How to Cite | Publication History | PlumX Article Matrix
Elsa Dilla Dertyasasa and Woro Anindito Sri Tunjung
Faculty of Biology, Universitas Gadjah Mada Jalan Teknika Selatan, Sekip Utara Yogyakarta, 55281 Indonesia.
Corresponding Author E-mail: wanindito@ugm.ac.id
DOI : http://dx.doi.org/10.13005/bbra/2566
ABSTRACT: Previous studies have reported that a number of organic compounds are present in kaffir lime (Citrus hystrix DC.) leaf extracts. Further research is needed to purify these compounds and determine which are biologically active. The objective of this study is to identify the volatile organic compounds of kaffir lime leaf crude extracts and fractions and to study their bioactivity. Fractionation was performed by the double maceration method, using hexane as the second solvent. TLC was performed to analyze the qualitative separation, whereas the individual constituents were detected using GC-MS. Our results showed that chloroform and ethyl acetate crude extracts contained various volatile organic compounds such as fatty acids, fatty alcohols, prenol lipids, sterol lipids, terpenoids and long chain alkanes. Fractionation separated these compounds into non-hexane fractions, which contained less volatile compounds, and hexane fractions. The volatile compounds of non-hexane fractions were identified to be long chain alkanes, meanwhile the hexane fractions contained terpenoids, fatty acids, fatty alcohols, prenol lipids and sterol lipids. Palmitic acid and terpenoids, such as citronellyl propionate, nerolidol, citronella and caryophyllene oxide were found to be the most dominant bioactive compounds in chloroform and ethyl acetate crude extract and their hexane fractions, which were reported to possess cytotoxicity against cancer cells. Meanwhile in non-hexane fractions, long chain alkanes such as triacontane and hentriacontane were found to be the most dominant bioactive compound which also possessed cytotoxic effect. In conclusion, fractionation using the double maceration method yielded different volatile organic compounds composition with different biological activities. The crude extracts and fractions of kaffir lime leaves were potential to be developed as a traditional medicine for cancer treatment.
KEYWORDS: Double Maceration; Fractionation; Kaffir Lime (Citrus Hystrix DC.); Volatile Organic Compounds
Download this article as:| Copy the following to cite this article: Dertyasasa E. D, Tunjung W. A. S. Volatile Organic Compounds of Kaffir Lime (Citrus Hystrix DC.) Leaves Fractions and Their Potency as Traditional Medicine. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Dertyasasa E. D, Tunjung W. A. S. Volatile Organic Compounds of Kaffir Lime (Citrus Hystrix DC.) Leaves Fractions and Their Potency as Traditional Medicine. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28143 |
Introduction
Traditional medicinal herbs have been widely used as a source of health treatments in many developed and developing countries.1 Many modern medicines are produced indirectly from medicinal herbs.2 Natural herbs provide easy availability, minimum cost, and minimum side effects,3 hence they are considered to be safer therapeutic agents. Kaffir lime (Citrus hystrix DC.) is a native medicinal herb in several Southeast Asia countries, including Indonesia. Its leaves have been traditionally used to treat headache, flu, fever, sore throats, bad breath and indigestion.4
Many kinds of organic compounds have been detected in kaffir lime leaves. Flavonoids, tannin, saponin, glycoside, coumarine, bergamottin and pinene have been identified in kaffir lime leaf extracts.5 In addition, phenolic acids, limonoids, glycerolipids and α-tocopherol were also reported as organic compounds from kaffir lime leaves.6 Citronella was revealed to be the major volatile compounds of fresh kaffir lime extract, followed by linalool, caryophyllene, squalene, dihydrogeraniol and β-citronellol.7 However there is still no information about the volatile organic compounds of kaffir lime leaves originating in Indonesia.
Previous studies reported that kaffir lime leaves exhibited anti-bacterial8 and antioxidant properties,7 antiviral action against the Herpes virus,9 cytotoxicity against cervix and neuroblastoma cancer cells,10 hepatoprotective action against paracetamol induced hepatotoxicity,6 usefulness as a mosquito repellent,11 and anti-inflammatory activities against P. acne12 and edema inducing compound on ICR mouse ears.13 In addition, kaffir lime leaf extracts are also reported to reduce acne scar formation and relieve acne blemishes.12
The individual organic compounds from of kaffir lime leaves might each have different biological activities. Fractionation is needed to separate and purify those compounds, in order to determine each compound’s biological activity and to determine which of these compounds appear to be more biologically active for specific health purposes. Many of the organic compounds of kaffir lime leaves detected from previous studies were non-polar. Hexane is used as the second solvent in double maceration method because of its very low polarity (0.009 out of 1.000),14 hence it is expected to separate the non-polar compounds such as lipids from its more polar constituents. Therefore, the objective of this study is to separate and identify the volatile organic compounds of the chloroform and ethyl acetate extracts of kaffir lime leaves and their fractions. Furthermore this study will also determine each extract’s potential as a natural medicine.
Materials and Methods
Sample Preparations and Extraction
Kaffir lime (Citrus hystrix DC.) leaves were collected from Candirejo Village, Borobudur, Magelang, Central Java, Indonesia. Only the spotless green to dark green leaves were harvested. The leaves were air dried at room temperature and ground to obtain simplicia powder. Extraction was done by maceration method. Leaf powder was soaked by 5 times volume of chloroform or ethyl acetate (Merck), as the first solvents, for 24 h with continuous shaking. The mixture was filtered and its residue was re-extracted three times using the same step. The total filtrate was evaporated to obtain crude extract paste.
Fractionation using Double Maceration
Double maceration was performed using hexane (e-Merck) as the second solvent. Hexane was added in 1:3 volume ratio compared to the accumulated filtrate volume from the first extraction. The crude paste and hexane mixture was homogenized for 30 min and then filtered using filter paper. The filtrate was evaporated to obtain hexane fraction, meanwhile the residue was collected as the non-hexane fraction. The crude extract paste and their fractions were gently blown under nitrogen gas for 5 minutes to remove any remaining solvent.
Thin Layer Chromatography
Thin Layer Chromatography (TLC, e-Merck) was performed to observe the separation profile of kaffir lime leaves after fractionation. A solvent system of hexane: diethyl ether: acetic acid = 80: 20: 2 was used as the mobile phase. Silica Gel 60 F254 was used as the stationary phase. The TLC chamber was saturated by the solvent system for minimum 1 hour before running. Prior to running, each sample was prepared by diluting 10 µg of paste in 50 µL of solvent (hexane for the hexane fraction and chloroform/ethyl acetate for the non-hexane fraction and crude extract). After running, the plate was left dried and then observed under visible light and UV light at 254 nm and 366 nm wavelength.
Gas Chromatography-Mass Spectrometry
The GC-MS analysis was performed using GCMS-QP2010 SE (Shimadzu, Japan) instrument with AGILENT HP 1 MS column (30 m x 0.25 ID x 0.25 um film). Helium was used as the carrier gas at a constant flow of 3.0 mL/min. The oven temperature was initially set for 700C for 5 min and then increased gradually with a rate of 50C/min up to 3100C as the injection temperature. Mass spectrometer was operated with electron ionization system with ionizing energy of 70 eV at a 2500C ion source temperature. Individual volatile compounds were identified by comparing their retention indices and mass spectra to the NIST 62 and WILEY 229 spectra libraries.
