of actinobacteria isolated from the chukchi shelf marine sediments in the Arctic Ocean. Marine drugs., 2014; 12(3): 1281-1297.
CrossRef - Cheng, C., MacIntyre, L., Abdelmohsen, U.R., Horn, H., Polymenakou, P.N., Edrada-Ebel, R., Hentschel, U. Biodiversity, Anti-Trypanosomal Activity Screening, and Metabolomic Profiling of Actinomycetes Isolated from Mediterranean Sponges. PloS one, 2015; 10(9): e0138528.
CrossRef - Prudhomme, J., McDaniel, E., Ponts, N., Bertani, S., Fenical, W., Jensen, P., et al. Marineactinomycetes: a new source of compounds against the human malaria parasite. PLOS ONE, 2008; 3:2335.
CrossRef - Olano, C., Lombo, F., Mendez, C., Salas, J.A. Improving production of bioactive secondary metabolites in actinomycetes by metabolic engineering. Metab. Eng., 2008; 10:281–292.
CrossRef - Asolkar, R.N., Kirkland, T.N., Jensen, P.R., Fenical, W. Arenimycin, an antibiotic effective against rifampin- and methicillin-resistant Staphylococcus aureus from the marine actinomycete Salinispora arenicola. J. Antibiot., (Tokyo), 2010; 63:37–9.
- Penn, K., Jensen, P.R. Comparative genomics reveals evidence of marine adaptation in Salinispora species. BMC genomics, 2012; 13(1): 86.
CrossRef - Donadio, S., Maffioli, S., Monciardini, P., Sosio, M., Jabes, D. Antibiotic discovery in the twenty-first century: current trends and future perspectives. J. Antibiot, 2010; 63:340–423.
CrossRef - Meena, B., Rajan, L.A., Vinithkumar, N.V., Kirubagaran, R. Novel marine actinobacteria from emerald Andaman & Nicobar Islands: a prospective source for industrial and pharmaceutical byproducts. BMC microbiol., 2013; 13(1): 1.
CrossRef - Venter, J.C., Remington, K., Heidelberg, J.F., Halpern, A.L., Rusch, D., Eisen, J.A., Wu, D., Paulsen, I., Nelson, K.E., Nelson, W., Fouts, D.E., Levy, S., Knap, A.H., Lomas, M.W., Nealson, K., White, O., Peterson, J., Hoffman, J., Parsons, R., Baden-Tillson, H., Pfannkoch, C., Rogers, Y.H., Smith, H.O. Environmental genome shotgun sequencing of the Sargasso Sea. Science, 2004; 304: 66–74.
CrossRef - Dastager, S.G., Damare, S. Marine actinobacteria showing phosphate-solubilizing efficiency in Chorao Island, Goa, India. Curr.Microbiol., 2013; 66(5): 421-427.
CrossRef - Sanghvi, G.V., Ghevariya, D., Gosai, S., Langa, R., Dhaduk, N., Kunjadia, P.D., Vaishnav, D.J., Dave, G.S. Isolation and partial purification of erythromycin from alkaliphilic Streptomyces werraensis isolated from Rajkot, India. Biotechnolo.Reports., 2014; 1:2-7.
- Govindarajan, G., Santhi, V.S., Jebakumar, S.R.D. Antimicrobial potential of phylogenetically unique actinomycete, Streptomyces sp. JRG-04 from marine origin. Biologicals, 2014; 42(6):305-311.
CrossRef - Thirumurugan, D., Vijayakumar, R. Characterization and structure elucidation of antibacterial compound of Streptomyces sp. ECR77 isolated from East Coast of India. Curr. Microbiol., 2015; 70(5):745-755.
CrossRef - Benouagueni, S., Ranque, S., Kirane, D.G. A non-polyenic antifungal produced by a Streptomyces yatensis strain isolated from Mellah Lake in El Kala, North-East of Algeria. Journal de Mycologie Médicale/J. of Medi. Mycolo., 2015; 25(1):2-10.
