Manuscript accepted on : 22 September 2017
Published online on: --
Mukhtar Aliyu1, Muhammad Atiku Kano2, Nasiru Abdullahi2 , Idris Aliyu Kankara3, Salihu Ismail Ibrahim4
, Idris Aliyu Kankara3, Salihu Ismail Ibrahim4 , Yusuf Yunusa Muhammad2
, Yusuf Yunusa Muhammad2 and Imam Abdullahi Abdulkadir1,2
and Imam Abdullahi Abdulkadir1,2
1Department of Biochemistry and Molecular Biology, Federal University, Dutsin-ma, Katsina State-Nigeria.
2Department of Biochemistry, Bayero University, Kano State-Nigeria.
3Department of Science Laboratory Technology, Federal Polytechnic, Kauran-Namoda, Zamfara State-Nigeria.
4Department of Biochemistry, Federal University Dutse Jigawa State-Nigeria.
Corresponding Author E-mail: aaimam.bch@buk.edu.ng
DOI : http://dx.doi.org/10.13005/bbra/2573
ABSTRACT: Fats and oils are part of food constituents, and may play a vital role in cosmetics and pharmaceuticals industries. There are several underutilized plants which their seeds have not been fully studied in terms of oil extraction and characterization. In this work two underutilized plants seeds Nymphaea lotus and Nymphaea pubescens were studied. Oils from these two seeds were extracted using soxhlet extraction with n-hexane. Gas chromatographic coupled mass spectrometry analysis of the N. lotus seed oil showed that linoleic (13.01%), palmitoleic (4.46%), arachidic (9.01%) and stearic (12.45%) acids were the major fatty acids whereas oleic (37.85%), palmitic (23.57%) and stearic (5.71%) were the major fatty acids detected in N. pubescens seed oil. In addition, oil extracted from N. pubescens seed was found to have better quality than N. lotus seeds. The fatty acids composition of N. pubescens seed oil is similar to palm and groundnut oil. Extracted oil from of N. pubescens seed is unsaturated which type is classified in the oleic – linoleic acid group. This work has shown that N. pubescens seed oils have great nutritional and industrial potentials.
KEYWORDS: Fatty Acids; Nymphaea lotus; Nymphaea Pubescens; Nutritional Qualities Seed Oils;
Download this article as:| Copy the following to cite this article: Aliyu M, Kano M. A, Abdullahi N, Kankara I. A, Ibrahim S. I, Muhammad Y. Y, Abdulkadir I. A. Extraction, Characterization and Fatty Acids Profiles of Nymphaea Lotus and Nymphaea Pubescens Seed Oils. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Aliyu M, Kano M. A, Abdullahi N, Kankara I. A, Ibrahim S. I, Muhammad Y. Y, Abdulkadir I. A. Extraction, Characterization and Fatty Acids Profiles of Nymphaea Lotus and Nymphaea Pubescens Seed Oils. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28106 |
Introduction
Oils extracted from plant seed are of nutritional importance, and some may have omega -3 fatty acids. Some plant oils play essential role in curbing disease conditions (Wang, 2004). Besides, some characterized seed oils have been proven to have fatty acids of nutritional as well as nutraceutical importance (Muibat et al., 2011). It is worth to note that more interest is growing in the area of seed oil, this may be associated to their bioactive components. Several bioactive components identified in seed oil are showing different health benefits. In addition fats and oils can extensively be used in food and other needs. Some of these oils have antioxidant properties, a factor strongly considered for therapeutic usage. Antioxidants tends to reduce fat oxidation by scavenging the free radicals generated. Two herbaceous aquatic plants (Nymphaea lotus and Nymphaea pubescens) were studied in this work, these plants are commonly known as white and red lily respectively. These two herbaceous plants are known as hydrophytes, they usually float in water with white or yellow flowers (Wasagu et al., 2015). This work is aimed at extracting characterizing and evaluating fatty acids compositions of Nymphaea lotus and Nymphaea pubescens seed oils and to compare their nutritional qualities with commonly consumed vegetable oils to ascertain their suitability or otherwise for human consumption and industrial applications.
Material and Methods
Sample Identification
Plant samples (Nymphaea lotus and Nymphaea pubescens seeds) were obtained from Guzu- Guzu Dam in Kabo Local Government Area, Kano state of Nigeria. The samples were authenticated by the Department of Plant Biology, Bayero University Kano with accession number BUKHAN 0356 and BUKHAN 0357 for Nymphaea lotus and Nymphaea pubescens seeds respectively.
