Manuscript accepted on : 07-10-2021
Published online on: 16-10-2021
Plagiarism Check: Yes
Reviewed by: Dr. Salim Albukhaty
Second Review by: Dr. Pamela Jha
Final Approval by: Dr. Ghulam Md Ashraf
Somke Pamela Madueke , Princewill Ikechukwu Ugwu
, Princewill Ikechukwu Ugwu , Chinaza Amarachi Uguru
, Chinaza Amarachi Uguru , Adaobi Pearl Okeke
, Adaobi Pearl Okeke , Okechukwu Maduabuchi Omire-Oluedo
, Okechukwu Maduabuchi Omire-Oluedo , Sandra Ugonne Ugwu
, Sandra Ugonne Ugwu , Vivian Ifeyinwa Nwannadi
, Vivian Ifeyinwa Nwannadi and Daniel Chukwu Nwachukwu
and Daniel Chukwu Nwachukwu
Department of Physiology, Faculty of Basic Medical Sciences, College of Medicine, University of Nigeria, Enugu Campus
Corresponding Author E-mail: princewill.ugwu@unn.edu.ng
DOI : http://dx.doi.org/10.13005/bbra/2942
ABSTRACT: BACKGROUND: Piper guineense seed is a well known spice consumed in many parts of West Africa as a result of its nutritional and medicinal properties. METHODS: Twenty Wistar rats were divided into four groups of five per group. The phytochemical analyses was done; different concentration of aqueous seed extract of Piper guineense was administered for 21 days to three experimental groups, Group 2 (25 mg/kg), Group 3 (50 mg/kg), Group 4 (100 mg/kg), while group 1 were given only rat feed and water. RESULTS: The investigation revealed that in low and medium dose groups, creatinine (62.3±7.3 to 51.1±4.5 and 51.1±8.1 respectively) and urea (6.6±1.3 to 5.2±0.8 and 4.8±1.0) levels decreased significantly while in high dose group, creatinine (62.3±7.3 to 66.9±11.0) and urea (6.6±1.3 to 7.0±0.8) increased significantly. There was a dose dependent increase in the serum electrolyte (sodium, potassium, chlorine and bicarbonate). Only bicarbonate (19.1±0.8 to 24.3±1.3) and chloride (102.4±3.8 to 107.0±1.6) had significant increase in their values. The histological study showed that at a low (25mg/kg) and medium dose (50mg/kg) of aqueous seed extract of Piper guineense the normal cyto-architecture of the kidney was maintained while in high dose group (100mg/kg) normal cyto-architecture of the kidney was distorted. CONCLUSION: The result obtained suggests that Piper guineense seed may not be harmful at a moderate dose; but high doses could be toxic. Caution should be taken on the quantity of Piper guineense seed consumed.
KEYWORDS: Aqueous; Kidney; Piper Guineense; Rats
Download this article as:| Copy the following to cite this article: Madueke S. P, Ugwu P. I, Uguru C. A, Okeke A. P, Omire-Oluedo O. M, Ugwu S. U, Nwannadi V. I, Nwachukwu D. C. Continuous Intake of High Doses of Piper guineense (Ashanti pepper) Aqueous Seed Extract Impairs Renal Function in Wistar Rats. Biosci Biotech Res Asia 2021;18(3). |
| Copy the following to cite this URL: Madueke S. P, Ugwu P. I, Uguru C. A, Okeke A. P, Omire-Oluedo O. M, Ugwu S. U, Nwannadi V. I, Nwachukwu D. C. Continuous Intake of High Doses of Piper guineense (Ashanti pepper) Aqueous Seed Extract Impairs Renal Function in Wistar Rats. Biosci Biotech Res Asia 2021;18(3). Available from: https://bit.ly/3FM4R0Z |
Introduction
Piper guineense is a spice plant. It belongs to the botanical family Piperaceae. In this family, it goes by the genus name piper. Piper guineense is found majorly in West African where it is known as a spice plant, and is commonly called Ashanti pepper.1 In Nigeria, The Ibos call it Uziza, while the Yorubas call it Iyere.1 With an ability to grow to a height of 20 meters, the plant is grouped as a vine which climbs up trees by the help of its adventitious roots. It usually has a life span of 2 years. It is easily identified by its typical heart-shaped leaves. P. guineense fruits occur in clusters. The fruits have red-brown when mature, and dark when dried.2
After childbirth, women in Nigeria consume P. guineense in large quantities (as spice in their food, and occasionally in raw form) because they believe that it facilitates uterine contractions – hence restoring the preconception size of the uterus, and helps to remove ‘bad blood’ that might be remaining inside the womb after delivery.3,4 In the Eastern parts of Southern Nigeria, young women have been reported to consume large quantities of Piper guineense in a bid to terminate unwanted pregnancies. Piper guineense It is believed to also have ameliorative effect on chronic joint pains suffered by some elderly folks in parts of West Africa. Traditional doctors in Some parts of West Africa prescribe Piper guineense fruit/seeds for asthmatic patients, men with low libido, and maidens desiring to lose weight.5,6,7,8 P. guineense is used as a treatment for abdominal discomfort.8 For locals in sub-Saharan Africa, Piper guineense leaves and fruits can be crushed and juiced to produce non-toxic insecticides and body colones.9,10
 |
Figure 1: Ripe seeds of Piper guineense. |
 |
Figure 2: Dry seeds of Piper guineense |
The popularity of use of plant extracts as herbal remedies is increasing globally. At least one quarter of patients with kidney diseases admit to use of ethno-botanicals, and medicinal herbal extracts.11 Locals, especially in West African, consume Piper guineense seed because they believe it has multi-medicinal properties, and also for the nutritional qualities it has. Consumption in many instances is not gauged, and can be for protracted periods. Hence, we sought to investigate the effects of aqueous extract of Piper guineense seed on the renal function of Wistar rats.
