How to Cite | Publication History | PlumX Article Matrix
Osama A. Abbas1, Hassan I. El-Sayyad2* and Zahraa A. Greash1
1Zoology Department, Faculty of Science, Port Said Univ, Egypt.
2Zoology Department, Faculty of Science, Mansoura Univ, Egypt.
Corresponding Author E-mail : elsayyad@mans.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2814
ABSTRACT: High cholesterol diet is associated cardiovascular disorder and other metabolic diseases represent the public health problem. However, little detail information about its effects on spinal cord structure and function. Barley is one of the cereal grains rich of nutrients and shows medicinal importance. The present study assessed its role on hypercholesterolemia induced toxicological aspects on spinal cord. In the present study, sixty virgin female and twenty adult male albino rats (Rattus norvegicus) weighing approximately 100±10gm body weight, at ratio of 1 male/3 females were used for experimental work. Virgin females were fed 4 months before matting on a diet containing 3 % cholesterol. The virgin females of both control and hypercholesterolemic females were mated with fertile males (1male/3 females) for overnight and observing sperm in vaginal smears in the next morning for determining onset of gestation. The pregnant were arranged into 4 groups (n=15) ; control (C), barley supplemented group (B) (10%), hypercholesterolemic-group (H) (3%) and experimental hypercholesterolemic & malted barley group (H+B). Dams of both control and experimental groups were sacrificed post 21 weeks post-partum and their cervical spinal cord were removed. The spinal tissues were processed for histological , immunohistochemical of caspase 3 and glial fibrillary acidic protein (GFAP) and transmission electron microscopy. In addition, biochemical assessments of antioxidant enzymes (SOD, GSH), malondialdhyde, phospholipids, iron, caspase 4, 8-hdG, neutrotransmitters (DA-5-HT and γABA),cytokines(IL6717), leptin, homocysteine and amyloid–β. The present findings clarified that dietary supplementation on malted barley for mothers fed on a high cholesterol diet throughout pregnancy and lactation period improved the picture of neurons at light and ultrastructural level, restored myelination and reduced caspase 3 and GFAP immunohistochemistry. At the levels of the assessed biomarkers, the malted barley restored the levels of the assayed of both the antioxidant enzymes and neurotransmitters and phospholipids and reduced the high level of caspase 3, 8-hdG, cytokines,leptin,homocysteine, amyloid–β and iron.Thus , the malted barley can be used in combination with other pharmaceutical drugs in improvement or prevention the neurotoxicity due to elevated blood cholesterol level.
KEYWORDS: Cardiovascular Disorder; High Cholesterol Diet; Immunohistochemistry; Metabolic Diseases; Toxicological Aspects
Download this article as:| Copy the following to cite this article: Abbas O. A, El-Sayyad H. I, Greash Z. A. Dietary Malted Barley Grain Improved Structure and Function of Spinal Cord of Mother Rats fed on a high cholesterol diet. Biosci Biotech Res Asia 2020;17(1). |
| Copy the following to cite this URL: Abbas O. A, El-Sayyad H. I, Greash Z. A. Dietary Malted Barley Grain Improved Structure and Function of Spinal Cord of Mother Rats fed on a high cholesterol diet. Biosci Biotech Res Asia 2020;17(1). Available from: https://bit.ly/33NPu5m |
Introduction
The spinal cord is involved in the transmission of the neuronal impulses from the brain and various motor and sensory neurons to the various appendicular regions (Grillner and Robertson, 2015). Rabbit fed on a high cholesterol diet had increased average fat deposits in the vertebral body and interferes with compromised blood supply and spinal damage caused by vertebral degeneration (Sasani et al., 2016). High-fat diet associated with obesity has been found to cause irregular changes in biosynthesis of cholesterol contributing to spine injury (Spann et al., 2017). Higher risk of Alzheimer’s disease is present in patients with spinal cord injury (Yeh et al., 2018). It is known that the intracellular cholesterol is important in biological function of endolysosomes and mitochondria via its passage across their membranes. Abnormal passage resulted in its accumulation in mitochondria and lysosomes and leading to the development of neurodegenerative diseases (Arenas et al., 2017). Increased extracellular cholesterol levels was associated with neurodegenerative diseases, such as Alzheimer’s disease and Parkinson’s disease (Zhang and Liu, 2015).Also, increased LDL cholesterol level predicted the development of breast cancer (Cedó et al., 2019). Consequently promising research studies based on chelating the increased cholesterol level by methyl- β-cyclodextrin as well as sensitizing either tamoxifen (Mohammad et al., 2014 ) or doxorubicin (Mohammad et al., 2015 ) to overcome the development of cancer cells in breast and skin.
Barley is one of the most cereal crops belong to Magnoliophyta (Class Liliopsida (Monocotyledons), order : Cyperales, Family : Poaceae (Grass), Genus : Hordeum vulgare) widely cultivated in the Middle East (Newman and Newman, 2008). The protein content attained to 10-17% of its total mass especially hordeins (prolamin) comprising 40% to 50% (Anderson, 2013; Magliano et al., 2014). Barley is rich in phenolic (benzoic and cinnamic acid derivates, flavonoids, chalcones, tannins, quinones, proanthocyanidins) (Dvorakova et al., 2008, Carvalho et al., 2015), hydroxyl cinnamic acid derivatives such as p-coumaric, caffeic, ferulic and sinapic acids (Liu et al., 2007; Kim et al., 2007), flavonoids, lignans, tocols, phytosterols, and folate ((Idehen et al., 2017) which exhibited strong antioxidant, antiproliferative, and cholesterol lowering activities (Idehen et al., 2017). β-glucans showed hypolepidemia especially of cholesterol and triglyceride levels in animal model (Kalra and Jood, 2000) and human (Wilson et al., 2004; AbuMweis et al., 2010; McRorie and McKeown , 2017) through activation of cholesterol excretion choleterol 7a-hydroxylase (Abumweis et al., 2010; Wang et al., 2017).