Results and Discussion
Hexane in the TLC system solvent acted as the mobile phase which migrated the non-polar compounds further from the starting point. On the other hand, more polar compounds migrated slower due to their greater affinity towards the polar Silica Gel plate. The spot that appeared near the starting line indicated a polar compound. The polarity property decreased as the spot appeared further from the starting line. However, each spot detected in the chromatogram might not represent a single compound, but rather a group of compounds. The chromatogram was observed under UV light with wavelength of 254 nm and 366 nm because no clear spot was detected under the visible light (Fig. 1a-c and Fig. 2 a-c).
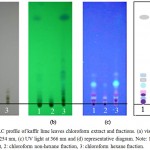 |
Figure 1: TLC profile of kaffir lime leaves chloroform extract and fractions. (a) visible light, (b) UV light at 254 nm, (c) UV light at 366 nm and (d) representative diagram.
|
Note: 1: chloroform crude extract, 2: chloroform non-hexane fraction, 3: chloroform hexane fraction.
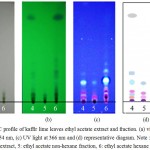 |
Figure 2: TLC profile of kaffir lime leaves ethyl acetate extract and fraction. (a) visible light, (b) UV light at 254 nm, (c) UV light at 366 nm and (d) representative diagram.
|
Note : 4: ethyl acetate crude extract, 5: ethyl acetate non-hexane fraction, 6: ethyl acetate hexane fraction.
The chromatogram of chloroform crude extract, non-hexane and hexane fractions showed the appearance of 7, 5 and 12 spots respectively (Fig. 1d). Meanwhile in the ethyl acetate crude extract, its non-hexane and hexane fractions obtained 7, 4 and 13 spots respectively (Fig. 2d). The number of spots detected in the hexane fractions were higher compared to the chlorofomm and ethyl acetate crude extract, hence fractionation using hexane increased the number of organic compounds in the hexane fractions.
The non-hexane fractions of both extracts obtained fewer spots, indicating that the hexane fractions contained less numbers of organic compounds, compared to the hexane fractions. The spots in the non-hexane fractions were mostly detected near the starting line, suggesting that the non-hexane fractions contain more polar compounds. In addition, the hexane fractions (Fig.1b and Fig.2b) showed non-polar spots migrating further away from the starting line. The very non-polar spot of each hexane fraction (Fig.1b and 2b) also appeared to be thicker compared to the crude extract; this may indicate an increasing content of non-polar compounds in the hexane fraction compare to crude extract.
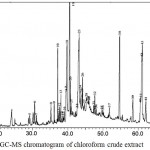 |
Figure 3: GC-MS chromatogram of chloroform crude extract
|
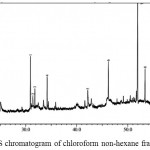 |
Figure 4: GC-MS chromatogram of chloroform non-hexane fraction
|
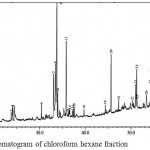 |
Figure 5: GC-MS chromatogram of chloroform hexane fraction
|
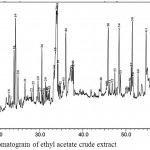 |
Figure 6: GC-MS chromatogram of ethyl acetate crude extract
|
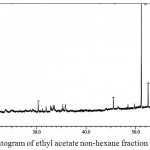 |
Figure 7: GC-MS chromatogram of ethyl acetate non-hexane fraction
|
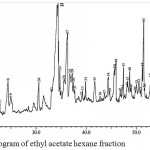 |
Figure 8: GC-MS chromatogram of ethyl acetate hexane fraction
|
The GC-MS chromatogram of kaffir lime leaf chloroform extract exhibited the presence of 45 peaks in crude extract (Fig.3), 9 peaks in non-hexane fraction (Fig.4) and 35 peaks in hexane fraction (Fig.5). Meanwhile the ethyl acetate crude extract, its non-hexane and its hexane fraction showed a total of 65 peaks (Fig.6), 7 peaks (Fig.7) and 70 peaks (Fig.8) respectively. The number of peaks differ in each extract because of the different polarity of each solvent. Ethyl acetate, which was more polar than chloroform, dissolved more organic compounds. Each peak detected in the GC-MS analysis represented an individual compound however, different peaks might result in similar compounds.
Table 1: Volatile compounds identified in chloroform crude extract and their biological activities
| Peak Area (%) | Compounds Name | Groups | Biological Activity |
| 17,24 | 9,12,15-octadecatrien-1-ol | Fatty alcohol | Antioxidant[15] |
| 9,77 | Citronellyl propionate | Terpenoids | Cytotoxicity and antioxidant[16] |
| 8,32 | Palmitic acid | Fatty acid | Cytotoxicity[17], anti-inflammatory [18], antibacterial and antifungal [19] |
| 6,18 | 1,5,9-decatriene,2,3,5,8-tetramethyl | Alkene hydrocarbon | |
| 4,76 | Heneicosane | Long chain alkane | Cytotoxicity, antibacterial[20] |
| 3,20 | Neophytadiene | Alkene hydrocarbon | Antioxidant, analgesic, antipuretic, anti-inflammatory and antimicrobial[21] |
| 2,75 | 14B-pregnane | Sterol lipid | |
| 2,06 | Squalene | Terpenoids | Antioxidant, antitumor against skin carcinogens, skin hydration[22], antibacterial[23] |
| 2,02 | Isophytol | Terpenoids | Antibacterial[24] |
| 1,98 | 9-tricosene | Alkene hydrocarbon | |
| 1,83 | citronellyl acetate | Terpenoids | Cytotoxicity, antioxidant, antiproliferative[25] |