- Braña, A.F., Fiedler, H.P., Nava, H., González, V., Sarmiento-Vizcaíno, A., Molina, A., Acuña, J.L., García, L.A., Blanco, G. Two Streptomyces species producing antibiotic, antitumor, and anti-inflammatory compounds are widespread among intertidal macroalgae and deep-sea coral reef invertebrates from the central cantabrian sea. Microbial ecology, 2015; 69(3):512-524.
CrossRef - Renner, M.K., Shen, Y.-C., Cheng, X.-C., Jensen, P.R., Frankmoelle, W., Kauffman, C.A., Fenical, W., Lobkovsky, E., Clardy, J. Cyclomarins AC, new antiinflammatory cyclic peptides produced by a marine bacterium (Streptomyces sp.). J. Am. Chem. Soc, 1999; 121:11273–11276.
CrossRef - Xu, B., Jin, Z., Wang, H., Jin, Q., Jin, X., Cen, P. Evolution of Streptomyces pristinaespiralis for resistance and production of pristinamycin by genome shuffling. Appl. Microbiol. Biotechnol., 2008; 80:261–267.
CrossRef - Rashad, F.M., Fathy, H.M., El-Zayat, A.S., Elghonaimy, A.M. Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiological research, 2015; 175:34-47.
CrossRef - Marinelli F, Marcone GL, Microbial secondary metabolites. Comprehensive biotechnology (second edition). Burlington, American: Academic Press; 2011. p. 285-297.
CrossRef - Barrios-Gonzalez J, Mejýa A. Production of antibiotics and other commercially valuable secondary metabolites. Current developments in solid-state fermentation. Heidelberg, New York: Springer; 2008. p. 302-336.
CrossRef - Sanchez S, Demain AL. Metabolic regulation of fermentation processes. Enzyme and Microbial Technology. 2002;31:895-906.
CrossRef - Vaishnav P, Demain AL. Unexpected applications of secondary metabolites. Biotechnol. Advan.,. 2010;29:223-229.
CrossRef - Wu, C., Zhu, H., van Wezel, G.P. Choi, Y.H. Metabolomics-guided analysis of isocoumarin production by Streptomyces species MBT76 and biotransformation of flavonoids and phenylpropanoids. Metabolomics, 2016; 12(5): 1-11.
CrossRef - Lombó, F., Velasco, A., Castro, A., de la Calle, F., Brana, A.F., Sánchez-Puelles, J.M., Méndez, C., Salas, J.A. Deciphering the biosynthesis pathway of the antitumor thiocoraline from a marine actinomycete and its expression in two Streptomyces species. Chembiochem, 2006; 7:366–376.
CrossRef - Donadio S, Monciardini P, Sosio M. Polyketide synthases and nonribosomal peptide synthetases: The emerging view from bacterial genomics. Natural Product Reports. 2007;24:1073-1109.
CrossRef - Ichikawa N, Sasagawa M, Yamamoto M, Komaki H, et al. Dobiscuit: A database of secondary metabolite biosynthetic gene clusters. Nucleic Acids Research. 2013;41:D408-D414.
CrossRef - Arias AA, Craig M, Fickers P. Science against microbial pathogens: Communicating current research and technological advances. In: Méndez Vilas A, editor. Gram-positive antibiotic biosynthetic clusters: a review. Badajoz, Spain: Formatex Research Center; 2011. p. 977-986.
- Osbourn A. Secondary metabolic gene clusters: Evolutionary toolkits for chemical innovation. Trends in Genetics. 2010;26:449-457.
CrossRef - Baltz RH. Renaissance in antibacterial discovery from actinomycetes. Curr. Opinion in Pharmacolo.,. 2008;8:557-563.
CrossRef - Kanoh, K., Matsuo, Y., Adachi, K., Imagawa, H., Nishizawa, M., Shizuri, Y. Mechercharmycins A and B, cytotoxic substances from marine-derived Thermoactinomyces sp. YM3-251. J. Antibiot, 2005; 58:289–292.
CrossRef - Perez, B.J., Canedo, L., Fernández, P.J., Silva, E.M. Thiocoraline, a novel depsipeptide with antitumor activity produced by a marine Micromonospora. II. Physico-chemical properties and structure determination. J. Antibiot, 1997; 50:738–741.
CrossRef - Romero, F., Espliego, F., Pérez, B.J., García, d.Q.T., Grávalos, D., De la Calle, F., Fernández-Puentes, J.L., Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J. Antibiot, 1997; 50:734–737.