Preparation of Samples
The samples were washed, drained and dried thoroughly. This was followed by grinding into powder with subsequent drying of the powdered samples at 250C. The samples were placed in clean plastics before analysis and labelled appropriately.
Extraction of Oil using Soxhlet Apparatus
n-Hexane (300 ml) was poured into a flask, with subsequent addition of 50 grams of the powdered sample. The mixture was heated at 600C and at boiling point, the vapour rises and condenses at the top of the Soxhlet apparatus. The condensed liquid drips down to the filter containing the oil to be extracted. The extract flows through the thimble thereby filling the tube and subsequently flows back to the flask. The set-up continued for 30 minutes, then removed removed, dried, cooled and weighed again to estimate the amount of extracted oil. The experiment was replicated with determination of the weight of extracted oil determined at intervals of 30 minutes. Resulting mixture containing the oil was distilled off using simple distillation to recover the solvent. Extracted oil was properly stored in labelled container prior to analysis (AOAC, 2006)
Percentage oil Yield Determination
The percentage oil yield was calculated by using following relation (AOAC, 2000).
![]()
Determination of physicochemical properties of Nymphaea lotus seed oil, Nymphaea pubescens seed oil, Palm oil and Groundnut oil
Determination of Acid Value
The oil sample (0.25g) was placed in flask with addition of 50 ml ethanol (95%). Three drops of 1% phenolphthalein indicator was added and the mixtures was allowed to boil. Titration was done using 0.1N NaOH. Resultant solution was shaked properly as titration proceeds. Appearance of red colour within the first ten seconds was taken as an end point, while the volume of alkali used was noted (Onwuka, 2005).
![]()
A=Volume of Alkali
N=Normality of NaOH
W= Weight of sample (g)
Determination of Saponification Value
The sample (0.26g) was poured in a flask and 25 ml of KOH was added. The mixture was boiled for an hour using a reflux condenser, while the mixture is swirled regularly at different intervals. Excess alkali was determined by titrating with 0.5 N HCl, 3 drops of phenolphthalein was used as as indicator. Blank was determined using KOH under same experimental procedure (AOAC, 2005).
![]()
N= Normality of KOH
A= Volume of HCL (ml) for the sample
B= Volume of HCL used (ml) for blank titration
W= Weight of sample taken (g)
Equivalent weight = molecular weight of KOH = 56.1
Determination of Iodine Value
The sample (0.26g) was placed in a flask and 10 ml of CCL4 together with 20 ml of Wij’s solution were mixed and shaked properly. The resulting mixture was kept safely in dark for thirty minutes at 370C. Potassium iodide (10 percent; 15 ml) and distilled water (100 ml) were later added to the flask. Resulting mixture was titrated against 0.1 M Na2S2O3. Starch was used as an indicator in this titration, the appearance of a colorless solution from a blue black coloration marks the end point. Blank titration was done using with 10 ml CCL4. Iodine value was calculated using the below formula (AOCS, 1998; Kyriakidis and Katsiloulis, 2000; Knothe, 2002; AOAC, 2005).
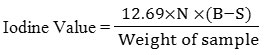
B = 0.1 N Na2S2O3 needed (ml) by blank
S = 0.1 N Na2S2O3 needed (ml) by sample
N = Normality of Na2S2O3
Peroxide Value Determination
The sample (0.26g) was placed in a flask with addition of 7.5 ml of acetic acid and 5 ml chloroform, the mixture was swirled properly until the sample was fully dissolved. Subsequent addition of saturated potassium iodide (0.25 ml) was done with vigorous shaking for a minute. This was followed with addition of 15 ml of distilled water. The reaction mixture was titrated with 0.05 N Na2S2O3 solution, until the appearance of a pale yellow color from the original brownish color Furthermore; Five ml of starch was then added with continuous shaking, the appearance of blue color is an indication of the end point (Onwuka, 2005).
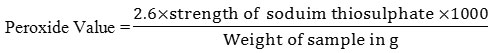
Determination of pH
The sample (2g) was placed in a dry clean beaker containing 13 ml distilled water (hot), it was stirred gently and allowed to cool at 250C. pH meter was inserted to the mixture and the reading was taken (Garba et al., 2015).
Determination of Density
The weight of an empty dried beaker was taken and exactly 50 cm3 of each of the oil sample were measured and pour into the beaker and weighed. The weights of the 50cm3 of the samples were recorded (Garba et al., 2015). The density was calculated thus;
![]()
GC-MS Analysis of Nymphaea lotus seed oil, Nymphaea pubescens seed oil, Palm oil and Groundnut oil
Procedure
Fatty acid Methyl Esters (FAMEs).