| 1,64 | tetradecanoic acid /myristic acid | Fatty acids | Anti-inflammatory[26], antioxidant, antibacterial[19] |
| 1,48 | Farnesol | Fatty alcohol | Cytotoxicity[27], antioxidant [27,28], antimicrobial [28] |
| 1,25 | 9-octadecanoic acid/ Oleic acid | Fatty acid | Reduce Her-2/NEU overexpression in breast cancer[29], antibacterial[19] |
| 2,18 | (CAS) Phytol | Fatty alcohol | Cytotoxicity[30], anti-inflammatory[31], anti-neoceptive, antioxidant[32] |
| 0,87 | Cyclooctacosane | Alicyclic hydrocarbon | |
| 0,81 | 3-eicosene | Alkene hydrocarbon | Cytotoxicity, antibacterial[20] |
| 0,76 | Citronella | Terpenoids | Cytotoxicity[33], antibacterial[34], Analgesic-like activity on mouse[35] |
| 0,75 | 6-octen-1-ol | Alcohols | |
| 0,74 | Phenol,3,5-bis(1,1-dimethylethyl)- | Phenolic | |
| 0,52 | 1-eicosanol | Fatty alcohols | |
| 0,48 | Benzene,1-methoxy-2-[(4-methoxyphenyl)methyl] | Aromatic hydrocarbon | |
| 0,46 | Spathulenol | Terpenoids | Cytotoxicity, anti-inflammatory, antioxidant[36] |
| 0,38 | trans-linalool oxide | Terpenoids | antioxidant and antibacterial[37] Anxiolytic-like effect[38] |
| 0,37 | hexanedioic acid/Adipic acid | Dicarboxylic acid | |
| 0,36 | Phytene | Diterpenoid alkene | |
| 0,34 | 1,2-benzenedicarboxylic acid,bis(2-ethylhexyl)ester | Aromatic ester | Antioxidant[39] |
| 0,24 | Styrene | Aromatic hydrocarbon | |
| 0,23 | Eicosane | Long chain alkane | Cytotoxicity, antibacterial[24] |
Table 2: Volatile compounds identified in chloroform non-hexane fraction and their biological activities
| Peak Area (%) | Compounds Name | Groups | Biological Activity |
| 30,02 | Triacontane | Long chain alkane | Cytotoxic against melanoma B16F10-Nex2 cancer cell line [40] |
| 29,85 | Octacosane | Long chain alkane | Cytotoxic against melanoma B16F10-Nex2 cancer cell line [40] |
| 10,17 | Octadecyne | Alkyne hydrocarbon | |
| 6,86 | Nonacosane | Long chain alkane | Antibacterial[41], antioxidant[39] |
| 6,27 | 1,7-nonadiene, 4,8-dimethyl | Alkene hydrocarbon | |
| 3,58 | 1-octadecyne | Alkyne hydrocarbon | |
| 1,73 | Eicosyne | Alkyne hydrocarbon |
Table 3: Volatile compounds identified in chloroform hexane fraction and their biological activities
| Peak Area (%) | Compounds Name | Groups | Biological Activity |
| 22.7 | Nerolidol | Terpenoids | Cytotoxicity, antibacterial[20] |
| 13.42 | Citronella | Terpenoids | Cytotoxicity[33], antibacterial[34], Analgesic-like activity on mouse[35] |
| 9.78 | Phytol | Fatty alcohol | Cytotoxicity[30], anti-inflammatory[31], anti-neoceptive, antioxidant[32] |
| 8.43 | Ergost-35-ene-3,5,6,12-tetrol | Sterol lipid | |
| 6.43 | Citronellol | Terpenoids | Cytotoxicity[42], antioxidant and antibacterial[37] |
| 7.9 | β-sitosterol | Sterol lipid | Cytotoxicity, antibacterial[24], anti-hypercholestrolemia[43] |
| 5.43 | Lupeol | Terpenoids | Cytotoxicity[44] |
| 3.43 | Hentriacontane | Long chain alkane | Cytotoxicity[45], anti-inflammatory[46] |
| 2.41 | Pentacosane | Long chain alkane | |
| 1.82 | Stigmasterol | Sterol lipid | Cytotoxicity and antioxidant[47]; antibacterial[48] |
| 1.14 | Spathulenol | Terpenoids | Cytotoxicity, anti-inflammatory, antioxidant[36] |
| 1.13 | Neophytadiene | Alkene hydrocarbon | Antioxidant, analgesic, antipuretic, anti-inflammatory and antimicrobial[21] |
| 1.06 | Caryophyllene oxide | Terpenoids | Cytotoxicity[49], analgesic-like activity on mouse[35], antimicrobial[50] |
| 0.94 | Squalene | Terpenoids | Antioxidant, antitumor against skin carcinogens, skin hydration[22], antibacterial[23] |
| 0.9 | 9-Eicosyne | Alkyne hydrocarbon | |
| 0.79 | 1,6-Octadiene, 2,5-dimethyl | Alkene hydrocarbon | |
| 0.77 | Myrcenol | Fatty alcohol | |
| 0.73 | Palmitaldehyde | Fatty aldehyde | |
| 0.67 | γ-tocopherol | Prenol lipids | Cytotoxicity[51], antibacterial[52], antioxidant, neuroprotective effect[53] |
| 0.67 | Farnesol | Fatty alcohols | Cytotoxicity[27], antioxidant [27,28], antimicrobial[28] |
| 0.51 | Palmitoleic acid | Fatty acids | Lipid lowering activity, anti-inflammatory[54] |
| 0.48 | Terpineol | Terpenoids | Cytotoxicity, apoptosis inducing activity, inhibiting cell cycle at G1 phase[55] |
The five dominant volatile compounds of chloroform crude extract were 9,12,15-octadecatrien-1-ol, citronellyl propionate, palmitic acid, 1,5,9-decatriene-2,3,5,8-tetramethyl and heneicosane (Table 1). These compounds belong to fatty alcohols, terpenoids, fatty acids and long chain alkane classes of compounds. However none of these compounds were detected in non-hexane or hexane fraction. The chloroform non-hexane fraction was dominated by long chain alkane compounds, which were of triacontane, octacosane and nonacosane (Table 2). Meanwhile the chloroform hexane fraction was dominated by terpenoids, phytosterol and fatty alcohol compounds which were nerolidol, citronella, phytol, ergost-35-ene-3,5,6,12-tetrol, citronellol and β-sitosterol (Table 3).
