Manuscript accepted on : 30-11-2021
Published online on: 07-12-2021
Plagiarism Check: Yes
Reviewed by: Dr. Francisco Solano
Second Review by: Dr. Khalid Rasib
Final Approval by: Dr. Ahmad Ali
Quercetin Modulates Behavioural and Biochemical Alterations in Stressed Mice
Anthony Taghogho Eduviere1 , Emuesiri Goodies Moke1
, Emuesiri Goodies Moke1 , Adrian Itivere Omogbiya1
, Adrian Itivere Omogbiya1 , Lily Oghenevovwero Otomewo1*
, Lily Oghenevovwero Otomewo1* , Juliet Nnenda Olayinka2
, Juliet Nnenda Olayinka2 , Faith Eninidiere Aboyewa1
, Faith Eninidiere Aboyewa1 and Atare Peace Ijeje1
and Atare Peace Ijeje1
1Department of Pharmacology, Delta State University, Abraka, Nigeria.
2Department of Pharmacology, AfeBabalola University, Ado-Ekiti, Nigeria.
Corresponding Author E-mail: ejirukomaotomewo@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2951
ABSTRACT:
Disruption of the active phase of sleep alters the physiological homeostasis of the body and results in oxidative breakdown which may trigger a wide array of defects. The central nervous system and the metabolic system are some of the most affected systems as described in several literatures. Some plant based compounds with antioxidant property have been previously described in the abrogation of the deleterious effects of active sleep disruption. One of such compounds is quercetin. This study was premeditated to expatiate on the probable neuroprotective effect of quercetin on mice exposed to 72hr active sleep disruption. Mice were allotted into five treatment groups (n = 6): group 1 served as control, group 2 received 10 mL/kg vehicle, groups 3 and 4 received 25 and 50 mg/kg quercetin respectively, and group 5 received 50 mg/kg astaxanthin. Treatment lasted for 7 days while groups 2-5 were exposed to the sleep deprivation protocol starting from day 4. Behavioural tests followed by biochemical assays and histopathological changes in the prefrontal cortex were evaluated. Data were analysed by ANOVA set at p<0.05 significance. The results revealed that quercetin, in both doses, significantly amplified memory performance, attenuated depression-like behaviour, replenished catalase and superoxide dismutase, attenuated nitric oxide levels in brain and liver of mice when compared to control group and protected against loss of prefrontal cortex neurons. In conclusion, quercetin possesses protective effects against sleep deprivation-induced brain damage.
KEYWORDS: Antioxidants; Hepatoprotective; Liver; Neuroprotective; Oxidative stress; Quercetin
Download this article as:| Copy the following to cite this article: Eduviere A. T, Moke E. G, Omogbiya A. I, Otomewo L. O, Olayinka J. N, Aboyewa F. E, Ijeje A. P. Quercetin Modulates Behavioural and Biochemical Alterations in Stressed Mice. Biosci Biotech Res Asia 2021;18(4). |
| Copy the following to cite this URL: Eduviere A. T, Moke E. G, Omogbiya A. I, Otomewo L. O, Olayinka J. N, Aboyewa F. E, Ijeje A. P. Quercetin Modulates Behavioural and Biochemical Alterations in Stressed MiceBiosci Biotech Res Asia 2021;18(4). Available from: https://bit.ly/3GiSJE2 |
Introduction
Acute stress such as insufficient sleep is aninfamous challenge in modern society, affecting a significant number of people at various points in their lives. Although occasional sleep disruptions are usually no more than a nuisance, persistent lack of sleep can lead to various alterations in bodily functions that may alter the quality of life1.Meanwhile, there are reports that acute stress increase oxidations and diminishes antioxidant protection particularly in the liver2.A similar ripple effect had previously been noted in the brain due to the relationship between the functional status of the liver and the brain3.