Oils extracted from the samples were subjected to heat in presence of NaOH, with subsequent addition of BF3 methanol for esterification. n-Heptane (5 ml) was poured to the reaction mixtures in order obtain methyl esters in organic phase. Further addition of NaCl was done while both the aqueous and organic layers were then separated. The upper part contains n-heptane which was pipetted out and stored (-40C) for GC-MS analysis. (AOAC, 1997). The preparation was done at the National Research Institute of Chemical Technology (NARICT), Zaria, Nigeria.
Fatty Acid Methyl Ester (FAME) Analysis (GC-MS)
The analysis was done using Shimadzu QP2010 quadrupole (GC-MS). The instrument has a capillary in which one microliter of sample was injected into it. The carrier gas used was helium, while the temperatures (injector and detector) were maintained at 280°C. While for the column the temperature was initially 50°C for a minute and left to increase steadily at 5°C per minute till it reached 280°C. Split mode (1:30) was allowed during injection. Separation of the esters were done at a constant pressure (100kPa). Mass spectra of identified peaks as well as their retention time were compared that of a database.
Statistical Analysis
One way analysis of variance (ANOVA) of the SPSS statistical package was used in determining significance difference between the results obtained. The mean ± standard deviation of the triplicates were presented in the results.
Results and Discussion
Physicochemical properties (pH, Density, Peroxide value, Iodine value, free fatty acid, saponification number and peroxide value) of Nymphaea lotus and Nymphaea pubescens seed oils were presented in Table 1. The results were compared with commonly consumed oils.
Table 1: Physicochemical properties of Nymphaea lotus seed oil, Nymphaea pubescens seed oil, Palm oil and Groundnut Oil.
| Parameters | N. lotus Seed Oil | N. pubescens Seed Oil | Palm Oil | GroundnutOil |
| % Oil yield | 13.23 | 9.28 | – | – |
| pH | 5.90 | 6.20 | 6.18 | 5.73 |
| Density (g/cm3) | 0.73 | 0.82 | 0.98 | 0.77 |
| Colour | Pale red | Red | Red | Light yellow |
| Odour | Pleasant | Pleasant | Pleasant | Pleasant |
| Iodine Value (gI2/100g) | 38.85±0.44a | 133.14±0.93a | 128.28±1.16a | 110.82±1.16a |
| Acid Value (mgKOH/g) | 3.19±0.02ab | 2.54±0.08a | 3.00±0.14 | 2.40±0.36b |
| Free fatty acid Value (%) | 1.59±0.01 | 1.27±0.04 | 1.51±0.07 | 1.11±0.04 |
| Saponification Value (mgKOH/g) | 138.98±1.77 | 186.81±161 | 198.68±0.40 | 141.61±0.96 |
| Peroxide Value (mEqO2/kg) | 3.18±0.22a | 5.11±0.40 | 4.62±0.29 | 7.89±0.23a |
Values are expressed as mean ± standard deviation. Same superscripts in a single row indicates statistical significance (p<0.05). Different superscripts indicates no statistical significance (p>0.05).