Table 4: Volatile compounds identified in ethyl acetate crude extract and their biological activities
| Peak Area (%) | Compounds Name | Groups | Biological Activity |
| 10.13 | Palmitic acid | Fatty acids | Cytotoxicity[17], anti-inflammatory[18], antibacterial and antifungal[19] |
| 6.67 | Caryophyllene oxide | Terpenoids | Cytotoxicity[49], analgesic-like activity on mouse[35], antimicrobial[50] |
| 6.67 | Citronella | Terpenoids | Cytotoxicity[33], antibacterial[34], Analgesic-like activity on mouse[35] |
| 6.27 | Lanost-7-en-3-one | Lipid sterol | |
| 9.59 | Citronellyl acetate | Terpenoids | Cytotoxicity, antioxidant, anti-proliferative[25] |
| 3.96 | Oleic acid | Fatty acids | Reduce Her-2/NEU overexpression in breast cancer[28], antibacterial[19] |
| 3.85 | Tetratetracontane | Long chain alkanes | |
| 4.1 | Phytol | Fatty alcohol | Cytotoxicity[30], anti-inflammatory[31], anti-neoceptive, antioxidant[32] |
| 4.59 | Citronellyl formate | Terpenoids | |
| 3.3 | Hexatriacontane | Long chain alkanes | |
| 3.52 | Farnesol | Fatty alcohol | Cytotoxicity[27], antioxidant[27,28], antimicrobial[28] |
| 3.58 | 1,7-Nonadiene, 4,8-dimethyl- | Alkene hydrocarbon | |
| 2.58 | Palmitaldehyde | Fatty aldehyde | |
| 2.23 | Nerolidol | Terpenoids | Cytotoxicity, antibacterial[20] |
| 2.4 | Germacrene | Terpenoids | Cytotoxicity[36] |
| 2.17 | Stearyl aldehyde | Fatty aldehyde | |
| 1.62 | Stearyl vinyl ether | ||
| 1.33 | Longipinenepoxide | Terpenoids | |
| 1.31 | 1,4-Heptadiene, 3,3,6-trimethyl | Alkene hydrocarbon | |
| 1.19 | β-Sitosterol | Sterol lipid | Cytotoxicity, antibacterial[24], anti-hypercholestrolemia[43] |
| 1.86 | Citronellol | Terpenoids | Cytotoxicity[42], antioxidant and antibacterial[37] |
| 1.07 | 1-Hexacosanal | Fatty aldehyde | |
| 1.07 | Globulol | Terpenoids | |
| 1.02 | a-Tocopherol | Lipid prenol | Cytotoxicity[51], antibacterial[52], antioxidant, neuroprotective effect[53] |
| 1.54 | Linalool-oxide | Terpenoids | |
| 0.91 | Limonene-oxide | Terpenoids | Analgesic-like activity on mouse[35] |
| 0.91 | Octacosane | Long chain alkanes | Cytotoxic against melanoma B16F10-Nex2 cancer cell line[40] |
| 0.88 | Patchulane | Hydrocarbon | |
| 1.17 | 1,6-Octadiene, 2,5-dimethyl | Alkane hydrocarbon | |
| 0.84 | 2-Dodecanol | Fatty alcohol | |
| 0.79 | Dichlorobenzene | Aromatic hydrocarbon | |
| 0.71 | Squalene | Terpenoids | Antioxidant, antitumor against skin carcinogens, skin hydration[22], antibacterial[23] |
| 0.66 | 9-Eicosyne | Alkyne hydrocarbon | |
| 0.65 | Dihydromyrcenol | Fatty alcohol | Antibacterial, antifungal, cytotoxicity against colorectal, hepatilc and breast cancer cell[56] |
| 0.54 | Myrcenol | Fatty alcohol | |
| 0.47 | Dihydrobrassicasterol | Sterol lipid | |
| 0.41 | Melonal | Fatty aldehyde | |
| 0.39 | Eicosane | Long chain alkanes | Cytotoxicity, antibacterial[24] |
| 0.37 | Geraniol | Terpenoids | |
| 0.32 | 1-octadecyne | Alkyne hydrocarbon | |
| 0.28 | a-Cubene | Terpenoids | |
| 0.27 | β-Pinene | Terpenoids | Cytotoxicity[57], analgesic-like activity on mouse and rat[35] |
| 0.27 | Geranyl Butyrate | ||
| 0.24 | Calarene | ||
| 0.24 | a-Cedrane | Terpenoids | |
| 0.23 | Cyclohexane | Alicyclic hydrocarbon | |
| 0.22 | a-Thujene | Terpenoids | |
| 0.2 | 2-Dodecanal | Fatty aldehyde | |
| 0.19 | a-Calacorene | ||
| 0.18 | 1,5,9-DECATRIENE, 2,3,5,8-TETRAMETHYL | Alkane hydrocarbon |
Table 5: Volatile compounds identified in ethyl acetate non-hexane fraction and their biological activities
| Peak Area (%) | Compounds Name | Groups | Biological Activity |
| 43.11 | Hentriacontane | Long chain alkane | Cytotoxicity[45], anti-inflammatory[46] |
| 35.37 | Nonacosane | Long chain alkane | Antibacterial[41], antioxidant[39] |
| 8.3 | Octacosane | Long chain alkane | Cytotoxic against melanoma B16F10-Nex2 cancer cell line[40] |
| 3.86 | 1,2,3-Propanetriol, monoacetate (Glycerol acetate) | Glycerolipid | |
| 2.87 | Tetradecanal (Myristaldehyde) | Fatty aldehydes | |
| 2.72 | Linalool-oxide | Terpenoids |
Table 6: Volatile compounds identified in ethyl acetate hexane fraction and their biological activities
| Peak Area (%) | Compounds Name | Groups | Biological Activity |
| 14.23 | Palmitic acid | Fatty acids | Cytotoxicity[17], anti-inflammatory[18], antibacterial and antifungal[19] |
| 7.08 | Lanost-7-en-3-one | Sterol lipids | |
| 6.24 | Phytol | Fatty alcohols | Cytotoxicity[30], anti-inflammatory[31], anti-neoceptive, antioxidant[32] |
| 4.65 | Citronella | Terpenoids | Cytotoxicity[33], antibacterial[34], Analgesic-like activity on mouse[35] |
| 4.47 | Tetratetracontane | Long chain alkanes | |
| 4.36 | 1,7-Nonadiene, 4,8-dimethyl- | Alkene hydrocarbon | |
| 3.2 | Hexatriacontane | Long chain alkanes | |
| 3.18 | Oleic acid | Fatty acids | Reduce Her-2/NEU overexpression in breast cancer[28], antibacterial[19] |
| 3.07 | Caryophyllene oxide | Terpenoids | Cytotoxicity[49], analgesic-like activity on mouse[35], antimicrobial[50] |
| 2.9 | Linoleidic acid methyl ester | Fatty acids Ester | |
| 2.55 | Thunbergol | Terpenoids | |
| 2.02 | Undecylenic aldehyde | Fatty aldehyde | |
| 1.74 | 2-Decyn-1-ol | Alcohol | |
| 1.56 | Globulol | Terpenoids | |
| 1.54 | 3-Buten-2-ol, 2,3-dimethyl- | Branched alcohol | |
| 1.5 | Palmitaldehyde | Fatty aldehyde | |
| 1.64 | Myrcenol | Fatty alcohols | |
| 4.02 | Citronellol | Terpenoids | Cytotoxicity[42], antioxidant and antibacterial[37] |
| 1.13 | Farnesol | Fatty alcohols | Cytotoxicity[27], antioxidant[27,28], antimicrobial[28] |
| 1.13 | Widdrol | Aromatic hydrocarbon | |
| 1.50 | Neophytadiene | Alkene hydrocarbon | Antioxidant, analgesic, antipuretic, anti-inflammatory and antimicrobial[21] |
| 1.06 | Stearic acid | Fatty acids | Antibacterial[19] |
| 0.97 | β-sitosterol | Sterol lipids | Cytotoxicity, antibacterial[24], anti-hypercholestrolemia[43] |
| 0.94 | Citronellyl propionate | Terpenoids | Cytotoxicity and antioxidant[16] |
| 0.91 | 2-Hexyl-1-decanol | Alcohols | |
| 0.79 | Squalene | Terpenoids | Antioxidant, antitumor against skin carcinogens, skin hydration[22], antibacterial[23] |
| 0.74 | 8-Hexadecanol | Fatty alcohol | |
| 0.67 | Eicosane | Long chain alkanes | Cytotoxicity, antibacterial[24] |
| 0.61 | Geranyl linalool | Terpenoids | |
| 0.61 | n-hentriacontanol | Fatty alcohol | |
| 0.47 | Heptacosane | Long chain alkanes | |
| 0.43 | Caryophyllene | Terpenoids | Antimicrobial and antioxidant[58] |
| 0.4 | Dihydromyrcenol | Fatty alcohols | Antibacterial, antifungal, cytotoxicity against colorectal, hepatilc and breast cancer cell[56] |
| 0.36 | Methyl Palmitate | Fatty acids Ester | |
| 0.34 | Dihidrobrassicosterol | Sterol lipids | |
| 0.32 | 3-Hexen-1-ol | Fatty alcohols | |
| 0.29 | Citronellyl acetate | Terpenoids | Cytotoxicity, antioxidant, antiproliferative[25] |
| 0.26 | α-tocopherol | Lipid Prenol | Cytotoxicity[51], antibacterial[52], antioxidant, neuroprotective effect[53] |
| 0.24 | α-copaene | Terpenoids | |
| 0.23 | 4,5-dimethyl-3-heptanol | Branched alcohol | |
| 0.23 | Germacrene | Terpenoids | Cytotoxicity[36] |
| 0.19 | Trans-dodec-5-enal | Fatty aldehyde | |
| 0.16 | Linalool | Terpenoids | Analgesic-like activity on mouse[35] |
| 0.25 | Dihydrocarveol | Terpenoids | |
| 0.15 | Methyl Citronellate | Terpenoids | |
| 0.15 | n-octyl phthalate | Dicarboxylic ester | |
| 0.11 | α-Muurolene | Terpenoids | |
| 0.1 | δ-Cadinene | Terpenoids |
The ethyl acetate crude extract was dominated by terpenoids and fatty alcohol compounds which were palmitic acid, citronellyl acetate, caryophyllene oxide, citronella and Lanost-7-en-3-one (Table 4). Similar compounds were detected in the hexane fraction however, with different peak areas. The ethyl acetate hexane fraction was dominated by palmitic acid, Lanost-7-en-3-one, phytol, citronella, caryophyllene oxide and citronellyl acetate (Table 6). Meanwhile the non-hexane fraction was dominated by long chain alkane hydrocarbons, which were octacosane, hentriacontane and nonacosane (Table 5). Hentriacontane and nonacosane, on the other hand, were not detected in the ethyl acetate crude extract.