On the other hand, there is literary evidence on the neuroprotective effect of quercetin4.Several scientific literatures have shown that quercetin can bestow neuroprotection and antagonize oxidative stress-mediated disorders in vivo. For example, oral quercetin was revealed to protect laboratory animals from oxidative stress and neurotoxicity which were induced by various insults5,6. Also, Bona et al.7, reported that quercetin reversed the increase in serum levels of liver enzymes in rats caused by inhaled chloroform, supporting the belief that quercetin possesses antioxidant, hepatoprotective and neuroprotective ability. However, there is paucity of information on the role of quercetin in sleep deprivation-induced stress. Therefore, this study is justified in that it contributes to the body of literature on the probable modulatory effect of quercetin on behavioural and biochemical alterations in the brain and liver of sleep deprived mice.
Material and Methods
Animals and Housing
Male Albino Swiss mice (n = 30; 22.0±2.0 g) used in this study were procured from the central animal facility, Faculty of BMS, Delta State University, Abraka. Mice were housed in plastic cages in groups of six and maintained under room temperature with a 12h light–dark cycle (lights on from 07:00 to 19:00 hours). They were allowed access to water and rodent chow adlibitum. Note that mice were acclimatized for about one week before the experiment. The experimental protocols were performed according to the NIH guideline for laboratory animals with due approval from the ethical committee of the institution (REF/FBMS/DELSU/21/105).
Drugs and Chemicals
Quercetin, astaxanthin and DTNB were obtained from Aldrich, Germany. Acetic acid was gotten from Sigma-Aldrich, Inc., St Louis, USA. TCA was obtained from Burgoyne Burbidge’s& Co., Mumbai, India.TBA and DMSOwere obtained from Guanghua Chemical Factory Co. Ltd., China.Tris-buffer was obtained from Hopkins& Williams Company, USA. NaHCO3,NaH2PO4.H2O, K2HPO4, K2Cr2O7, KCl and Na2HPO4.H2O were obtained from BDH Chemicals Ltd, Poole, England. Sodium Carbonate was obtained from Fisons, LoughboroughLeics, England. NaOH was obtained from J.T Baker Chemicals Co., Phillipsburg, N.J., USA.
Drug Preparation and Treatment Groups
The concentrations of quercetin used in this study were obtained following a serial dilution of 100 mg quercetin in 20 mL of 0.5% DMSO. The mice were indiscriminately distributed into five (5) treatment groups (n = 6) based on the drug they received: group 1 received vehicle (10 mL/kg 0.5%DMSO, p.o), group 2 received vehicle in addition to being sleep-deprived, while group 3 received low-dose quercetin and group 4 received high-dose quercetin(25 mg/kg and 50 mg/kg p.o, respectively) in addition to being sleep-deprived, group 5 received astaxanthin (50 mg/kg) in addition to being sleep-deprived. Total treatment duration was seven (7) days. From the fourth day, mice in groups 2-5 were subjected to 72 hr sleep deprivation.
Experimental Design
Deprivation of the activestage of sleep was carried out in linewith the method of Shinomiya and colleagues8 with moderate modifications.
At the end of the 72 hr sleep deprivation duration, 1 hr after the last treatment, the effect of active sleep deprivation on behaviour, hepatic/brain oxidative stress parameters and lipid profile was assessed. Also, animals were euthanized on the seventh day. Liver and brain tissues were harvested and specific tissues were kept aside for histopathological evaluation.
Behavioural Tests
Open field test (OFT)
The OFT was used to determine the spontaneous motor activity (SMA) of mice following the method described9,10. Numberof square lines crossed and duration of ambulation of each mouse was recorded within a10 min period.
Tail suspension test(TST)
The TST was carried out in accordance with the procedure described11 with slight changes. A mouse was adjudged to be non-mobile if it made no movements with its head above water level.
Novel object recognition test
The novel object recognitionmemory test explores the animal’s preference for novelty. In this study, the method described12 was followed. The percentage preference, which was used as anindex of recognition memory, was calculated as the total time spent by a mouse in exploring the novel object divided by the summation of total time spent exploring both the familiar and novel objects multiplied by 100%.