Materials and Methods
Collection and Identification of Plant Specimen
Fresh seeds of P. guineense were harvested from a rural farm land in Ngwo, Enugu State, Nigeria. Botanical identification was then carried out at the Department of Botany, University of Nigeria, Nsukka, Enugu State, Nigeria. The seeds collected were sun-dried for 2 weeks and then ground into powder using an electric mill. The ground powder was then stored in air tight container while awaiting extraction.
Plant Extraction and Phytochemical analysis
One hundred and fifty grams of air dried seed of Piper guineense was gotten from the harvested lot, blended and submerged in 900 millimeters (900ml) of portable water for 48 hours. The mixture was shaken intermittently at room temperature. On the third day, the mixture was sieved using a muslin cloth into a clean container and then was vacuum filtered through Whatman No.1 filter paper and concentrated using rotary evaporator at 600C. The resultant solution was then poured into a container and refrigerated at 40C and the concentration to be used was prepared. Aqueous extraction was chosen to mimic the state in which the plant is consumed normally. Qualitative and quantitative phytochemical analyses were done using an already established method.12
Animal Care and Use
Twenty male Wistar rats weighing between 150 – 220g were purchased from the animal house of Faculty of Basic Medical Sciences, University of Nigeria, Enugu Campus and used for the experiment. The animals were acclimatized for two weeks before the experiment, after which administration of the plant extract commenced. Their cages were kept clean to prevent infections and diseases.
Experimental Design
The animals were grouped into 4 groups (n = 5). Group 1 served as the Control group, and received standard rat chow and water only during the experiment. Groups 2, 3 and 4 in addition to standard rat chow and water were administered the prepared extract at 25 mg/kg, 50 mg/kg and 100 mg/kg respectively. The extract was administered using oral gavage. Administration of Piper guineense seed extract lasted for 21 days.
Blood Sample Collection
After the 21 days of extract administration, blood samples were gotten through the retro-bulbar plexus of the median canthus vein of the eye using micro-capillary tube, which was carefully inserted into the medial canthus of the eye to puncture the retrobulbar plexus to enable outflow of blood. At least 3 mL of blood was collected into clean glass tubes for serum biochemistry determination. Blood in tubes was left for 30 minutes at room temperature after which it was centrifuged for 15 minutes. Serum was collected using a pipette and stored in a freezer at -200C awaiting biochemical assay.
Biochemical Assay
Serum urea was measured by the diacetyl monoxine method.13 Serum creatinine Serum creatinine was measured using the Jaffe-Slot method.14 Serum electrolytes, (sodium, potassium, chloride and bicarbonate) were measured using an ion selective electrode analyzer.15
Histological Analysis
The kidneys were also identified and excised immediately after blood sample collection. The tissue was stored in 10% formal saline. Subsequently, slides were prepared and the haematoxylin and eosin staining was used. The slides were examined under a light microscope and photomicrographs were taken.
Statistical Analysis
All data was expressed as mean ± standard deviation (SD). Statistical package for social science, (SPSS) version 20 was used to analyze the data obtained. One- way analysis of variance, (ANOVA) was used to determine the difference between the means of various groups. Values with p < 0.05 were considered significant.
Ethical Approval
Ethical clearance approval for this study was obtained from the research and ethics committee of the College of Medicine, University of Nigeria, Enugu Campus. The protocol number is: 031/08/2017.