Fractionation by the double maceration method separated the organic compounds of kaffir lime leavf extracts based on like-dissolve-like principle. The non-polar constituents were more soluble in the hexane fraction. The more polar constituents, on the other hand, were left insoluble as the non-hexane fraction.
The GC-MS analysis in this study was performed to detect volatile organic compounds of kaffir lime leaf extracts. Each compound has a different boiling point and polarity, resulting in different retention times and mass spectra which are then used to identify a single compound. The number of peaks detected in ethyl acetate crude extract was higher compared to chloroform crude extract. Ethyl acetate has a polarity index of 4.4 whereas chloroform’s polarity index is 4.1.59 Both solvents are considered as moderately polar.60 Ethyl acetate dissolved more organic compounds due to its hydrogen bonding acceptor and high dipolarity-polarizability properties,making it a stronger solvent.59
TLC and GCMS results showed similar phenomena. TLC chromatograms suggested that the non-hexane fraction contained more polar compounds, while the hexane fraction contained more non-polar compounds. Kaffir lime leaves have been reported to contain glycosides, flavonoids, saponin and tannin5 which are considered more polar than terpenoids. In addition, other polar compounds such as flavonone glycosides, namely hesperidine and neohesperidine,61 as well as some phenolic acids such as vanillic acid, coumaric acid and benzoic acid62 were also detected in kaffir lime leaves. However, none of these polar compounds were detected in this study. This may be because polar compounds tend to be less volatile due to their high boiling point. Other detection methods, such as LC-MS might be needed for further analysis in order to determine the non-volatile compounds of kaffir lime fractions.
In this study, the diversity of volatile compounds detected in the non-hexane fractions of both extracts were lower compared to the hexane fractions. This may suggest that the majority of organic compounds contained in the non-hexane fractions were polar and non-volatile compounds, hence they were not detected using GC-MS.
Hexane fractions of both chloroform and ethyl acetate extract were revealed to contain more varieties of non-polar volatile compounds compared to the non-hexane fractions. Fatty acids, terpenoids, fatty alcohols and sterol lipids composed the majority of volatile organic compounds detected. These compounds possess various biological activities. Palmitic acid, the most dominant saturated fatty acid detected in this study, is reported to induce apoptosis in human leukemic cell lines.17 Saturated fatty acids in general were also reported to possess antibacterial activity against methicillin-resistant Staphylococcus aureus. 63 Phytol, as the major fatty alcohol detected, is reported to have various medicinal bioactive properties, including anti-allergy, anti-inflammatory,64 anti-schistosomiasis,65 antineoceptive, anti-oxidant activities and anticancer activities32
Terpenoids were detected as the major constituents of volatile secondary metabolites in kaffir lime leaves. Terpenoids have been reported to exhibit anticancer activity in cervical cancer cells,66 oral squamosa carcinoma67 and induce cell death in breast and prostate cancer.68 In addition, terpenoids, as the major components of essential oils in plant, were reported to have anti-oxidant, antimicrobial69 repellent, insecticidal70 antifungal71 antihelminthic against Haemonchus contortus72 antiviral against HPV (Herpes simplex virus)73 and antibacterial activity against respiratory tract pathogens.74
Sterol lipids, or phytosterols specifically, also made up a large component of kaffir lime leaf extract fractions. Phytosterols are reported to have lipid lowering activities and thus reduce the risk of coronary and cardiovascular diseases.75 Phytosterols reduce the total cholesterol and low density lipoprotein (LDL) levels in blood by inhibiting cholesterol absorption in intestinum.75 In addition, phytosterols also inhibited cancer cell growth and induced apoptosis in cancer cell,76 thus making its potential as anticancer agent.
Some minor compounds were also detected in the hexane fractions which were fatty aldehydes, prenol lipids and aromatic hydrocarbons. Prenol lipids such as tocopherol is widely reported to possess cytotoxicity,51 antioxidant and anti-bacterial activity52 and also exhibit a neuroprotective effect against H2O2 and BSO oxidative stress.53 However, no biological activity information of the fatty aldehydes and aromatic hydrocarbon compounds have been reported.
The non-hexane fractions of both extracts revealed the presence of aliphatic hydrocarbon such as long chain alkanes, which have also been reported to possess several biological activities. Triacontane, octacosane and hentriacontane have potential as anticancer agents. Triacontane and octacosane were reported to be cytotoxic against melanoma B16F10-Nex2 cancer cell line,40 meanwhile hentriacontane induced cell death in lymphoma cancer cell line45 as well as possessing anti-inflammatory activity46 by inhibiting the activation of caspase-1 pro-inflammatory agents.45 In addition, another long chain alkane detected in both non-hexane fractions, nonacosane, was also reported to have anti-bacterial and antioxidant properties.39,41
Groups of fatty acids, fatty alcohols, prenol lipids, terpenoids, phytosterols and long chain alkenes were also detected in the crude extracts. However they appear in different amounts and composition compared to their fractions. Several compounds were only detected in their fractions and were missing in the crude extracts. It has been suggested that fractionation using the double maceration method yields different volatile organic compounds, possibly because different solubility in hexane separates the larger and complex molecules of crude extracts into their simpler constituents. This may be followed by further fractionation to purify a single compound. In addition, double maceration also affected the composition of kaffir lime volatile compounds through the increased or decreased abundance (peak area) of some individual compounds.