There is a little of work illustrated the hypercholesterolemia associated the development of histopathology and cytological lesions of the spinal cord coincides with the biochemical markers of lesions. At the same time illustrating the phytomedicinal importance of dietary inclusion of malted barley in improving high cholesterol diet associated the neurotoxicity.
Materials and Methods
Induction of Hypercholesterolemia
The high cholesterol diet is composed of 3% cholesterol plus 7% animal fat plus 2% cholic acid and 2% thiouracil in conjugation with other components of standard diet according to Enkhmaa et al. (2005). Rats were fed on it for 4 months prior to conception and throughout pregnancy and lactation period..
Diets Containing Malted Barley
Malted barley was prepared three days before mixing with normal diet by Wettingbarley grains with water at a ratio of 20 percent. Throughout pregnancy and lactation time the hypercholesterolemic pregnant groups were fed on a diet that included fermented barley.
Experimental Animal
This research and all procedures had been authorised by the Egyptian Committee’s Animal Care and Bioethics.Sixty virgin female and twenty adult male albino rats (Rattus norvegicus) weighing approximately 100±10gm body weight, obtained from Helwan Breading Farm, Ministry of Health, Egypt were used for experimentation.
They were allowed in good aerated room with nearly 12 hours of light and dark cycle for acclimatization. Free access of food and water were allowed ad libitum. Pregnancy was carried out by keeping each normal fertile male with three females for overnight and examining vaginal smears in the next morning for observing sperm and zero date of pregnancy was determined. The pregnant were arranged into 4 groups (n=15) as follows: Control (C), barley supplemented group (B) (10%), experimental hypercholesterolemic-group (H) (3%) and experimental hypercholesterolemic & fermented barley group (H+B). Dams of both control and experimental groups were sacrificed at 1, 2 and 3 weeks post-partum.
The Following Parameters Were Investigated
Histological Investigation
Cervical spinal cord of mother rats of the studied groups were separated and fixed immediately in 10% phosphate buffered formalin (pH 7.4), dehydrated in ascending series of ethyl alcohol, cleared in xylene and mounted in molten paraplast at 58-62ºC. Five µm histological sections were cut, stained with Hematoxylin& eosin (Weesner, 1968) and examined under a bright field light microscope.
Transmission electron microscopy (TEM)
Cervical spinal cord of the studied groups were fixed in 2.5 % glutaraldehyde buffered in 0.1M cacodylate buffer (pH 7.4). These was followed by post-fixed in 1% osmium tetraoxide at 4°C, dehydration in ascending concentrations of ethyl alcohol, cleared in propylene oxide and embedded in epoxy–resin. Ultrathin sections were cut on a LKB Ultratome IV (LKB Instruments, Bromma, Sweden) and mounted on grids, stained with uranyl acetate and lead citrate, and examined under a Joel 100CX transmission electron microscope at Mansoura University Lab, Egypt.
Immunohistochemistry for PCNA and GFAP
Five μm histological sections of formalin-fixed, paraffin-embedded cerebrum tissue were placed on polylysine-coated glass slides. After overnight packing at 65°C, tissue sections were deparaffinized in xylene and rehydrated in descending grades of alcohol. Endogenous peroxidase activity was removed by incubation of tissue sections in 3% H2O2 for 10 minutes at room temperature. The tissue sections were placed in digested media composed of 0.05 % trypsin (pH 7.8) for 15 minutes at 37°C and incubated with the primary monoclonal mouse antibody of glial fibrillary acidic protein GFAP (DAKO, clone MIB5, 1:50, mouse) and primary antibody against proliferating cell nuclear antigen PCNA (DAKO, clone MIB5, 1:50, mouse) at 1:50 overnight at 4°C. After washing, the slides were incubated with a secondary biotin linked anti-mouse antibody for 50 minutes at room temperature; and with the streptavidin-peroxidase complex for 50 minutes. Sections were then washed and incubated with developing solution (diaminobenzidine-hydrogen peroxide; DAKO), and counterstained with hematoxylin. The immune reaction was visualized as brown nuclear or cytoplasmic labeling counterstained with hematoxylin. Sections incubated with 1% nonimmune serum phosphate buffer solution (PBS) solution served as negative controls. Finally, the sections were examined under bright field light Olympus microscope with a digital canon camera.
Biochemical Assays
Cervical spinal cord tissue of both the control and experimental groups were homogenized in Tris buffer at pH 7.5 and separated, their supernatant stored in a deep freeze.
Determination of Superoxide Dismutase and Glutathione-S-Reductase Activities
It was determined according to Niskikimi et al. (1972) and based on the inhibition of nitroblue tetrazolium (NBT). The reduction of NBT by superoxide radicals to blue colored formazan was assayed at 560 nm. Glutathione S-transferases (GSH) is determined according to Habig et al., (1974). It is carried out by measuring the conjugation of 1- chloro- 2,4- dinitrobenzene with reduced glutathione and the product is measured spectrophotometrically at absorbance of 340 nm.
Determination of Lipid Peroxidation end Product Malonaldhyde (Thiobarbituric Acid Reactive Substances, TBARS) Level
It is carried according to Ohkawa et al. (1979) based on the developed reddish pink color and estimated at 532nm which indicates the extent of peroxidation and expressed as nmol/ mg protein.
Determination of 8-Hydroxy-2-Deoxy Guanosine (8-hdG)
The amount of 8-HdG was determined using the Bioxytech 8-HdG -ELISA Kit (OXIS Health Products, Portland, OR, USA, Catalog. No. KOG-200S/E) according to the manufacturer’s instructions. The reaction was terminated and the absorbance was measured using a FLUO star OMEGA microplate reader (BMG LABTECH Ltd., Germany) at a wavelength of 450nm (Attia, 2012). Values of 8-OHdG are expressed as mg/ml.