Results and Discussion
This present study investigated the effect of aqueous extract of P. guineense seed on indices of renal function. The phytochemical analysis done showed that the aqueous extract of P. guineense seed contains various phyto-constituents such as reducing sugars, terpenoid, phenol and alkaloid. These were highly present whereas steroid was the least observed constituent. Previous studies have shown that the seeds and leaves of P. guineense contain abundant phytochemical constituents. Although ethanolic extracts were studies, the seed (compared to the leaves) of P. guineense has been reported to have, and retain over time, a better share of these phytochemical substances.16 These phyto-constiuents possess nutritive and medical properties (Table 1).
Table 1: Qualitative and quantitative phytochemical result of Piper guineense seed
| Phytochemical | Qualitative result | Quantitative result, (mg/100 g) |
| Reducing sugar | ++ | 1034.79 ± 0.00 |
| Hydrogen cyanide | + | 2.06 ± 0.00 |
| Soluble carbohydrate | + | 2.11 ± 0.00 |
| Tannin | + | 5.82 ± 0.00 |
| Alkaloid | ++ | 191.87 ± 0.00 |
| Steroid | + | 0.52 ± 0.00 |
| Terpenoid | ++ | 1060.67 ± 0.00 |
| Phenol | ++ | 2558.70 ± 0.00 |
| Flavonoid | + | 345.68 ± 0.00 |
| Saponin | + | 2.51 ± 0.00 |
| Glycoside | + | 46.75 ± 0.00 |
+ = present
++ = highly present
The renal function test done shows that there was a significant reduction in the level of serum urea and creatinine at low and medium doses. This implies that aqueous extract of P. guineense seed was not toxic to the kidney at moderate doses (Table 2). There was a significant increase in both serum urea and creatinine level in the group that was given high dose of P. guineense seed extract (Table 2). P. guineense has previously been known to have toxic effect on hepatic and renal tissue of some species of fish.17 This result however supports some other previous work done which suggested that P. guineense may not be toxic at moderate doses but should be taken with caution because when high dose.18
Table 2: Assay for biomarkers of kidney function in serum
| Biomarkers
|
Group1 Group 2 Group 3 Group 4
(Control) |
|||
| Creatinine, (µmol/l) | 62.3±7.3 | 51.1±4.5 | 51.1±8.1* | 66.9±11.0* |
| Urea, (mmol/l) | 6.6±1.3 | 5.2±0.8
|
4.8±1.0
|
7.0±0.8* |
*p < 0.5 (significant when compared to control)
There was a dose dependent increase in serum Na+, K+, HCO3–, Cl– levels. However, the increase in bicarbonate, (HCO3–) and chlorine, (Cl–) in the group treated with the highest dose of the extract was statistically significant, (p ≤ 0.05). This indicates that P. guineense may cause HCO3– and Cl– imbalance. A previous study done on diabetic wistar rats showed that methanolic extract of P. guineense, given in moderate doses, had ameliorative effect on the serum electrolyte derangements caused by diabetes.19
Table 3: Assay of serum electrolytes
| Serum electrolytes | Group1 Group 2 Group 3 Group 4
(Control) |
|||
| Na+, (mmol/l) | 139.6±2.7 | 138.2±2.0 | 137.3±1.3 | 140.3±3.2 |
| K+, (mmol/l) | 4.2±0.8 | 4.5±0.3 | 4.7±0.5 | 4.8±0.6 |
| HCO–3, (mmol/l) | 19.1±0.8 | 19.7±1.1 | 22.9±1.2 | 24.3±1.3* |
| Cl–, (mmol/l) | 102.4±3.8 | 104.9±1.6 | 104.0±1.5 | 107.0±1.6* |
*p < 0.5 (significant when compared to control)
The histological analysis revealed that the control group had normal kidney architecture, while in the experimental group, there was a dose dependent changes in the cyto-architecture of the kidney (Figure 3). When the highest dose of P. guineense was administered, there was some level of cyto- architectural distortion of the renal tissue. There was also evidence of glomerular collapse and inflammatory cellular infiltration at the peri-glomerular and peri-tubular regions. There was also marked deposition of intra-luminal eosinophilic tubular casts (Figure 4). This may be due certain chemicals contained in the P. guineense, like phenol. Though, phenols found mainly in plants are believed to protect humans from tumors and viruses, it also produces side effect such as irritation, scarring, and tissue necrosis.20 Therefore, when the dose of the P. guineense is increased, it could be toxic to the body.