Generally, the volatile organic compounds of kaffir lime leaf crude extracts and fractions possess various biological activities, including anticancer (cytotoxicity, apoptosis inducing activity, anti-proliferative), antimicrobial (antibacterial, antifungal, antiviral), antioxidant, anti-inflammatory, lipid lowering effect, anxiolytic-like effect, anti-neoceptive and analgesic-like effect which make each of them potential to be developed as a protective or therapeutic agents for various health care applications. However further research still needs to be done in order to examine their biological activities through both in-vitro and in-vivo studies.
Conclusion
In conclusion, the double maceration method separated the volatile organic compounds of kaffir lime crude extracts into non-hexane and hexane fractions. Non-hexane fractions contained more long chain alkanes. Meanwhile the hexane fractions contained more secondary metabolite terpenoids and lipids (fatty acids, fatty alcohol, fatty aldehydes, prenol lipids and sterol lipids). Fractionation using the double maceration method yielded different volatile organic compounds with different biological activities, and it will be useful to further investigate these for their potential use as medicinal therapeutic agents.
Acknowledgements
We sincerely acknowledge Faculty of Biology, Universitas Gadjah Mada, for funding our study through BPPTNBH research grant 2016 (to W.A.S.T)
References
- Modak M, Dixit P, Londhe J, Ghaskadbi S, Devasagayam T.P.A. Indian herbs and herbal drugs used for the treatment of diabetes. J. Clin. Biochem. Nutr. 2007;40(3):163-73.
CrossRef - Gomathi D, Kalaiselvi M, Ravikumar G, Devake K, Uma C. GC-MS analysis of bioactive compounds from the whole plant ethanolic extract of Evolvulus alsinoides (L.) L. J.. Food. Sci. Technol. 2015;52(2):1212-17.
CrossRef - Ghosh G, Panda P, Rath M, Pal A, Sharma T, Das D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacognosy Res. 2015;7(1):110-13.
CrossRef - Hutadilok-Toawatana N, Chaiyamutti P, Panthong K, Mahabusarakam W, Rukachaisinkul V. Antioxidative and free radical scavenging activities of some plants used in Thai folk medicine. Pharm. Biol. 2006; 44(3):221-8.
CrossRef - Butryee C, Sungpuag P, Chitchumroonchokchai C. Effect of Processing on the Flavonoid Content and Antioxidant Capacity of Citrus hystrix Leaf. Int. J. Food Sci. Nutr. 2009;2:162–74.
CrossRef - Abirami A, Nagarani G, Siddhuraju P. Hepatoprotective effect of leaf extracts from Citrus hystrix and C. maxima against paracetamol induced liver injury in rats. Food science and human wellness. 2015; 4(1):35-41.
CrossRef - Ratseewo J, Tangkhawanit E, Meeso N, Kewseejan N, Siriamornpun S. Changes in antioxidant properties and volatile compounds of kaffir lime leaf as affected by cooking processes. Int. Food. Res. J. 2016;23(1):188-96.
- Kooltheat N, Kamuthachad L, Anthapanya M, Samakchan N, Sranujit R.P, Potup P, Ferrante A, Usuwanthim K. Kaffir lime leaves extract inhibits biofilm formation by Streptococcus mutans. Nutrition 2016; 32(4):486-90.
CrossRef - Chowdhurry A, Alam M.A, Rahman M.S, Hossain M.A, Rashid M.M. Antimicrobial, antioxidant and cytotoxic activities of Citrus hystrix DC. Fruits. J. Pharm. Sci. 2009;8(2):177-80.
- Tunjung W.A.S, Cinatl Jr.J, Michaelis M, Smales C.M. Anti-cancer effect of kaffir lime (Citrus hystrix DC.) leaf extract in cervical cancer and neuroblastoma cell lines. Procedia Chem. 2015;14:465-8.
CrossRef - Tawtsin A, Wratten S.D, Scott R, Thavara U, Techadamrongsin Y. Repellency of volatile oils from plants against three mosquito vectors. N. Engl. J. Med. 2001;26:76-82.
CrossRef - Lertsatitthanakorn P, Taweechaisupapong S, Aromdee C, Khunkitti. In vitro bioactivities of essential oils used for acne control. Int. J. Aromather. 2006;16:43-49.
CrossRef - Murakami A, Nakamura Y, Koshimizu K, Ohigashi H. Glyceroglycolipids from Citrus hystrix, a traditional herb in Thailand, potently inhibit the tumorpromoting activity of 12-O-tetradecanolphorbol 13-acetate in mouse skin. J. Agric. Food. Chem. 1995;43:2779-2783.
CrossRef - Chueahongthong F, Ampasavate C, Okono S, Tima S, Anuchapreeda S. Cytotoxic effects of crude kaffir lime (Citrus hystrix DC.) leaf fractional extracts on leukemic cell lines. J. Med. Plant. Res. 2011;5(14):3097-3105.
- Wang L, Yang Z, Wang S, Wang S, Liu J. Antioxidant and antibacterial activities of Camptotheca acuminate D. seed oil. Afr. J. Microbiol. 2011; 5(32):5854-5862.
- Fayed S.A. Antioxidant and anticancer activities of Citrus reticulate (Petitgrain Mandarin) and Pelargonium graveolens (Geranium) essential oils. Res. J. Agric. Biol. Sci. 2009;5(5):740-747.
- Harada H, Yamashita U, Kurihara H, Fukushi E, Kawabata J, Kamei Y. Antitumor activity of palmitic acid found as selective cytotoxic substance in a marine red alga. Anticancer Res. 2002;22(5):2587-2590.
- Aparma V, Dileep K.V, Mandal P.K, Karthe P, Sadasivan C, Haridas M. Anti-inflammatory property of n-hexadecanoic acid: structural evidence and kinetic assessment. Chem. Biol. Drug. Des. 2012;80(3):434-439.
CrossRef - Agoramoorthy G, Chandrasekaran M, Venkatesalu V, Hsu M.J. Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from india. Braz. J. Microbiol. 2007;38:739-742.
CrossRef - Hsouna A.B, Trigu M, Mansour R.B, Jarrava R.M, Damaka M, Jaoua S. Chemical composition, cytotoxicity effect and antimicrobial activity of Ceratonia siliqua essential oil with preservative effects against Listeria inoculated in minced beef meat. Intl. J. Food. Microbiol. 2011;148(1): 66-72.
CrossRef - Raman B.V, Samuel L.A, Pardha S.M, Narashimha R.B, Naga V.K.A, Sudhakar M. Radhakrishnan T.M. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012;5(2):99-106.
- Huang Z.R, Lin Y.K, Fang J.Y. Biological and pharmacological activities of squalene and related compounds : potential uses in cosmetic dermatology. Molecules. 2009;14:540-554.
CrossRef - Rajeswari G, Murugan M, Mohan V.R. GC-MS analysis of bioactive components of Hugonia mystax L. (Linaceae). Res. J. Pharm. Biol. Res. Sci. 2012;3(4):301-308.