Determination of Caspase-3
It is determined colorimetrically by using a Stressgen kit (catalog No. 907-013). The cleavage of the peptide can be quantitated spectrophotometrically at a wavelength of 405 nm. The level of caspase enzymatic activity in the cell lysate is directly proportional to the color reaction.
Determination of Dopamine (DA), Serotonin (5-HT) and γ-Aminobutyric Acid (GABA) Neurotransmitters
γ-amino-butyric acid, dopamine and serotonin were measured by high-performance liquid chromatography (HPLC) using the precolumn PTC derivatization technique according to the method of Heinrikson and Meredith (1984). The assay conditions were as follows: Temperature: 46oC; wave-length: 254 nm; flow rate: 1ml/min.Dopamine and serotonin were assayed HPLC according to the method described by Pagel et al. (2000). It is based on removal of trace element and lipid from the tested samples by solid phase extraction CHROMABOND column NH2 phase Cat. No. 730031 and injected directly into an AQUA column 150 54.6 mm (Phenomenex, USA).
Determination of Interleukin Level
Interleukin 6 (catalogue No. SEA079Ra), 10 (catalogue No. SEA056Ra) and interleukin 17 (Catalogue No.SEA063Ra) were estimated in cerebrum tissue by using Enzyme-linked Immunosorbent Assay Kit of Cloud-Clone Corp.
Assessments of Phosphatidylethanolamine, Sphingomyelin and Polyenylphosphatidylcholine
Phosphatidylethanolamine (PE), Sphingomyelin (SM) and polyenylphosphatidylcholine (PPC) were estimated in spinal cord tissues by Parker and Peterson (1965).
Determination Leptin, Homocysteine and Amyloid–β
Leptin (LEP, catalogue no. SEA084Ra), Homocysteine (Hcy, catalogue no. CSB-E13376r) and amyloid-B42 (Ab1-42, catalogue no. CEA946Ra) were estimated by using Enzyme-linked Immunosorbent Assay Kit of Cloud-Clone Corp.
Determination of Iron Concentration
Cervical spinal cord samples of the studied groups were digested by 1mL of nitric acid at highest purity and diluted with 4mL bi-distilled water and their iron concentration was measured by atomic absorption spectrometry (Scancar et al., 2000).
Statistical Analysis
The means value obtained in the different groups were compared by one-way post-hoc analysis of variance (ANOVA) test. All results were expressed as mean±standard error (SE) and significance was recorded at p <0.05.
Results
Light and Ultra-Structural of Mother
At light microscopic level, the cervical spinal cord of mother rat fed on a high cholesterol diet showed damaged epithelial cells lining the ependymal canal as compared to the control (Fig.1A&A1).Necrotic spots have been identified. The multipolar neuronal cells become shrinked and enclosed in a halo spaces. The ground grey matter become fibrous and fragile (Fig. 1 B&B1. Nevertheless, there was a detected improvement and restoration of nearly the normal pattern structure of ependymal canal, multipolar neuronal cells, and gray elements (Fig. 1C&C1).
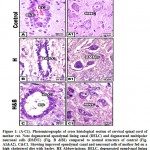 |
Figure 1: (A-C1). Photomicrographs of cross histological section of cervical spinal cord of mother rat. |
At transmission electron microscopy, mother rat fed on a high cholesterol diet showed neurons with abnormal pyknotic nuclei. In the cytoplasm, vesicuolated rough endoplasmic reticulum and atrophied mitochondria were detected. Many of the nerve axons become demyelinated (Fig.2 A1-C1). On the other hand, mother fed on a high cholesterol diet containing barley possessed a detected comparative improvement of the neuronal cells. Rough endoplasmic reticulum and mitochondria restored their normal level. The neuronal axons become myelinated and their neuropil appeared rich of mitochondria (Fig.2 A2-C2). Regard the control (Fig. 1 A-C ).
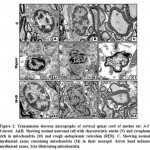 |
Figure 2: Transmission electron micrographs of cervical spinal cord of mother rat. |
A1-C1. Mother fed on a high cholesterol diet. A1&B1. Showing neuron cells with pyknotic nuclei (PN) and cytoplasm containing damaged mitochondria (DM) and vesicuolated rough endoplasmic reticulum. C1. Showing demyelinated axons with damaged mitochondria (DM) in the neuropil. Arrowhead showing demyelinated axons Star showing damaged mitochondria.
A2-C2. Mother fed on a high cholesterol diet with barley. A2. Showing improved neuronal cell with characterized nuclei (N) surrounding by nuclear envelope (NE) and cytoplasm containing few vacuoles (V) and intact mitochondria (M). B2. Showing less improved neuronal cell containing nuclei (N) with convoluted nuclear envelope (NE) and normal myelinated axons (MA) through the neuropil. C2. Showing improved myelinated axons (MA) containing mitochondria (M).Star showing normal mitochondria in the inner compartment of myelinated axon. Arrow head showing myelinated axon. Abbreviations; M, mitochondria; MA, myelinated axons; N, nucleus; NE, nuclear envelope; V, vacuoles. Star showing normal mitochondria in the inner compartment of myelinated axon. Arrowhead showing myelinated axon.