Table 2 showed the fatty acids detected in Nymphaea lotus and Nymphaea pubescens seed oils using GC-MS analysis. Saturated and unsaturated fatty acids were present in varied proportions
Table 2: GC-MS detected fatty acids in extracted oils of Nymphaea lotus and Nymphaea pubescens seeds
| Nymphaea lotus seed oil | ||||||||||
| RT | Fatty acid | Trivial name | Formula | Mol. Weight | Area% | |||||
| 15.492 | Pentadecanoic acid | – | C15H32O2 | 270 | 6.42 | |||||
| 17.167 | 9,12-Octadecadienoic acid | Linoleic acid | C18H34O2 | 294 | 13.01 | |||||
| 17.342 | 11-Octadecenoic acid | Vaccenic acid | C18H36O2 | 296 | 2.42 | |||||
| 17.442 | Octadecanoic acid | Stearic acid | C18H38O2 | 298 | 12.45 | |||||
| 19.150 | 9-Hexadecenoic acid | Palmitoleic acid | C16H30O2 | 254 | 4.46 | |||||
| 19.217 | Eicosanoic acid | Arachidic acid | C20H42O2 | 326 | 9.01 | |||||
| 20.842 | Docosanoic acid | Behenic acid | C22H46O2 | 354 | 1.10 | |||||
| Nymphaea pubescens seed oil | ||||||||||
| 13.983 | Tetradecanoic acid | Myristic acid | C14H28O2 | 228 | 0.65 | |||||
| 16.158 | Hexadecanoic acid | Palmitic acid | C16H32O2 | 256 | 23.57 | |||||
| 17.908 | 9-Octadecenoic acid | Oleic acid | C18H34O2 | 282 | 37.85 | |||||
| 18.025 | Octadecanoic acid | Stearic acid | C18H36O2 | 284 | 5.71 | |||||
| 19.442 | 9,12-Octadecadienoic acid | Linoleic acid | C18H34O2 | 294 | 0.78 | |||||
| 19.650 | Eicosanoic acid | Arachidic acid | C20H42O2 | 312 | 1.02 | |||||
Table 3 showed the fatty acids detected in Palm oil and Groundnut oil by GC-MS analysis. Saturated and unsaturated fatty acids were present in varied proportions
Table 3: GC-MS detected fatty acids in Palm oil and Groundnut oil
| Palm oil | |||||
| RT | Fatty acid | Trivial name | Formular | Mol. Weight | Area% |
| 10.950 | Octanoic acid | Caprylic acid | C8H16O2 | 144.21 | 0.01 |
| 16.640 | Dodecanoic acid | Lauric acid | C12H24O2 | 200.32 | 0.04 |
| 19.190 | Tetradecanoic acid | Myristic acid | C14H28O2 | 228 | 1.35 |
| 21.750 | Hexadecanoic acid | Palmitic acid | C16H32O2 | 256 | 31.05 |
| 23.634 | 9-Octadecenoic acid | Oleic acid | C18H34O2 | 282 | 33.79 |
| 23.650
24.069 25.265 25.375 |
9,12- Octadecadienoic acid
6-Octadecenoic acid 11-Octadecenoic acid Cis-13-Octadecenoic acid |
Linoleic acid
Petroselinic acid Vaccenic acid – |
C18H34O2
C18H34O2 C18H36O2 C18H34O2 |
294
282.47 296 282.46 |
10.15
11.19 0.22 0.19 |
| Groundnut oil | |||||
| 17.966 | Tetradecanoic acid | Myristic acid | C14H28O2 | 228 | 0.03 |
| 18.025 | Octadecanoic acid | Stearic acid | C18H36O2 | 284 | 3.38 |
| 19.150 | 9-Hexadecenoic acid | Palmitoleic acid | C16H30O2 | 254 | 0.11 |
| 19.217 | Eicosanoic acid | Arachidic acid | C20H42O2 | 326 | 20.00 |
| 19.650
21.562 |
Cis-13-Eicosanoic acid
Hexadecanoic acid |
Paulinnic acid
Palmitic acid |
C20H42O2
C16H32O2 |
312
256 |
0.09
1.04 |
| 23.724 | 9-Octadecenoic acid | Oleic acid | C18H34O2 | 282 | 19.43 |
| 23.154 | 9,12-Octadecadienoic acid | Linoleic acid | C18H34O2 | 294 | 37.54 |
| 24.532 | 9,12,15-Octadecatrienoic acid | Linolenic acid | C18H32O2 | 312 | 0.34 |
Table 4 shows the comparison of the relative abundance of common fatty acids detected by GC-MS analysis in Nymphaea lotus seed oil, Nymphaea pubescens seed oil, palm oil and groundnut oil. Saturated and unsaturated fatty acids were present in varied proportions
Table 4: Comparison of the Fatty acids Profiles of the Nymphaea seed oils extracts with Palm and Groundnut oils
| Fatty Acid | N. lotus Seed oil | N. pubescens Seed oil | Palm oil | Groundnut oil |
| Caprylic acid
Lauric acid Myristic acid |
ND
ND ND |
ND
ND 0.65 |
0.01
0.04 1.35 |
ND
ND 0.03 |
| Palmitic acid
Stearic acid |
4.46
12.45 |
23.57
5.71 |
31.05
ND |
1.04
3.38 |
| Arachidic acid | 9.01 | 1.02 | ND | 20.0 |
| Palmitoleic acid | ND | ND | ND | 0.11 |
| Oleic acid | ND | 37.85 | 33.79 | 19.43 |
| Linoleic acid | 13.01 | 0.78 | 10.15 | 37.54 |
| Linolenic acid | ND | ND | ND | 0.34 |
| Vaccenic acid
Behenic acid Pentadecanoic acid Paulinnic acid Petroselinic acid Cis-13-Eicosenoic acid |
2.42
1.10 6.42 ND ND ND |
ND
ND ND ND ND ND |
0.22
ND ND ND 11.19 0.19 |
ND
ND ND 0.09 ND ND |
ND: means not detected
Table 5 presents saturated fatty acids (SFA), monounsaturated fatty acids (MUFA) and polyunsaturated fatty acids (PUFA) as well as polyunsaturated to saturated fatty acids ratios (P/S index) of Nymphaea lotus seed oil, Nymphaea pubescens seed oil, palm oil and groundnut oil
Table 5: SFA, MUFA, PUFA and P/S Index values of the extracted oils, Palm oil and Groundnut oil values are presented.