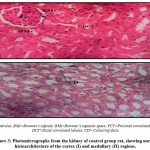 |
Figure 3: Photomicrographs from the kidney of control group rat, showing normal histoarchitecture of the cortex (I) and medullary (II) regions. |
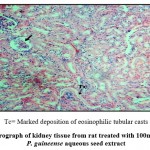 |
Figure 4: Photomicrograph of kidney tissue from rat treated with 100mg/kg body weight of P. guineense aqueous seed extract |
Conclusion
The result of this study suggests that aqueous extract of P. guineense seed did not negatively affect the indices of kidney functions when consumed in low and medium doses; signifying that it is not toxic to the organs at low and medium doses. However when consumed in high dose, especially for a period of time, it may be toxic to the organs like the kidney. Caution should be employed in the consumption of P. guineense seed.
Acknowledgement
The authors wish to thank the Department of Botany, University of Nigeria for their efforts in identifying the plant and the pictures provided. The authors also thank the laboratory staff at the Department of Physiology, University of Nigeria, Enugu Campus for the animal handling and bench work.
Conflict of Interest
The authors declare no conflict of interest.
Funding source
The authors received no external funding for the study.
References
- Katzer and Gernot. Gernot Katzer Spice pages, 2015.
- Okwute K. Plant derived pesticidal and antimicrobial agents for use in Agriculture. A review of phytochemical and biological studies on some Nigerian plants. J. Agricul. Sci. Technol, 1992;2(1):62–70.
- Achinewhu C., Ogbonna C. C. and Hart A. D. Chemical composition of indigenous wild herbs, spices, fruits, nuts and leafy vegetables used as foods. Plant Food Hum Nutr, 1995;48(4):341-348.
CrossRef - Sofowora Medicinal plant and traditional medicine in Africa. John wiley and sons, NewYork. 1982; p. 44.
- Mba A. Effect of dietary intake of P. guineense on growth and indices of fitness in Rattus rattus. Isc. Innoa., 1994;4:383-388.
- Noumi Amvam Z. P. H. and Lontsi D. Aphrodisiac plants used in Cameroon. Fitoter, 1998;69:5-34.
- Mbongue G. Y., Kantchouing P. Essame O. J. L., Yewah P. M. et al. Effect of the aqueous extract of dry fruits of Piper guineense on the reproductive function of adult male rats. Indian J. Pharm., 2005;37(1):30-32.
CrossRef - Bep, O. Medicinal plants in Nigeria. J. of Agric and Food Sci, 1999;7:218-225.
- Purseglove N., Brown E. G. and Robin R. J. Spices. Longmans Scientific and Technical Publishers. 1996;2:16.
- Okigbo N. and Ogbonnaya O. U. Antifungal effects of two tropical plant extract Ocimum gratissimumand Afromomum meleguetaon post harvest yam. Dicorea SPP. Afr. J. Biotech., 2006;5(9)727–731.
- Liwa A. C. and Jaka H. M. Renal Diseases and Use of Medicinal Herbal Extracts: A Concise Update of Reported Literature in Africa. Nephrol. Ren. Ther., 2016;2:008
CrossRef - Trease G. and Evans S. M. Pharmacognosy. 16th edition. Edinburg: Harciut Publishers Limited. 1989;2022
- Friedman H. S. Modification of the determination of urea by the diacetyl monoxime method. Chem., 1953;25:662.
CrossRef - Slot C. Plasma creatinine determination a new and specific reaction method 2009;17(4):381-387.
CrossRef - Roche diagnostics ISE/Na+, K+, Cl– package insert, 2009-03, V6
- Imo C., Yakubu O. E., Imo N. G., Udegbunam I. S., Tatah S. V. and Onukwugha O. J. 2018. Proximate, mineral and phytochemical composition of Piper guineense seeds and leaves. Biol. Sci., 2018;18: 329-337
CrossRef - Okon A. O. The Effect of Ethanolic Extract of Piper guineense on the Histology of some Organs of Oreochromis niloticus (Limn) Pisces: Cichlidae). J. Pure Appl. Sc., 2002;8(2):193-196.
CrossRef - Ebeye O. A., Emore E., Enaibe B. U. and Igbigbi P. S. Histological Effect of Piper guineense Extract on Wistar Rats. J. Biol. Sci., 2007;7(8):1484-1487.
CrossRef - Amadi, G., Iwuji, S. C., Azeez, T. O., Nwaokoro, C. J., and Wodu, C. O. Biochemical Effects of Piper Guineense (African Black Pepper) in Female Diabetics: Opportunities for Diabetes Treatment. J. Transl. Med. Res. Pub. H., 2019;3(1):59–65.
CrossRef - Pandey K. B. and Rizvi S. I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev., 2009;2(5):270-278.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