CrossRef - Miller, E.D., Kauffman, C.A., Jensen, P.R., Fenical, W., Piperazimycins: cytotoxic hexadepsipeptides from a marine-derived bacterium of the genus Streptomyces. J. Org. Chem, 2007; 72:323–330.
CrossRef - Moore, B.S., Trischman, J.A., Seng, D., Kho, D., Jensen, P.R., Fenical, W. Salinamides, antiinflammatory depsipeptides from a marine streptomycete. J. Org. Chem, 1999; 64:1145–1150.
CrossRef - Cho, J.Y., Williams, P.G., Kwon, H.C., Jensen, P.R., Fenical, W. Lucentamycins AD, cytotoxic peptides from the marine-derived actinomycete Nocardiopsis lucentensis. J. Nat. Prod, 2007; 70:1321–1328
CrossRef - Fiedler, H.P., Bruntner, C., Riedlinger, J., Bull, A.T., Knutsen, G., Goodfellow, M., Jones, A., Maldonado, L., Pathom-Aree, W., Beil, W. Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete Verrucosispora. J. Antibiot, 2008; 61:158–163.
CrossRef - Schneider, K., Keller, S., Wolter, F.E., Röglin, L., Beil, W., Seitz, O., Nicholson, G., Bruntner, C., Riedlinger, J., Fiedler, H.-P. Proximicins A, B, and C—antitumor furan analogues of netropsin from the marine actinomycete Verrucosispora induce upregulation of p53 and the cyclin kinase inhibitor p21. Angew. Chem. Int. Ed. 2008, 47:3258–3261.
CrossRef - Falagas, M.E., Grammatikos, A.P., Michalopoulos, A. Potential of old-generation antibiotics to address current need for new antibiotics. Expert.Rev .Anti. Infect. Ther., 2008; 6(5):593–600.
CrossRef - Jukes, T.H. Some historical notes on chlortetracycline. Rev. Infect. Dis., 1985; 7(5):702–7.
CrossRef - Fujiwara, A., Hoshino, T., Westley, J.W. Anthracycline antibiotics. Crit. Rev.Biotech., 1985; 3(2):133–57.
CrossRef - Majumdar, P., Wu, H., Tipton, P., Glaser, R. Oxanosine is a substrate of adenosine deaminase. Implications for the quest for a toxicological marker for nitrosation activity.Chem. Res. Toxicol., 2005; 18(12):1830–41.
CrossRef - Wu, S.J., Fotso, S., Li, F., Qin, S., Laatsch, H. Amorphane sesquiterpenes from a marine Streptomyces sp. J. Nat. Prod, 2007; 70:304–306.
CrossRef - Asolkar, R.N., Maskey, R.P., Helmke, E., Laatsch, H. Chalcomycin B, a new macrolide antibiotic from the marine isolate Streptomyces sp. B7064. J. Antibiot, 2002; 55:893–898.
CrossRef - Oaks, S.C., Mitchell, V.S., Pearson, G.W., Carpenter, C.C.J. Malaria: obstacles and opportunities. Washington: National Academy Press, 1991.
- Olliaro, P., Cattani, J., Wirth, D. Malaria, the submerged disease. JAMA, 1996; 275:230–3.
CrossRef - Maskey, R.P., Helmke, E., Kayser, O., Fiebig, H.H., Maier, A., Busche, A., et al. Anti-cancer and antibacterial trioxacarcins with high anti-malaria activity from a marineStreptomycete and their absolute stereochemistry. J Antibiot., 2004; :771-9.
CrossRef - Sosovele, M.E., Bergmann, B., Lyimo, T.J., Hosea, K.M., Mueller, B.I. Antimalarial activity of marine actinomycetes isolated from dares salaam mangrove sediments. Int. J. of Resear. in Biologi. Sci., 2012; 2:177-181.
- Olano, C., Mendez, C., Jose, A.S. Antitumor compounds from actinomycetes: from gene clusters to new derivatives by combinatorial biosynthesis. Nat. Prod. Rep., 2009; 26:628-660.