A-C Control. A&B. Showing normal neuronal cell with characteristic nuclei (N) and cytoplasm rich in mitochondria (M) and rough endoplasmic reticulum (RER). C. Showing normal myelinated axons containing mitochondria (M) in their neuropil. Arrow head indicate myelinated axons. Star illustrating mitochondria. A1-C1. Mother fed on a high cholesterol diet. A1&B1. Showing neuron cells with pyknotic nuclei (PN) and cytoplasm containing damaged mitochondria (DM) and vesicuolated rough endoplasmic reticulum. C1. Showing demyelinated axons with damaged mitochondria (DM) in the neuropil. Arrowhead showing demyelinated axons Star showing damaged mitochondria. A2-C2. Mother fed on a high cholesterol diet with barley. A2. Showing improved neuronal cell with characterized nuclei (N) surrounding by nuclear envelope (NE) and cytoplasm containing few vacuoles (V) and intact mitochondria (M). B2. Showing less improved neuronal cell containing nuclei (N) with convoluted nuclear envelope (NE) and normal myelinated axons (MA) through the neuropil. C2. Showing improved myelinated axons (MA) containing mitochondria (M).Star showing normal mitochondria in the inner compartment of myelinated axon. Arrow head showing myelinated axon. Abbreviations; M, mitochondria; MA, myelinated axons; N, nucleus; NE, nuclear envelope; V, vacuoles. Star showing normal mitochondria in the inner compartment of myelinated axon. Arrowhead showing myelinated axon.
Immunohistochemistry Observations of Mother
In mother fed on hypercholesterolemic diet, there was a marked reduction of PCNA immunohistochemical reaction in neuronal cells (Fig.3 B). However, in those fed on a high cholesterol diet containing barley, there was a comparatively improved immunohistochemical reaction expressed by a moderate dark-brown immunohistochemical reaction (Fig.3C).Regard the control (Fig.3 A). Image analysis revealed decreased expression of PCNA in cervical cord of experimental hypercholesterolemic mothers compared to moderate reaction in those fed on a high cholesterol diet containing barley and control (Fig.4).
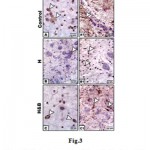 |
Figure 3: Photomicrographs of formalin-fixed, paraffin-embedded cervical spinal cord of mother rat. A-C. |
Note decreased staining in mother fed on a high cholesterol diet (B) compared to control (A) and improved in mother fed on a high fat diet containing barley (C).A1-C1. Spinal cord of mother rats immunohistochemically stained with GFAP- antibody. Note decreased staining in mother fed on a high cholesterol diet (B1) compared to control (A1) and improved in mother fed on a high fat diet containing barley (C1). Arrow head indicates the increased immunohistochemical reaction.
On the other hand, mothers fed on a high cholesterol diet group possessed decreased expression of GFAP in the neuronal cells (Fig.3 A1) compared to control (Fig.3 B1). However, in those fed on a high cholesterol diet containing barley, there was a detected moderate dark-brown immunohistochemical reaction (Fig.3 C1)). Image analysis revealed appeared increased of the mentioned immunostaining in mother fed on a high cholesterol diet compared to the other studied groups (Fig.4).
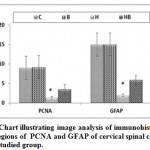 |
Figure 4: Chart illustrating image analysis of immunohistochemical reactive regions of PCNA |
Abbreviations; B, barley supplemented mother; C, control mother; H, mother fed on a high cholesterol diet; HB, mother fed on a high cholesterol diet containing barley. Star indicates a marked depletion of the immunoreaction.
Biochemical Observations
Mother rats fed on a high cholesterol diet, the cervical spinal cord contents of both glutathione-s-reductase (GSH) and superoxide dismutase (SOD) activities were markedly decreased. On the other hand, the cervical spinal cord contents of MDA, caspase 3 (Casp-3) and 8-hydroxy-deoxyguanosine (8-HdG) were markedly increased. The mentioned dramatic alterations were improved in those of mothers fed on a high cholesterol diet containing barley, but were still varied from the control values (Table 1).
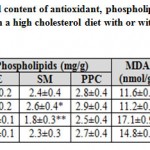 |
Table 1: Cervical spinal cord content of antioxidant |
Each result represents the mean±SE of n=5. One star means significant at P < 0.05; double stars mean high significant at P < 0.01. Abbreviations; B, barley supplemented; C, control; Casp3, caspase-3; GSH, glutathione-s-reductase; H, high cholesterol diet; HB, high cholesterol diet containing barley; 8-HdG, 8-hydroxydeoxyguanosine; MDA, malondialdhyde; PE, phosphatidylethanolamine; PPC, polyenylphosphatidylcholine; SM, sphingomyelin; SOD, superoxide dismutase.
In addition, there was a detected depletion of dopamine, serotonin and γ-aminobutyric acid in cervical spinal cord of mother fed on a high cholesterol diet compared to the control. These measurements were parallel with marked depletion of interleukin 10 and marked increased the content of interleukin 6. At the same time, the cervical spinal cord contents of the assayed neurotransmitters and interleukin 10 were markedly increased as well as improved the level of interleukin 6 in those of mothers fed on a high cholesterol diet containing barley. However, their level were not matched with the control values (Table 2).
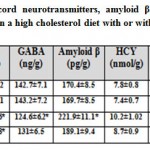 |
Table 2: Cervical spinal cord neurotransmitters |
Each result represents the mean±SE ( n=5). One star means significant at P < 0.05; double stars mean high significant at P < 0.01. Abbreviations; B, barley supplemented; C, control; DA, dopamine; GABA, γ-aminobutyric acid; H, high cholesterol diet; HB, high cholesterol diet containing barley; HCY, Homocystine; 5-HT, serotonin; IL6, interleukin 6; IL10, interleukin 10; IL17, interleukin 17.
Discussion
From the present findings, mother rats fed on a high cholesterol diet interfered with the antioxidative defense leading to depletion of glutathione reductase and superoxide dismutase and increased of MDA parallel with widespread of neuronal cell damage, degeneration of ependymal lining cells, clumping chromatin materials of many of the neuronal cells, fragmentation of RER and atrophy of mitochondria.