CrossRef - Akapuaka A, Kwenchi M.M.E, Dashak D.A, Dildar A. Biological activities of characterized isolates of n-hexane extract of Azadirachta indica A. Juss (Neem) leaves. Nat. Sci. 2013;11(5):141-147.
CrossRef - Ramadan M.M, El-Ghorab A.H, Ghanem K.Z. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. J. Arab. Soc. Med. Res. 2015;10(2):56-64.
CrossRef - Sharma N, Samarakoon K.W, Gyawali R, Park Y, Lee S, Oh S.J, Lee T, Jeong D.K. Evaluation of the antioxidant, anti-inflammatory and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014;19:14567-14581.
CrossRef - Vinholes J, Goncalves P, Martel F, Coimbra M.A, Rocha S.M. Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem. 2014;156:204-2011.
CrossRef - Derengowski L.S, De-Souza-Silva C, Braz S.V, Mello-De-Sousa T.M, Bao S.N, Kyaw C.M, Silva-Pereira I. Antimicrobial effect of farnesol, a Cancida albicans quorum sensing molecule, on Paracoccidiodes brasiliensis growth and morphogenesis. Ann. Clin. Microbiol. Antimicrob. 2009;8:13. doi: 10.1186/1476-0711-8-13.
CrossRef - Menendez J.A, Velon L, Colomor R, Lupu L. Oleic acid, the main monounsaturated fatty acid of olive oil, suppresses HER-2/neu (erbB-2) expression and synergistically enhances the growth inhibitory effects of trastuzumab (Herceptin) in breast cancer cells with HER-2/neu oncogene amplification. Ann. Oncol. 2005;16(3):359-371.
CrossRef - Pejin B, Kojic V, Bogdanovic G. An insight into the cytotoxic activity of phytol at in vitro conditions. Nat. Prod. Res. 2014;28(22):2053-2056.
CrossRef - Silvia R.O, Sousa F.B, Damasceno S.R, Carvalho N.S, Silvia V.G, Oliveira F.R, Sousa D.P, Aragao K.S, Barbosa A.L, Freitas R.M, Medeiros J.V. Phytol a diterpene alkohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014;28(4):455-464.
CrossRef - Santos C.C. d.M.P, Salvadori M.S, Mota V.G, Costa L.M, de Almeida A.A.C, de Oliveira G.A.L, Costa J.P, de Sousa D.P, de Freitas R.M, de Almeida R.N. Antiineoceptive and antioxidant activities of Phytol in vivo and in vitro models. J. Neurosci. 2013; doi:10.1155/2013/949452.
CrossRef - Stone S.C, Vasconcellos F.A, Leonardo E.J, doAmaral R.C, Jacob R.G, Leivas-Leite F.P. Evaluation of potential use of Cymbopogon sp. essential oils, (R)- citronellal and N-citronellylamine in cancer chemotherapy. Inl. J. Appl. Res. Nat. Prod. 2008;6(4):11-15.
- Lucas G.C, Alves E, Pereira R.B, Perina F.J, de Souza R.M. Antibacterial activity of essential oils on Xanthomonas vesicatoria and control of bacterial spot in tomato. Pesq. Agropec. Bras. 2012;47(3):351-359.
CrossRef - de Sousa D.P. Analgesic-like activity of essential oils constituents. Molecules. 2011;16:2233-2252.
CrossRef - Miguel M.G. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. 2010;15:9252-9287.
CrossRef - Pansanit A, Pripdeevech P. Constituents, antibacterial and antioxidant activities of essential oils from Trachelospermum jasminoides flowers. Nat. Prod. Commun. 2014;9(12):1791-1794.
- Souto-Maior F.N, deCarvalho F.L, deMorais L.C.S, Netto S.M, deSousa D.P. Anxiolytic-like effects of inhaled linalool oxide in experimental mouse anxiety models. Pharmacol. Biochem. Behav. 2011;100(2):259-63.
CrossRef - Marrufo T, Nazzaro F, Mancini E, Fratianni F, Coppola R, deMartino L, Agostinho A.B, deFeo V. Chemical composition and biological activity of the essential oil from leaves of Moringa oleifera Lam. Cultivated in Mozambique. Molecules. 2013;18:10989-11000.
CrossRef - Figueiredo C.R, Matsuo A.L, Pereira F.V, Rabaca A.N, Farias C.F, Girola, N, Massaoka M.H, Azevedo R.A, Scutti J.A.B, Arruda D.C, Silva L.P, Rodrigues E.G, Lago J.H.G, Travassos L.R, Silva R.M.G. Pyrustegia venusta heptane extract containing saturated aliphatic hydrocarbons induces apoptosis on B16F10-Nex2 melanoma cells and display antitumor activity in vivo. Pharmacogn. Mag. 2014;10(Suppl 2): S363-76.
CrossRef - Konovalova O, Gergel E, Herhel V. GC-MS analysis of bioactive components of Shepherdia argentea (Pursh.) Nutt. From Ukrainian flora. Pharma Innovation. 2013;2(6):7-12.
- Yousefzadi M, Heidari M, Akbarpour M, Mirjalili M.H, Zeinali A, Parsa M. In vitro cytotoxic activity of the essential oil of Dorema ammoniacum D.Don. Middle East J. Sci. Res. 2011;7(4):511-514.
- Saeidnia S, Azadeh M, Ahmad G, Mohammad R.A. The story of beta-sitosterol: a review. European J. Med. Plants. 2014;4(5):590-609.
CrossRef - Chudzik M, Korzonek-Szalacheta I, Krol W. Triterpenes as potentially cytotoxic compounds. Molecules. 2015;20:1610-1625.
CrossRef - Quintanilla-Licea R, Morad-Castillo R, Gomez-Flores R, Laatsch H, Verde-Star M.J, Hernandez-Martinez H, Tamez-Guerra P, Tamez-Guerra R, RodriguezPadilla C. Bioassay-guided isolation and identification of cytotoxic compounds from Gymnosperm glutonisum leaves. Molecules. 2012;17:11229-11241.
CrossRef - Kim S.J, Chung W.S, Kim S.S, Ko S.G, Um J.Y. Antiinflammatory effect of Oldenlandia diffusa and its constituent, hentriacontane, through suppression of caspase-1 activation in mouse peritoneal macrophages. Phytother. Res. 2011;25(10):1537-1546.
CrossRef - Karau G.M, Njagi E.N.M, Machocho A.K, Wangai L.N, Nthinga M.J. Chemical composition and in-vitro antioxidant activities of Ocimum americanum. Adv. Anal. Chem. 2015;5(2):42-49.
- Swamy M.K, Arumugam G, Kaur R, Ghasemzadeh A, Yusoff M.M, Sinniah U.R. GC-MS based metabolite profiling, antioxidant and antimicrobial properties of different solvent extracts of Malaysian Plectranthus amboinus Leaves. Evid. Based Complement. Alternat. Med. 2017; doi:10.1155/2017/15176833.
- Miraj S, Azizi N, Kiani S. A review of chemical components and pharmacological effects of Melissa officinalis L. Der Pharmacia Lettre 2016;8(6):229-237.