CrossRef - Galm, U., Martin, H.H., Steven, G.V.L., Jianhua, J., Jon, S.T. Antitumor Antibiotics: Bleomycin, Enediynes, and Mitomycin. Chem. Rev., 2005;105:739-758
CrossRef - Fujiwara, A., Hoshino, T., Westley, J.W. Anthracycline Antibiotics. Critical Reviews in Biotechnol., 1985; 3:133-157.
CrossRef - Naik, S.R., Harindran, J., Varde, A.B. Pimprinine, an extracellular alkaloid produced by Streptomyces CDRIL-312: fermentation, isolation and pharmacological activity. J Biotechnol., 2006; 88:1-10.
CrossRef - Kadiri, S., Yarla, N.S., Vidavalur, S. Isolation and Identification of A Novel Aporphine Alkaloid SSV, An Antitumor Antibiotic from Fermented Broth of Marine Associated Streptomyces sp. KS1908. J. Marine. Sci. Res. Dev., 2013; 3:1-5.
CrossRef - Smith, I. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev., 2003; 16,463-496.
CrossRef - Pauli, G.F., Case, R.J., Inui, T., Wang, Y., Cho, S., Fischer. N.H., Fransblau, G. New perspectives on natural products in TB drug research. Life Sci., 2005;78:485-94.
CrossRef - Vohra, R., Gupta, M., Chaturvedi, R., Singh, Y. Attack on the scourge of tuberculosis: patented drug targets, recent patents on anti-infective drug discovery 1, 2006;95-106.
- Hankey ME, Hoen MH, Antimicrob. Agents. Chemother. 1967;349.
- Mimouni, M., Khardli, F.Z., Warad, I., Ahmad, M., Mubarak, M.S., Sultana, S., Hadda, T.B. Antimicrobial Activity of Naturally occurring Antibiotics Monensin, Lasalocid and their Metal Complexes. J. Mater. Environ. Sci., 2014;5:207- 214.
- Yassien, M.A., Abdallah, H.M., El-Halawany, A.M., Jiman-Fatani, A.A. Anti-tuberculous activity of Treponemycin produced by a Streptomyces strain MS-6-6 isolated from Saudi Arabia. Molecul., 2015; 20(2):2576-2590.
CrossRef - Arai, M., Kamiya, K., Pruksakorn, P., Sumii, Y., Kotoku, N., Joubert, J.P., Moodley, P., Han, C., Shin, D., Kobayashi, M. Anti-dormant mycobacterial activity and target analysis of nybomycin produced by a marine-derived Streptomyces sp. Bioorganic & medicinal chem., 2015; 23(13):3534-3541.
- Wang, J.F., Dai, H.Q., Wei, Y.L., Zhu, H.J., Yan, Y.M., Wang, Y.H., Long, C.L., Zhong, H.M., Zhang, L.X. Antituberculosis agents and an inhibitor of the para-aminobenzoic acid biosynthetic pathway from Hydnocarpus anthelminthica seeds. Chem. Biodivers., 2010;7:2046-2053.
CrossRef - Liu, M., Wael, M., Mageed, A., Ren, B., Wenni, H., Huang, P., Xiaolin Li.,. Endophytic Streptomyces sp. Y3111 from traditional Chinese medicine produced antitubercular pluramycins. Appl. Microbiol. Biotechnol., 2013;98(3):1077-85.
CrossRef - Bailey, C.J., Day, C. Antidiabetic drugs. Br J Cardiol., 2003;10:128-136
- De Melo, E.B., Gomes, A.D.S., Carvalho, I. ±- and ²-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron, 2006; 62:10277-10302.
CrossRef - Kameda, Y., Asano, N., Yoshikawa, M., Takeuchi, M., Yamaguchi, T., Matsui, K., Horii, S., Fukase, H. Vaholamine, a new ±-glucosidase inhibiting aminocyclitol. produced by Streptomyces hygroscopicus. J. Antibiot., 1984;7:1301-1307.
CrossRef - Mahmud, T. The C7N aminocyclitol family of natural products. Nat. Prod. Rep., 2003; 20:137-166
CrossRef - Truscheit, E., Frommer, W., Junge, B., Muller, L., Schmidt, D.D., Wingender, W. Chemistry and Biochemistry of Microbial –Glucosidase Inhibitors. Angew. Chem. Int. Ed Engl., 1981;20:744–761.