| Parameters | N. lotus seed oil | N. pubescens seed oil | Palm oil | Groundnut oil |
| Total SFA (%) | 28.98 | 30.95 | 32.45 | 24.54 |
| Total MUFA (%) | 6.88 | 37.85 | 45.20 | 19.54 |
| Total PUFA (%) | 13.01 | 0.78 | 10.15 | 37.88 |
| P/S Index (%) | 0.45 | 0.03 | 0.31 | 1.54 |
| P+M/S (%) | 0.67 | 1.24 | 1.69 | 2.33 |
The results for Nymphaea lotus seed oil, Nymphaea pubescens seed oil, palm and groundnut oils were presented. The Nymphaea lotus seed oil was pale red and Nymphaea pubescens seed oil was red in colour. The oil contents of Nymphaea lotus and Nymphaea pubescens seeds were 13.23% and 9.28% respectively. The value of oil yield obtained for Nymphaea lotus seed was greater than the literature value reported by Musa et al., (2012) and Wasagu et al., (2015) respectively. Variation in the percentage oil yield might be due to intra species variations, extraction procedures and climatic or soil condition (Raja et al., 201; Mahale and Goswami-Giri, 2012).
Comparing the oil yield of Nymphaea lotus seed oil and Nymphaea pubescens seed oil with known vegetable oil of plant origin such as groundnut (46%) (Adebayo et al., 2012), soybean seed (18%) (Akbar et al., 2009), Shea butter (34%) (Kyari, 2008). Besides the result of the oil yield is similar with other underutilized plant seed, this includes Detarium microcarpum (Nzikou et al., 2009) and Pearsea americana (Sam et al., 2008). Furthermore, the density at 30°C for Nymphaea lotus seed oil, Nymphaea pubescens seed oil and the commercial samples (palm oil and groundnut oil) were presented in table 1. Lower difference were seen when the densities of commercial oil were compared with that of the extracted oils from the samples used. Even though, the high relative densities might suggest an increase molecular weight as well as unsaturation (Onyeka et al., 2005)
The acidity or alkalinity of the samples were done by determining the pH, from the results as presented in Table 1, the samples were slightly acidic, a good indication that free fatty acids (small amount) are present, thus, making them fit for consumption. In situation were the free fatty acids are in higher quantity, might rendered the oil non edible, due to reduction in its shelf life and palatability. In terms of iodine value which is a degree of unsaturation as reported by Hamilton, (1999). Nymphaea pubescens oil contains the highest iodine value (Table 1), as such it is the most unsaturated when compared to the common oils used in this work. Nymphaea lotus oil which had the least iodine value (Table 1) is the most saturated oil, a clear evidence could be seen when the oil is at ambient temperature. Iodine value is directly proportional to the number of saturated bonds (Aremu et al, 2006). Furthermore, the lesser the iodine value the lesser susceptible it is to rancidity (oxidative).
Liquid oils are oils that cannot dry, and are usually not suitable for paint and ink production, although they can be applied in soap production. (Kochhar, 1998). Drying oils needs an iodine value of 130gI2/100g or higher. Nymphaea pubescens seed oil has saponification number similar to palm oil. The saponification number of Nymphaea lotus seed oil can be compared with the values obtained for groundnut oil. Oils needed in production of shampoos and ice-cream require higher saponification value.