It is known that In biological systems there is a balance between the production and neutralization of reactive oxygen species (ROS). This balance is maintained by the presence of the assayed antioxidant enzymes superoxide dismutase and glutathione s reductase, SOD converts the highly reactive radical O2-. to the less reactive radical H2O2,meanwhile glutathione s reductase reduced the variety of hydroperoxides (Taylor et al., 1993).
The enhancement of lipid peroxidation or a decrease in antioxidant protection can frequently induce the reaction with the nucleophilic centers of the DNA, RNA and proteins leading to irreversible damage such as cytotoxicity, mutagenicity and carcinogenicity.
The observed neuronal damage was assessed by increased neuronal casp3,8-hdG. Like other neuronal tissues, the spinal cord is considered to be metabolic active organ containing reactive oxygen species. The free radicals and antioxidant enzymes maintained the balance of the antioxidant defense (Shim and Kim, 2013). The abnormal imbalance of antioxidants associated with cholesterol toxicities in neuronal tissues led to increased oxidative stress and breakdown of neuronal tissues. The present findings are consistent with Lim et al. (2014) who reported increased oxidative stress in neurodegenerative disorders. At the same time, the observed damage of mitochondria and vesicuolated rough endoplasmic reticulum marked the increased oxidative stress. The present findings are consistent with El-Sayyad et al. (2017) and paul et al. (2017) following assessment the involvement of hypercholesterolemia in oxidative stress of neuronal tissues.
From the present findings, a high cholesterol diet was found to alter the metabolism of phospholipids assessed by reduction of phosphatidylethanolamine, polyenylphosphatidylcholine and sphingomyelin
It is known that phospholipids may involve in transmission and relay signals from the membrane to intracellular compartments or to other cells.Also, it is the main components of myelin (Grigletto et al., 2017). Depletion of membrane lipids may contribute to the pathogenesis of depression and anxiety disorders (Müller et al., 2015). The assayed phospholipid fractions (phosphatidylethanolamine, polyenylphosphatidylcholine and sphingomyelin) were markedly depleted in spinal cord tissue of hypercholesterolemia and retained nearly normal level after barley supplementation added in diet.
Also, The increased brain content of lipid enhanced lipid peroxidation and cholesterol oxidation and the subsequent development of metabolites such as 4-hydroxynonenal and oxysterols, respectively from the two processes. The chronic inflammatory events observed in the AD which activates inflammatory molecule and free radical release. Oxidative stress is closely associated to neuroinflammation, which re intern activated oxidative stress leading to progress neuronal damage. Taking in consideration the higher activity of oxysterol to cross the blood brain barrier, it is the main contributor of the neuronal damage (Gamba et al., 2015).
The increased oxidative stress associated with hypercholesterolemia were assessed by the upregulation of the inflammatory cytokines IL6 and 17 and a decrease of IL10. Similar findings have been reported by Yi et al. (2012) in hypothalamus post a higher fat diet.
Beside Structural and functional disorganization, there was a detected decrease of VEGF immunohistochemistry and demyelination of nerve axons which was confirmed by depletion of neurotransmitters of dopamine, serotonin and α-aminobutyric acid this may led to impair cognitive function and delayed the function of synapses.
It is known that DA and 5-HT, play an a great role in managing the sensory, motor and autonomic functions in the spinal cord (Zhang, 2016; Wei et al., 2014). Neurodegenerative lesions can be resulted from depletion of DA and 5-HT, neuronal loss, inflammation and caspase activation within the spinal cord at both post- and pre-synaptic sites (Malcangio and Bowery, 1996). γABA was found to be increased in the hyperglycemic stroke (Guyot et al., 2001). The observed demyelinated nerve axons confirmed the work of Saher et al. (2005) who mentioned that cholesterol is the main elements of myelin membranes and decreased metabolism of cholesterol is involved for demyelination (Raddatz et al., 2016).
Wang and Zheng (2015) reported that rabbit fed on a high cholesterol diet. The authors showed impaired of synaptic function characterized by increased mushroom spine density and decreased thin spine density.
Also, the neurodegenerative lesions of the spinal cord were confirmed by Increased level of homocysteine and amyloid-β.
Similar findings were reported in in Alzheimer’s disease model mice (Matsumura et al. 2015) and rabbit (pan et al. 2018). The mentioned authors stated that hypercholesterolemia increased the burden of intraneuronal Aβ oligomers and synapses loss. At the same time increased level of homocysteine was found to initiate oxidative stress leading to inflammation and endoplasmic reticulum stress (Moretti and Caruso, 2019) as well as activated N-methyl-d-aspartate receptor leading to accumulation of amyloid and tau protein, apoptosis, and neuronal death (Obeid and Herrmann, 2006). Also, homocysteine levels are elevated in patients with spinal cord injury (SCI) due to fat distribution changes and sympathetic dysfunction (Latifi et al., 2013; Hao et al., 2014).
The observed finding revealed that the damaged spinal cord was confirmed by increased level of iron. Iron accumulation was found to increase oxidative stress associated spinal cord injury (Meng et al., 2017). Also increased iron level led neurodegenerative diseases such as Alzheimer’s disease through bounding to transferrin, and crossing the brain blood barrier (Moos and Morgan, 2000). It is known that monocytes is the main cell components of the central nervous system. The iron-containing monocytes facilitate migration and transform into macrophages, and degenerate and consequently phagocytosis of this cell debris including iron may transmit it to another neuronal region promoting the formation of free radicals and induced neurodegenerative disorders (Andersen et al., 2014).
On the other hand, mother rats fed on a high fat diet plus barley possessed marked improvement of their spinal tissues.