CrossRef - Yokose, K., Ogawa, M., Ogawa, K. New ±-amylase inhibitor, Trestatins III. Structure determination of New Trestatin Components Ro 09-0766, Ro 09-0767 and Ro 09-0768. J. Antibiot., 1984;37:182-186.
CrossRef - Schmidit, D.D., Frommer, W., Junge, B., Muller, L., Wingender, W., Truscheit, E., Schafer, D. ±-Glucosidase inhibitors. Naturwissenschaften, 1977;64:535-536.
CrossRef - Xu, H., Yang, J., Bai, L., Deng, Z., Mahmud, T. Genetically engineered production of 1,1’-bis-valienamine and validienamycin in Streptomyces hygroscopicus and their conversion to valienamine. Appl. Microbiol. Biotechnol. 2009;81(5):895-902.
CrossRef - Nakas, J.P. and C.Hagedorn. Biotechnology of Plant Microbe Interactions. McGraw–Hill, New York, 1990.
- Canaday, C.H. Biological and cultural tests for control of plant diseases. American Photopathological Society, St.Paul, MN, 1995.
- Hussain AA, Mostafa SA, Ghazal SA, Ibrahim SY. Studies on antifungal antibiotic and bioinsecticidal activities of some actinomycete isolates. African J.Mycol . Biotechnol . 2002;10:63-80.
- Sundarapandian, S. , Sundaram, M.D. , Tholkappian, P. , Balasubramanian, V. Mosquitocidal properties of indigenous fungi and actinomycetes against Culex quinquefasciatus Say. J. Biol. Control., 2002;16:89-91.
- Bream, A.S., Ghazal, S.A., Abd el-Aziz, Z.K., Ibrahim, S.Y. Insecticidal activity of selected actinomycete strains against the Egyptian cotton leaf worm Spodoptera littoralis (Lepidoptera:Noctuidae). Meded. Rijksuniv. Gent. Fak. Landbouwkd Toegep. Biol. Wet., 2001; 66:503-512.
- Gadelhak, G.G., EL-Tarabily, K.A., AL-Kaabi, F.K. Insect control using chitinolytic soil actinomycetes as biocontrol agents. Int. J. Agri. Biol., 2005; 7:627-633.
- Sonya, H.M., Osman, G., Mostafa, S. Antagonistic and insecticidal activities of some Streptomyces isolate. Pak. J. Biotechnol., 2007;4:65-71.
- Dhanasekaran, D., Sakthi, V., Thajuddin, N., Panneerselvam, A. Preliminary evaluation of anopheles mosquito larvicidal efficacy of mangrove actinobacteria. Int. J. of Appl. Biol. and Pharmaceutical Technol., 2010;1:374-381.
- El-Khawagh, M.A., Hamadah, Kh. Sh., El- Sheikh, T.M. The insecticidal activity of actinomycetes metabolites, against the mosquito Culex pipieins. Egypt. Acad. J. biolog. Sci., 2011;4(1):103-113.
- Bream, A.S., Ghazal, S.A., Abd el-Aziz, Z.K., Ibrahim, S.Y. Insecticidal activity of selected actinomycete strains against the Egyptian cotton leaf worm Spodoptera littoralis (Lepidoptera:Noctuidae). Meded. Rijksuniv. Gent. Fak. Landbouwkd Toegep. Biol. Wet., 2001; 66:503-512.
- Sonya, H.M., Osman, G., Mostafa, S. Antagonistic and insecticidal activities of some Streptomyces isolate. Pak. J. Biotechnol., 2007;4:65-71.
- El-khawaga, M.A. and M.M.M. Megahed. Antibacterial and insecticidal activity of actinomycetes isolated from sandy soil of (Cairo- Egypt). Egypt. Acad. J. Biolog. Sci., 2012;4:53- 67.
- Yasuhara-Bell, J., & Lu, Y. (2010). Marine compounds and their antiviral activities. Antiviral research, 86(3), 231-240.