- Matasyoh J.C, Kiplimo J.J, Karubio N.M, Hailstroks T.P. Chemical composition and antimicrobial activity of essential oil of Tarchonanthus camphoratus. Food Chem. 2007;101(3):1183-1187.
CrossRef - Jiang Q, Wong J, Fyrst H, Saba J.D, Ames B.N. γ-Tocopherol or combinations of vitamin E forms induce cell death in human prostate cancer cells by interrupting sphingolipid synthesis. Proc. Natl. Acad. Sci. 2004;101(51):17825-30.
CrossRef - Al-Salih D.A.A.K, Aziz F.M, Mshimesh B.A.R, Jehad M.T. Antibacterial effect of vitamin E : in vitro study. Res. J. Biol. 2013;7(2):22-23.
- Sue-Mian T, Ngah W.Z.W, Top G.M, Mazlan M. Comparison of the Effects of α-Tocopherol and γ-Tocotrienol against Oxidative Stress in Two Different Neuronal Cultures. Sains Malays. 2010;39(1):145-156.
- Morse N. Lipid lowering and anti-inflammatory effect of palmitoleic acid: evidence from preclinical and epidemiological studies. Lipid Technol. 2015;27(5):107-111.
CrossRef - Noor R, Astuti I, Mustofa. Cytotoxicity of a-terpineol in HeLa cell line and its effects to apoptosis and cell cycle. J. Med. Sci. 2014;46(1):1-9.
- Al-Gendy A.A, Nematallah K.A, Zaghloul S.S, Ayoub N.A. Glucosinolates profile, volatile constituents, antimicrobial, cytotoxic activities of Lobularia libyca. Pharm. Biol. 2016;54(12):3257-63.
CrossRef - Bakurnga-Via I, Hzounda J.B, Fokou P.V.T, Tchokouaha L.R.Y, Gary-Bobo M, Gallud A, Garcia M, Walbadet L, Secka Y, Dongmo P.M.J, Boyon F.F, Menut C. Composition and cytotoxic activity of essential oils from Xylopia aethiopica (Dunal) A. Rich, Xylopia parviflora (A. Rich) Benth.) and Monodora myristica (Gaertn) growing in Chad and Cameroon. BMC Complement. Altern. Med. 2014;14:125. doi: 10.1186/1472-6882-14-125.
CrossRef - Silverio M.S, Del-Vechio-Vieira G, Pinto M.A.O, Alves M.S, Sousa O.V. Chemical composition and biological activities of essential oils of Eremanthus erytropappus (DC) McLeisch (Asteraceae). Molecules 2013;18:9785-96.
CrossRef - Hansen S.H, Pedersen-Bjergaard S. (Eds): Bioanalysis of pharmareuticals: sample preparation, separation techniques and mass spectrometry. West Sussex: John Wiley and Sons. 2015;87-88.
CrossRef - Leonard J, Lygo B, Procter G. (Eds): Advanced practical organic chemistry, 3rd edn. Florida: CRC Press. 2013;139.
- Niamthiang S, Sawasdee P. Cholinesterase inhibitors from the leaves and roots of Citrus hystrix DC. Pure and applied chemistry international conference. 2013.
- Jamilah B, Gedi M.A, Suhaila M, Zaidul I.S.Md. Phenolics in Citrus hystrix levaes obtained using supercritical carbon dioxide extraction. Intl. Food Res. J. 2011;18(3):941-948.
- Kitahara T, Koyama N, Matsuda J, Aoyama Y, Hirakata Y, Kamihira S, Kohno S, Nakashima M, Sasaki H. Antimicrobial activity of saturated fatty acids and fatty amines against methicillin-resistant Staphylococcus aureus. Biol. Pharm. Bull. 2004;27(9):1321-1326.
CrossRef - Ryu K.R, Choi J.Y, Chung S, Kim D.H. Anti-scratching behavioral effect of the essential oil and phytol isolated from Artemisia princeps Pamp. in mice. Planta Med. 2011;77(1):22-26.
CrossRef - de Moraes J, deOliveira R.N, Costa J.P, Junior A.L.G, deSousa D.P, Freitas R.M, Allegretti S.M, Pinto P.L.S. Phytol, a diterpene alcohol from chlorophyll, as a drug against neglected tropical disease Schistosomiasis mansoni. PLoS Negl. Trop. Dis. 2014;8(1):e2617. doi.10.1371/journal.pntd.0002617.
CrossRef - Aryanti, Ermayanti T.M, Mariska I, Bintang M. Isolation of anticancer compound of Artemisia cina hairy root and its inhibitor activity on cervix cancer cell. Indonesian J. Pharm. 2005;16(4):192-96.
- Arundina I, Budhy S.T.I, Luthfi M, Indrawati R. Identifikasi Kromatografi Lapis Tipis Sudamala (Artemisia vulgaris L.). Maj. Ked. Gi. Ind. 2015;1(2):167-71.
- Yang H, Dou Q.P. Targeting Apoptosis Pathway with Natural Terpenoids: Implications for Treatment of Breast and Prostate Cancer. Curr. Drug Targets 2010;11(6):733-744.
CrossRef - Joshi S, Chanotiya C.S, Agarwal G, Prakash O, Pant A.K, Mathela C.S. Terpenoid compositions, and antioxidant and antimicrobial properties of the rhizome essential oils of different Hedychium species. Chem. Biodivers. 2008;5(2):299-309.
CrossRef - Karpouhtsis I, Pardali E, Feggou E, Kokkini S, Scouras Z.G, Mavragani-Tsipidou P. Insecticidal and genotoxic activities of oregano essential oils. J. Agric. Food. Chem. 1998;46:1111-1115.
CrossRef - Fontenelle R.O, Morasi S.M, Brito E.H, Brilhante R.S, Cordeiro R.A, Nascimento N.R, Kerntopf M.R, Sidrim J.J. Antifungal activity of essential oils of Croton species from the Brazilian caatinga biome. J. Appl. Microbiol. 2008;104:1383-1390.
CrossRef - Pessoa L.M, Morais S.M, Bevilaqua C.M, Luciano J.H. Antihelminthic activity of essential oil of Ocinum gratissimum Linn. and eugenol against Haemonchus contortus. Vet. Parasitol. 2002;109:59-63.
CrossRef - Khan M.T, Ather A, Thompson K.D, Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005;67:107-119.
CrossRef - Srisukh V, Tribuddharat C, Nukoolkarn V, Bunyapraphatsara M, Chokephaibulkit K, Phoomniyom S, Chuanphuang S, Srifuengfung S. Antibacterial activity of essential oils from Citrus hystrix (makrut lime) against respiratory tract pathogens. Sci. Asia. 2012;38:212-217.
CrossRef - Ogbe R.J, Ochalefu D.O, Mafulul S.G, Olaniru O.B. A review on dietary phytosterols: their occurrence, metabolism and health benefits. Asian J. Plant. Sci. Res. 2015;5(4):10-21.
- Woyengo T.A, Ramprasath V.R, Jones P.J.H. Anticancer effects of phytosterols. Eur. J. Clin. Nutr. 2009;63(7):813-820
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