These seemed to be resulted from the hypolipidemic activity of barley β-glucans in animal model (Kalra and Jood, 2000) and human (Wilson et al., 2004; AbuMweis et al., 2010; McRorie and McKeown, 2017). It is known that bile acids are derivatives of cholesterol, and their excretion activates choleterol 7a-hydroxylase which . increase the transport of LDL cholesterol into hepatocytes for conversion into bile acids thus lowering the serum cholesterol and LDL cholesterol levels in the body (Maki et al., 2010; Wang et al., 2017). Also, barley β-glucans was influenced in the production of short chain fatty acids via either its fermentation in the human intestinal by microflora (Barsanti et al., 2011; Othman et al., 2011) or through improving gut microflora especially Lactobacilli for synthesis it (Morrison and Preston, 2016), leading to inhibition of hepatic cholesterol biosynthesis (Hara et al., 1999) and depleting plasma triacylglycerol and cholesterols from week 3 to 7 (Mikkelsen et al., 2017).
Also, barley β-glucan was found to improve the brain and sciatic nerve of the streptozotocin-induced diabetic rat (Alp et al., 2012) and reduced the number of degenerated neurons in the ovariectomized rats (Selli et al., 2016). Ferulic acid and Coumaric acids, the main components of barley grain showed high antioxidant activity (Kováčová and Malinová, 2007) and consquenly improve neuronal injuries (Szwajgier et al., 2017). Barley seeds exhibited the presence of 3, 4-Dihydroxybenzaldehyde, which inhibits oxidative DNA damage and apoptosis via scavenging H2O2 (Jeong et al., 2009; Aggarwal et al., 2011).
Tocotrienol (member of vitamin E) – treatment was found to reduce oxidative stress in Alzheimer disease through reducing free-radicals and promote mitochondrial function and cellular repair. It also inhibited glutamate-induced neurotoxicity in the neuronal cells (Chin and Tay, 2018).
Finally, the present study concluded that high cholesterol diet is involved in the development inflammatory reactions and oxidative stress associated impairment of function. Barley supplementation exerted improvement of the assessed histopathological, immunohistochemical and biochemical pictures. This findings clarifying that the high barley contents of antioxidants and nutrients are of great importance in scavenging the free radicals and decreasing the oxidative stress and consequently restore the spinal cord structure and function.
References
- AbuMweis SS, Jew S, Ames NP (2010) β-glucan from barley and its lipid-lowering capacity, a meta-analysis of randomized, controlled trials. Eur J Clin Nutr. 64(12):1472-1480.
CrossRef - Aggarwal BB, Prasad S, Reuter S, et al (2011) Identification of novel anti-inflammatory agents from Ayurvedic medicine for prevention of chronic diseases, “reverse pharmacology” and “bedside to bench” approach. Curr Drug Targets. 12(11):1595-1653.
CrossRef - Alp H, Varol S, Celik MM, et al (2012) Protective Effects of Beta Glucan and Gliclazide on Brain Tissue and Sciatic Nerve of Diabetic Rats Induced by Streptozosin. Experimental Diabetes Research. 2012:1-7.
CrossRef - Andersen HH, Johnsen KB, Moos T (2014) Iron deposits in the chronically inflamed central nervous system and contributes to neurodegeneration. Cell Mol Life Sci. 71(9):1607-1622.
CrossRef - Anderson OD (2013) (The B-hordein prolamin family of barley. Genome. 2013 ;56(3):179-85.
CrossRef - Arenas F, Garcia-Ruiz C, Fernandez-Checa JC (2017) Intracellular cholesterol trafficking and impact in neurodegeneration. Front Mol Neurosci.10:382.
CrossRef - Attia SM (2012) Influence of resveratrol on oxidative damage in genomic DNA and apoptosis induced by cisplatin. Mutat Res. 741(1-2): 22-31.
CrossRef - Barsanti L, Passarelli V, Evangelista V, et al (2011) Chemistry, physico-chemistry and applications linked to biological activities of β-glucans. Nat Prod Rep. 28(3): 457-466.
CrossRef - Carvalho DO, Curto AF, Guido LF (2015) Determination of Phenolic Content in Different Barley Varieties and Corresponding Malts by Liquid Chromatography-diode Array Detection-Electrospray Ionization Tandem Mass Spectrometry. Segura-Carretero A, Arráez-Román D, eds. Antioxidants. 4(3):563-576.
CrossRef - Cedó L, Reddy ST, Mato E et al. (2019) HDL and LDL: Potential New Players in Breast Cancer Development. J Clin Med. 14;8(6):853.
CrossRef - Chin KY, Tay SS (2018) A Review on the Relationship between Tocotrienol and Alzheimer Disease. Nutrients. 10(7): 881.
CrossRef - Dvořáková M, Guido LF, Dostálek P et al. (2008) Antioxidant properties of free, soluble ester and insoluble-bound phenolic compounds in different barley varieties and corresponding malts. J. Inst. Brew. 114:27–33.
CrossRef - El-Sayyad HI, Amin AH, El-Ghawet HA, et al (2017) Role of Pomegranate Juice and Atorvastatin in Ameliorating Spinal Neurotoxicity of Wistar Rats Maternally Fed on Hypercholesterolemic Diet. J Mol Biomark Diagn. 8: 316.
CrossRef - Enkhmaa B, Shiwaku K, Anuurad E, et al (2005) Prevalence of the metabolic syndrome using the Third Report of the National Cholesterol Educational Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (ATP III) and the modified ATP III definitions for Japanese and Mongolians. Clin Chim Acta. 352(1-2): 105-113.
CrossRef - Gamba P, Testa G, Gargiulo S, et al (2015) Oxidized cholesterol as the driving force behind the development of Alzheimer’s disease. Front Aging Neurosci.7:119.