CrossRef - Wei, Y., Fang, W., Wan, Z., Wang, K., Yang, Q., Cai, X., Shi, L., Yang, Z. Antiviral effects against EV71 of pimprinine and its derivatives isolated from Streptomyces sp. Virology journal, 2014; 11(1):1.
CrossRef - Imada, C. Enzyme inhibitors of marine microbial origin with pharmaceutical importance. Mar. Biotech., 2004; 6(3):193–8.
CrossRef - Imada, C. Enzyme inhibitors and other bioactive compounds from marine actinomycetes. Antonie Van Leeuwenhoek, 2005; 87(1):59–63.
CrossRef - Aoyagi, T., Hatsu, M., Imada, C., Naganawa, H., Okami, Y., Takeuchi, T. Pyrizinostat: a new inhibitor of pyroglutamyl, peptidase. J. Antibiot, 1992; 45(11):1795–6.
CrossRef - Raja, S., Ganesan, S., Sivakumar, K., Thangaradjou, T. Screening of marine actinobacteria for amylase enzymes inhibitors. Ind. J. Microbiol., 2010; 50(2):233–7.
- Ganesan, S., Raja, S., Sampathkumar, P., Sivakumar, K., Thangaradjou, T. Isolation and screening of α-glucosidase enzyme inhibitor producing marine actinobacteria. Afr. J. Microbiol. Res., 2011; 5(21):3437–45.
CrossRef - Nolte, O., Morscher, J., Weiss, H. E., Sonntag, H. G. Autovaccination of dairy cows to treat post partum metritis caused by Actinomyces pyogenes. Vaccine, 2001; 19(23): 3146-3153.
CrossRef - Weiss, H-E., Bertl, F., Geßler, K. Heilerfolge durch therapeutischen Einsatz von Pseudomonas Aeruginosa autovakzinen in der HNO Infektionskasuistik der Kleintierpraxis. Eine Alternative auch fur den Menschen? Tieraztl Umschau, 1998; 53(1):38–43.
- Jost, B. H., Songer, J. G., Billington, S. J. An Arcanobacterium (Actinomyces) pyogenes mutant deficient in production of the pore-forming cytolysin pyolysin has reduced virulence. Infection and immunity, 1999; 67(4):1723-1728.
- Baltz, R.H. Mutagenesis in Streptomyces. In: Demain AL, Soloman NA (eds) Manual of industrial microbiology and biotechnology. American Society for Microbiol., Washington, DC, 1986;184–190.
- Matsushima, P., Baltz, R.H. Protoplast fusion. In: Demain AL, Soloman NA (eds) Manual of industrial microbiology and biotechnology. American Society for Microbiol., Washington, DC, 1986; 170–183.
- Chen, X., Wei, P., Fan, L., Yang, D., Zhu, X., Shen, W., Xu, Z., Cen, P. Generation of high-yield rapamycin-producing strains through protoplasts-related techniques. Appl. Microbiol. Biotechnol., 2009; 83:507–512.
CrossRef - Baltz, R.H. Gene expression in recombinant Streptomyces. Bioprocess Technol, 1995; 22:309–381.
- Baltz, R.H. New genetic methods to improve secondary metabolite production in Streptomyces. J. Ind. Microbiol. Biotechnol., 1998; 20:360–363.
CrossRef - Baltz, R.H. Genetic methods and strategies for secondary metabolite yield improvement in actinomycetes. Antonie Van Leeuwenhoek, 2001; 79:251–259.
CrossRef - Baltz, R.H. Genetic manipulation of secondary metabolite biosynthesis for improved production in Streptomyces and other actinomycetes. J. of Industr. Microbiol. & Biotechnol., 2015; 1-28.
- Li, L., Ma, T., Liu, Q., Huang, Y., Hu, C., & Liao, G. Improvement of daptomycin production in Streptomyces roseosporus through the acquisition of pleuromutilin resistance. BioMed research international, 2013.
- Baltz, R.H. Streptomyces and Saccharopolyspora hosts for heterologous expression of secondary metabolite gene clusters. J Ind Microbiol Biotechnol, 2010; 37:759–772.
CrossRef - Gomez‐Escribano, J.P., Bibb, M.J. Engineering Streptomyces coelicolor for heterologous expression of secondary metabolite gene clusters. Microbial Biotechnology, 2011; 4(2): 207-215.