Acid value of Nymphaea lotus oil, Nymphaea pubescens oil, and common oils are presented in Table 1. The measurement of the extent of which triacylglycerides are decomposed by lipase is known as the acid value (Inekwe et al., 2012). Most often in indicates edibility of an oil, in this work low acid values were obtained in Nymphaea lotus and Nymphaea pubescens seed oils. The result is comparable to palm oil and groundnut oil. It is worthy to note that oil with low level of acidity can serve as quality indicator of oil (Yousefi et al., 2013). According to FAO/WHO (1991), edible oils should exceed 4 mgKOH/g in terms of acid value. It is striking to note that the samples studied in this work had there acid values within the recommended range for edible oils
Peroxide values of Nymphaea lotus seed oil, Nymphaea pubescens seed oil, palm and groundnut oils are presented in table 1.
A measurement of deterioration levels of lipids due to oxidation is considered as peroxide value (Inekwe et al., 2012). It is a good indicator in determining the usability of oils, when the peroxide value is low, then the stability and quality of oil tends to be high (Yousefi et al., 2013). A low peroxide value of Nymphaea lotus and Nymphaea pubescens seed oils as compared to palm oil shows that the seed oils are stable to relative oxidation. The FAO/WHO (1994) gave a maximum permissible limit for peroxide level which should not exceed 10 milliequivalent of oxygen/kg of the oils. Here we reported the samples used were within the specification recommended by FAO and are suitable for consumption.
The Gas-chromatography coupled with mass spectrometry analysis of free fatty acids in the extracted oil of Nymphaea lotus and Nymphaea pubescens as well as palm oil and groundnut oil were recorded in table 2 and 3 respectively. Table 4 presents comparison of the relative abundance of common fatty acids of Nymphaea lotus seed oil, Nymphaea pubescens seed oil and commonly used vegetable oils. Palmitic acid is predominant saturated acid in Nymphaea pubescens seed oil and palm oil, while arachidic acid was the predominant saturated fatty acid identified in Nymphaea lotus seed oil and groundnut oil. Palmitic acid is an antioxidant, with nematicidal activity commonly used in soap making. Myristic acid was absent in Nymphaea lotus seed oil but present in a lower amount in Nymphaea pubescens seed oil, palm and groundnut oils. There is appreciable high amount of myristic and palmitic acids which rise blood cholesterol (Zock et al., 1994). In addition saturated fatty acids (C12:0-C16:0) have atherogenic effects while stearic acid as neutral effects (Aro et al., 1997; Hu et al., 1999). In Nymphaea lotus seed oil, there was an unusual detection of pentadecanoic acid as well as vaccenic acid. These acids are rarely seen in common vegetable oils, eventhough they are can be seen in animal fats. (Shoji et al., 2005).
Nymphaea pubescens seed oil has high amount of oleic acid (37.85) and trace amount of linoleic acid (0.78%). Studies have shown that the presence of good amount of oleic in diet might serve to decrease the development of atherosclerosis. In addition it may also lower the level of cholesterol in serum, as well as promoting antioxidant defense. Even though oleic acid have been associated to an increase in the permeability of alveolar cells to solutes, as a result of an increase in intracellular calcium levels. The overall effect leads to change in membrane fluidity (Wang et al., 1994; Davidson et al., 2000; Vadaz, 2005).
Linoleic acid was the predominant fatty acids in the extracted oils samples. In Nymphaea lotus seed oil it was 13.01%, this was higher than palm oil (10.15%). Although, groundnut oil was reported to have higher (37.54) linoleic acid. Linoleic acid is important in production of some hormonal substance which may exert different functions including blood clotting and lipid levels. Most beauty products use linoleic acid as part of their ingredients (Shanks and Heise, 1993; Egmond et al., 1996). Linolenic acid was absent in the extracted oils but present in trace amount in groundnut oil
Total unsaturated fatty acid was observed to be higher when compared to the total saturated fatty acids in the samples except in Nymphaea lotus seed oil (table 4). The result conforms to the report that plant oils contains predominantly unsaturated fatty acids ranging between 73.94% of total lipid content (Wardlaw, 2003). Presence of high amount of unsaturated fatty acid than saturated acids could be of nutritional advantage.
The relationship between polyunsaturated fatty acid and saturated fatty acid is presented in Table 4. The relationship is represented as P/S and it is a very good indicator in understanding quality and nutritional value of some oils. Most often oils with P/S value greater than 1 might be of nutritional advantage. On the other hand oils with higher P/S value is an indication that lower lipids are deposited in the body (Lawton et al., 2000). The recommended mean ratio of PUFA/SFA is 0.45 (Da Silva et al., 2002). It is worth to note that the P/S index of Nymphaea lotus seed oil and Nymphaea pubescens seed oil were found to be lower than in palm oil and groundnut oil.