CrossRef - Grigoletto J, Pukaß K, Gamliel A, et al (2017) Higher levels of myelin phospholipids in brains of neuronal α-Synuclein transgenic mice precede myelin loss. Acta Neuropathol Commun.5(1):37.
CrossRef - Grillner S, Robertson B (2015) The basal ganglia downstream control of brainstem motor centres–an evolutionarily conserved strategy. Curr Opin Neurobiol. 33: 47–52.
CrossRef - Guyot LL, Diaz FG, O’Regan MH, et al (2001) The effect of streptozotocin-induced diabetes on the release of excitotoxic and other amino acids from the ischemic rat cerebral cortex. Neurosurgery. 48(2):385-390.
CrossRef - Habig WH, Pabst MJ, Jakoby WB (1974) Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 249(22): 7130-7139.
CrossRef - Hao M, Yan Zhang, Shuangxing Hou, et al (2014) Spinal cord demyelination combined with hyperhomocysteinemia, a case report. Neuropsychiatr Dis Treat. 10: 2057–2059.
CrossRef - Hara H, Haga S, Aoyama Y, Kiriyama S (1999) Short-chain fatty acids suppress cholesterol synthesis in rat liver and intestine. J Nutr. 129(5): 942-948.
CrossRef - Heinrikson RL, Meredith SC (1984) Amino acid analysis by reverse-phase high-performance liquid chromatography, precolumn derivatization with phenylisothiocyanate. Anal Biochem.136(1): 65-74.
CrossRef - Idehen E, Tang Y, Sang S. (2017). Bioactive phytochemicals in barley.J Food Drug Anal. 25(1):148-161.
CrossRef - Jeong JB, Hong SC, Jeong HJ (2009) 3,4-dihydroxybenzaldehyde purified from the barley seeds (Hordeum vulgare) inhibits oxidative DNA damage and apoptosis via its antioxidant activity. Phytomedicine.16(1): 85-94.
CrossRef - Kalra S, Jood S (2000) Effect of Dietary Barley β-Glucan on Cholesterol and Lipoprotein Fractions in Rat. J. Cereal Sci, London 31(2):141-145.
CrossRef - Kim MJ, Hyun JN, Kim JA et al. (2007) Relationship between phenolic compounds, anthocyanins content and antioxidant activity in colored barley germplasm. J Agric Food Chem. 55(12):4802-9.
CrossRef - Kováčová M, Malinová E (2007) Ferulic and coumaric acids, total phenolic compounds and their correlation in selected oat genotypes. Czech j Food Sci. 25: 325–332.
CrossRef - Latifi S, Koushki D, Norouzi Javidan A, et al (2013) Changes of Leptin concentration in plasma in patients with spinal cord injury, A Meta-analysis. Spinal Cord. 51(10):728–731.
CrossRef - Lim JL, Wilhelmus MM, de Vries HE, et al (2014) Antioxidative defense mechanisms controlled by Nrf2: state-of-the-art and clinical perspectives in neurodegenerative diseases. Arch Toxicol. 88(10):1773-1786.
CrossRef - Liu RH (2007) Whole grain phytochemicals and health. J Cereal Sci. 46: 207–219.
CrossRef - Magliano PN, Prystupa P, Gutiérrez-Boem FH (2014) Protein content of grains of different size fractions in malting barley. J. Inst. Brew. 120: 347–352.
CrossRef - Maki KC, Beiseigel JM, Jonnalagadda SS, et al (2010) Whole-grain ready-to-eat oat cereal, as part of a dietary program for weight loss, reduces low-density lipoprotein cholesterol in adults with overweight and obesity more than a dietary program including low-fiber control foods. J Am Diet Assoc. 110(2):205-214.
CrossRef - Malcangio M, Bowery NG (1996) GABA and its receptors in the spinal cord. Trends Pharmacol Sci.17(12): 457-462.
CrossRef - Matsumura A, Emoto MC, Suzuki S, et al (2015) Evaluation of oxidative stress in the brain of a transgenic mouse model of Alzheimer disease by in vivo electron paramagnetic resonance imaging. Free Radical Biology and Medicine. 85:165–173.
CrossRef - McRorie JW, McKeown NM (2017) Understanding the Physics of Functional Fibers in the Gastrointestinal Tract, An Evidence-Based Approach to Resolving Enduring Misconceptions about Insoluble and Soluble Fiber. J Acad Nutr Diet. 117(2): 251-264.
CrossRef - Meng FX, Hou JM, Sun TS (2017) Effect of oxidative stress induced by intracranial iron overload on central pain after spinal cord injury. J Orthop Surg Res. 12(1):24.
CrossRef - Mikkelsen MS, Jensen MG, Nielsen TS (2017) Barley beta-glucans varying in molecular mass and oligomer structure affect cecal fermentation and microbial composition but not blood lipid profiles in hypercholesterolemic rats. Food Funct. 8(12): 4723-4732.
CrossRef - Mohammad N, Malvi P, Meena AS et al. (2014). Cholesterol depletion by methyl-β-cyclodextrin augments tamoxifen induced cell death by enhancing its uptake in melanoma. Mol Cancer. 13:204.
CrossRef - Mohammad N, Singh SV, Malvi P et al. (2015) Stratefy to enhance the efficacy of doxorubicin in solid tumor cells by methyl-β-cyclodextrin: Involvement of p53 and Fas receptor ligand complex. Sci Rep. 5:11853.
CrossRef - Moos T, Morgan EH (2000) Transferrin and transferrin receptor function in brain barrier systems. Cell Mol Neurobiol. 20: 77–95.
CrossRef - Moretti R, Caruso P (2019) The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. Int J Mol Sci. 20(1): 231.
CrossRef - Morrison DJ, Preston T (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes. 7(3):189-200.
CrossRef - Müller CP, Reichel M, Mühle C, et al (2015) Brain membrane lipids in major depression and anxiety disorders. Biochim Biophys Acta.1851(8):1052-1065.