CrossRef - Maloney, K.N., MacMillan, J.B., Kauffman, C.A., Jensen, P.R., DiPasquale, A.G., Rheingold, A.L., Fenical, W. Lodopyridone, a structurally unprecedented alkaloid from a marine actinomycete. Organic letters, 2009; 11(23):5422-5424.
CrossRef - Macherla, V.R., Liu, J., Sunga, M., White, D.J., Grodberg, J., Teisan, S., Lam, K.S., Potts, B.C. Lipoxazolidinones A, B, and C: antibacterial 4-oxazolidinones from a marine Actinomycete isolated from a Guam marine sediment. J. Nat. Prod., 2007; 70:1454–1457.
CrossRef - McArthur, K.A., Mitchell, S.S., Tsueng, G., Rheingold, A., White, D.J., Grodberg, J., Lam, K.S., Potts, B.C. Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marine Actinomycete. J. Nat. Prod., 2008; 71:1732–1737.
CrossRef - Wang, Q., Song, F., Xiao, X., Huang, P., Li, L., Monte, A., Abdel-Mageed, W.M., Wang, J., Guo, H., He, W., Xie, F., Dai, H., Liu, M., Chen, C., Xu, H., Liu, M., Piggott, A.M., Liu, X., Capon, R.J., Zhang, L. Abyssomicins from the South China Sea deep-sea sediment Verrucosispora sp.: natural thioether Michael addition adducts as antitubercular prodrugs. Angew Chem. Int. Ed. Engl., 2013; 52:1231–1234.
CrossRef - Shirai, M., Okuda, M., Motohashi, K., Inoto, M., Furihata, K., Matsuo, Y., Shizuri, Y., Seto, H. Terpenoids produced by Actinomycetes: isolation, structural elucidation and biosynthesis of new diterpenes: gifhornenolones A and B from Verrucosispora gifhornensis YM28- 088. J. Antibiot, 2010; 63:245–250.
CrossRef - Wyche, T.P., Hou, Y., Braun, D., Cohen, H.C., Xiong, M.P., Bugni, T.S. First natural analogs of the cytotoxic thiodepsipeptide thiocoraline A from a marine Verrucosispora sp. J. Org. Chem., 2011; 76:6542–6547.
CrossRef - Abdel-Mageed, W.M., Milne, B.F., Wagner, M., Schumacher, M., Sandor, P., Pathom-aree, W., Goodfellow, M., Bull, A.T., Horikoshi, K., Ebel, R. Diederich, M. Dermacozines, a new phenazine family from deep-sea dermacocci isolated from a Mariana Trench sediment. Organic & biomolecular chemistry, 2010; 8(10):2352-2362.
CrossRef - Engelhardt, K., Degnes, K.F., Kemmler, M., Bredholt, H., Fjærvik, E., Klinkenberg, G., Sletta, H., Ellingsen, T.E. and Zotchev, S.B. Production of a new thiopeptide antibiotic, TP-1161, by a marine Nocardiopsis species. Appl. Environ. Microbial., 2010; 76(15):4969-4976.
CrossRef - Arumugam, M., Mitra, A., Jaisankar, P., Dasgupta, S., Sen, T., Gachhui, R., Kumar Mukhopadhyay, U., Mukherjee, J. Isolation of an unusual metabolite 2-allyloxyphenol from a marine actinobacterium, its biological activities and applications. Appl. Microbiol. Biotechnol, 2010; 86:109–117.
CrossRef - Jørgensen, H.; Degnes, K. F.; Dikiy, A.; Fjaervik, E.; Klinkenberg, G.; Zotchev, S. B. Insights into the evolution of macrolactam biosynthesis through cloning and comparative analysis of the biosynthetic gene cluster for a novel macrocyclic lactam, ML-449. Appl. Environ. Microbiol., 2010; 76(1)283-29.
CrossRef - Xie, Q. Y., Qu, Z., Lin, H. P., Li, L., Hong, K. Micromonospora haikouensis sp. nov., isolated from mangrove soil. Antonie van Leeuwenhoek, 2012; 101(3):649-655.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