Conclusion
Nymphaea lotus and Nymphaea pubescens seeds are good sources of oils. Nymphaea pubescens seed oil is similar to the common oils used for cooking whereas Nymphaea lotus seed oil is not suitable for human consumption due to its saturated nature. But it could be used in industries as raw material for cosmetics, candles and shoe polish due to its low iodine and saponification values.
Acknowlegdements
We will like to acknowledge the help of the locals at Guzu- Guzu Dam in Kabo Local Government Area, Kano state of Nigeria.
Conflict of Interest
We declare no conflict of interest in this work
Funding Source
The research was supported by a grant from the Federal University, Dutsin-ma, Katsina State-Nigeria.
References
- Adebayo S.E, Orhevba B.A, Adeoye P. A, Fase O.J and Musa J.J. Solvent Extraction and Characterization of Oil From African Star Apple (Chrysophyllum Albidum) Seeds. Academic Research International. 2012;3(2):178-183.
- Akbar E, Zahira Y, Siti K.K, Mana I and Jumat S. Characteristics and composition of Jatropha curcas oil seed from Malaysia and its potential as biodiesel feedstock. European Journal of Scientific Research. 2009; 3:396-403.
- Oils and Fats, in William H. (Ed.), Official Methods of Analysis of AOAC International, Maryland, USA: AOAC International. 2000;1-69.
- Official Method of Analysis of the Association of Official Analytical Chemists, Washington D. C, AOAC. Asian Fisheries Society, Manila Philippines Edition. 2006;18th:810-931.
- Official methods and recommended. Journal of Science Research. 1998; 29(3):396-403.
- Official Methods of Analysis, 2005. Association of Official Analytical Chemists, Washington D.C.
- Aremu M.O, Olaofe O and Akintayo E.T. Chemical composition and physicochemical characteristics of two varieties of bambara groundnut (Vigna subterrenea) flours. Journal Applied Sciences. 2006;6(9):1900– 1903.
CrossRef - Aro A, Jauhiainen M, Partanen R, Salminen I and Mutanen M. Stearic acid, transfatty acids, and dairy fat: effects on serum and lipoprotein lipids, apolipoproteins, lipoprotein (a), and lipid transfer proteins in healthy subjects. Journal of the American Oil Chemists’ Society. 1997; 65(5):1419–1426.
- Silva D.R.G, Prado D.N.I, Matsuhita M and Souza D.N. E. Dietary effects on muscle fatty acid composition of finished heifers. Pesquisa Agropecuaria Brasileira. 2002;37(1):95-101.
CrossRef - Davidson K.G, Bersten A.D, Barr H.A, Dowlin, K.D, Nicholas T.E and Doyle I.R. Lung function, permeability, and surfactant composition in oleic acid-induced acute lung injury in rats. American Journal Physiological Lung Cell and Molecular Physiology. 2000;279:1091-1102.
CrossRef - Egmond A.W, Kosorok M.R, Koscki R, Laxova A and Farrell P.M. Effect of linoleic acid intake on growth of infants with cystic fibrosis. The American Journal of Clinical Nutrition. 1996;63(5):746-752.
CrossRef - FAO/WHO. Fats and oils in human nutrition report of expert committee, Food and Nutrition Paper No.57, FAO 1994; Rome, Italy.
- Flintoff-Dye N.L and Omaye S.T. Antioxidant effects of conjugated linoleic acid isomers human low- density lipoproteins. Nutritional Resource. 2005;1-12
- Garba A.A, Medugu D.W, Gwaski P.A and Amusat R.O. Extraction and characterization of moringa oleifera seed oil. Applied Research Journal. 2015;1(9):473-477.
- Hamilton A. Hand book of Nutritional Biochemistry. Bhati (Eds) Applied Sciences Publishers, London. 1999;193.
- Hu F.B, Stampfer M.J, Manson J.E, Ascherio A, Colditz G.A, Speizer F. E, Hennekens C.H and Willet W.C. Dietary saturated fats and their food sources in relation to the risk of coronary heart disease in women. Journal of the American Oil Chemists’ Society. 1999;70(6):1001–1008.
- Inekwe U.V, Odey M.O, Gauje B, Dakare A.M, Ugwumma C.D and Adegbe E.S. Fatty acid composition and physicochemical properties of jatropha curcas oils from Edo and Kaduna states of Nigeria and India. Annals of Biological Research. 2012;3:4860-4864.
- Knothe G. Structure indices in fatty acid chemistry: How relevant is the iodine value. Journal of American Oil Chemical Society. 2002;l9:847-853.