CrossRef - Newman RK, Newman CW (2008) Barley for food and health: science, technology, and products. A John Willey & Sons, INC., Publication.
CrossRef - Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun. 46(2): 849-854.
CrossRef - Obeid R, Herrmann W (2006) Mechanisms of homocysteine neurotoxicity in neurodegenerative diseases with special reference to dementia. FEBS Lett. 580(13):2994–3005.
CrossRef - Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95(2): 351-358.
CrossRef - Othman RA, Moghadasian MH, Jones PJ (2011) Cholesterol-lowering effects of oat β-glucan. Nutr Rev. 69(6): 299-309.
CrossRef - Pagel P, Blome J, Wolf HU (2000) High-performance liquid chromatographic separation and measurement of various biogenic compounds possibly involved in the pathomechanism of Parkinson’s disease. J Chromatogr B Biomed Sci Appl. 746(2): 297-304.
CrossRef - Pan Y, Xu J, Chen C, et al (2018) Royal Jelly Reduces Cholesterol Levels, Ameliorates Aβ Pathology and Enhances Neuronal Metabolic Activities in a Rabbit Model of Alzheimer’s Disease. Front Aging Neurosci. 10:50.
CrossRef - Parker F, Peterson NF (1965) Quantitative analysis of phospholipids and phospholipid fatty acids from silica gel thin-layer chromatograms. J Lipid Res. 6:455-460.
CrossRef - Paul R, Choudhury A, Chandra Boruah D, et al (2017) Hypercholesterolemia causes psychomotor abnormalities in mice and alterations in cortico-striatal biogenic amine neurotransmitters, Relevance to Parkinson’s disease. Neurochem Int. 108:15-26.
CrossRef - Raddatz BB, Sun W, Brogden G, et al (2016) Central Nervous System Demyelination and Remyelination is Independent from Systemic Cholesterol Level in Theiler’s Murine Encephalomyelitis. Brain Pathol. 26(1):102-119.
CrossRef - Saher G, Brügger B, Lappe-Siefke C, et al (2005) High cholesterol level is essential for myelin membrane growth. Nat Neurosci. 8(4):468-475.
CrossRef - Sasani M, Aydın AL, Aytan N, et al (2016) Effect of a hypercholesterolemia as a starting factor on spinal degeneration in rabbits and role of Vitamin E (α-tocopherol). Surg Neurol Int.7:36.
CrossRef - Scancar J, Milacic R, Benedik M, Bukovec, P (2000) Determination of trace elements and calcium in bone of the human iliac crest by atomic absorption spectrometry. Clin Chim Acta. 293(1-2):187-197.
CrossRef - Selli J, Unal D, Mercantepe F, et al (2016) Protective effects of beta glucan in brain tissues of post-menopausal rats, a histochemical and ultra-structural study. Gynecol Endocrinol. 32(3):234-239.
CrossRef - Shim SY, Kim HS (2013) Oxidative stress and the antioxidant enzyme system in the developing brain. Korean J Pediatr.56(3):107-111.
CrossRef - Spann RA, Lawson WJ, Grill RJ, et al (2017) Chronic spinal cord changes in ahigh –fat diet-fed male rat model of thoracic spinal contusion. Physiol Genomics. 49(9):519-529.
CrossRef - Szwajgier D, Borowiec K, Pustelniak K (2017) The Neuroprotective Effects of Phenolic Acids: Molecular Mechanism of Action. Nutrients. 9(5):477.
CrossRef - Taylor SD, Davenport LD, Speranza MJ, et al (1993) Glutathione peroxidase protects cultured mammalian cells from the toxicity of adriamycin and paraquat. Arch Biochem Biophys. 305(2):600-605.
CrossRef - Vichiansiri R, Saengsuwan J, Manimmanakorn N, et al (2012) The prevalence of dyslipidemia in with spinal cord lesion in Thailand. Cholesterol. 2012:847462.
CrossRef - Wang D, Zheng W (2015) Dietary cholesterol concentration affects synaptic plasticity and dendrite spine morphology of rabbit hippocampal neurons. Brain Res. 1622:350-360.
CrossRef - Wang Y, Harding SV, Thandapilly SJ, et al (2017) Barley β-glucan reduces blood cholesterol levels via interupting bile acid metabolism. Br J Nutr.118(10):822-829.
CrossRef - Weesner FM (1968) General zoological microtechniques, Williams and Wilkins company, Baltimore, Indian Edition, Scientific Book Agency, Calcutta.
- Wei K, Glaser JI, Deng L, et al (2014) Serotonin affects movement gain control in the spinal cord. J Neurosci. 34(38):12690-12700.
CrossRef - Wilson TA, Nicolosi RJ, Delaney B, et al (2004) Reduced and high molecular weight barley beta-glucans decrease plasma total and non-HDL-cholesterol in hypercholesterolemic Syrian golden hamsters. J Nutr.134(10): 2617-2622.
CrossRef - Yeh TS, Ho YC, Hsu CL, Pan SL (2018) Spinal cord injury and Alzheimer’s disease risk: a population-based, retrospective cohort study. Spinal Cord. 56(2):151-157.
CrossRef - Yi CX, Al-Massadi O, Donelan E, et al (2012) Exercise protects against high-fat diet-induced hypothalamic inflammation. Physiol Behav.106(4):485-490.
CrossRef - Zhang J, Liu Q (2015) Cholesterol metabolism and homeostasis in the brain. Protein Cell. 6(4):254-264.
CrossRef - Zhang M (2016) Two-step production of monoamines in monoenzymatic cells in the spinal cord, a different control strategy of neurotransmitter supply?. Neural regeneration research (NRR) journal. 11(12):1904-1909.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