- Kochhar S .L. Economic Botany in the Tropics. 2nd Edition, Macmillan India Ltd. 1998;354–355.
- Kyari M. Z. Extraction and characterization of seed oils. International Agrophysics. 2008;22:139-142.
- Kyriakidis N.B and Katsiloulis T. Calculation of iodine value from measurements of fatty acid. methyl esters of some oils: comparison with the relevant American oil chemists society method. Journal of American Oil Chemical Society. 2000;77:1235-1238.
CrossRef - Lawton C.L, Delargry H.J, Brockman J, Simith R.C and Blundell J.E. The degree of saturation of fatty acids of fatty acids influences in post ingestive satiety. British Journal of Nutrition. 2000;83(5):473-482.
- Mahale S.M and Goswami-Giri A.S. Composition and Characterization of Refined Oil Compared withIts Crude Oil from Waste Obtained from Mangifera indica.Asian Journal of Research Chemistry. 2012;4(9):1415-1419.
- Muibat O.B, Temitope L.A, Deborah O. A and AbdulKabir O.O. Physicochemical Properties and Fatty Acids Profile of Seed Oil of Telfairia occidentalis .International Journal of Basic & Applied Sciences. IJBAS-IJENS. 2011;11:06.
- Musa A, Birnin-Yauri A, Muhammad C.and Umar A. Proximate composition and mineral analysis of. Nymphaea lotus African Journal of Food Science and Technology. 2012;3(7):4.
- Nzikou J.M, Kimbonguila A, Matos L, Loumouamou B, Pambou-Tobi N. P.G, Ndangui C.B, Abena A. A, Th S, Scher J and Desobry S. Extraction and characteristics of seed kernel oil from mango (Mangifera indica). Research Journal of Environmental and Earth Sciences. 2009;2(1):31-35.
- Onwuka G.I. Food Analysis and Instrumentation: Theory and Practice. Naphthali Prints, Lagos. 2005;89-98.
- Onyeka E.U, Onuegbu N, Onuoha N.U and Ochonogor F. Effect of Extraction Pretreatment on the composition and characteristics of seed and pulp oil of African Black Pear (Dacryodes edulis). Nigerian Food Journal. 2005;23 (1):13-20.
- Sam S.M, Akonye L.A, Mensah S.I and Esenowo G.J. Extraction and Classificationof Lipids From Seeds of Persea americana Miller and Chrysophyllum albidum Don. Scientia Africana. 2008;7(2):35-38.
- Shanks M and Heise H. Plant oils: Tropical application and anti-inflammatory effects (Croton oil test)”. Monatccchr. 1993;179:173.
- Shoji K, Kazutaka Y and Masatoshi N. Rapid discrimination of fatty acid composition in fats and oils by electrospray ionization mass spectrometry. Analytical Sciences. 2005; 21(12):1457–1465.
CrossRef - Vadaz I, Morty R.E, Kohstall M.G, Olschewski A, Grimminger F, Seeger W and Ghofrani H.A. Oleic acid inhabits alveolar fluid reabsorption: a role in acute respiratory distress syndrome? American Journal. Respiriration Critical Care Medecine. 2005;171:469-479.
CrossRef - Wang L.Y, Ma J.K, Pan W.F, Teledovelasquez D, Malanga C.J and Rojanasakul, Y. Alveolar permeability enhancement by oleic acid and related fatty acids: evidence for a calcium-dependent mechanism. Pharmacology Research. 1994;11:513-517.
CrossRef - Zock P.L, Vries D and Katan M.B. Impact of myristic acid versus palmitic acid on serum lipid and lipoprotein levels in healthy women and men. Arteriosclerosis and Thrombosis. 1994;14:567-575.
CrossRef - Wardlaw G.M. Contemporary Nutrition.5th ed., Mc Graw Hill. Higher Education, New York. 2003;143-159.
- Wasagu R.S.U, Lawal M, Galadima L.G and Aliero A.A. Nutritional composition, Anti-nutritional factors, Elemental analysis of Nymphaea lotus (Water lily). Bayero Journal of Pure and Applied Sciences. 2015; 8(1):1–5.
CrossRef - Yousefi M, Nateghi L and Rezaee K. Investigation of physicochemical properties, fatty acids profile and sterol content in Malaysian coconut and palm oil. Annals of Biological Research. 2013;4:214-219.

This work is licensed under a Creative Commons Attribution 4.0 International License.