Assessment of lipid profile
Serum triglyceride levels
Serum triglyceride level in mouse serum was determined by enzymatic colorimetric method according to the protocol earlier described13. Concentration of triglyceride (mg/dL) in the sample was obtained from the equation
CX = [K(AX-Ab) + Cb] x IF
Where:
Cx= Concentration of sample
K = Concentration factor
Ax = Mean of absorbance of Sample
Ab = Mean of absorbance of blank
Cb = concentration of blank
IF = instrument factor (or dilution correction)
High-density lipoproteins
High-density lipoproteins (HDL)level was determined in mouse serum by enzymatic colorimetric method according to the protocol described13. Concentration of HDL(mg/dL) in the sample is obtained from the equation:
CX = [K(AX-Ab) + Cb] x IF
Where:
Cx= Concentration of sample
K = Concentration factor
Ax = Mean of absorbance of Sample
Ab = Mean of absorbance of blank
Cb = concentration of blank
IF = instrument factor (or dilution correction)
Biochemical Assays
Superoxide dismutase (SOD) activity approximation
The level of SOD activity in the brain and liver was approximated according to the method modified by Umukoro and his colleagues14 which involves the use of adrenaline. This activity was expressed in units of adrenaline consumed per minute per mg protein.
Estimation of catalase (CAT) activity
Brain and liver catalase activity was determined according to the Hadwanmethod15.The catalase activity was expressed in μmol.
Estimation of nitric oxide
Brain and liver nitrite concentration was estimated following the method of Green et al.16, which involves the use of Greiss reagent.
Statistical Analysis
Data sets were presented as Mean ± S.E.M. The results were analysed using the one-way ANOVA technique, and a specific post hoc test (Student’s Newman–Keuls) was carried out to determine the criterionof significance using Graph Pad Biostatistics software. Significance for all tests was then established at p<0.05.
Results and Discussion
This study was undertaken to assess the effect of active sleep deprivation alone and in combination with quercetin supplementation on brain and liver oxidative stress status and behavioural phenotypes in mice. There are many mechanisms via which sleep deprivation can be responsible for oxidative consequences and liver toxicity, but these effects are probably not attributable to a single night of sleep deprivation. In this study, we measured the effects of 72 hr sleep deprivation on lipid profile parameters, hepatic and brain oxidative stress biomarkers, anxiety-like symptoms, depression-like behaviour and recognition memory consolidation in mice.
The results of this study showed that sleep deprivation significantly diminished recognition memory, as it decreased the percentage preference for the familiar object in mice (Figure 1). Recognition memory was impaired in the sleep deprived group with a significant reduction in percentage preference in the novel object recognition paradigm when compared to the non-sleep-deprived group. Traditionally, the usefulness of this test in memory assessment is based on the innate inclination of animals (particularly rodents) for unfamiliar objects12. The preference of mice for a novel object as compared to a familiar one indicates the existence of the object’s familiarity in the animals’ memory. Thus, the duration of exploration depends on the degree of residual memory of the object. In this test, quercetin increased the duration of exploration of the novel object, which suggests memory improvement12.
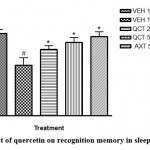 |
Figure 1: Effect of quercetin on recognition memory in sleep deprived mice. |
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
Quercetin also protects neuronal cells. This was substantiated in this study by histomorphometric analysis of the prefrontal cortex of mice17. The data revealed that sleep deprived mice had significant necrosis of brain cells (slide B) when compared with the non-sleep deprived group (slide A). This was similar to the observed changes in neuronal densitities of the prefrontal cortex of mice in all groups18. This was reversed by quercetin supplementation (slides C and D; Figure 2 and 3) as effectively as astaxanthin (slide E). This could also play a role in the memory impairment observed since a study by Umukoro and Eduviere19had implicated the prefrontal cortex as one of the notable brain regions with a role in recognition memory. Although more preclinical studies are necessary before commenting on how quercetin improves memory in mice, the present data suggest modulatory effect of quercetin on sleep deprivation-induced recognition memory impairment.
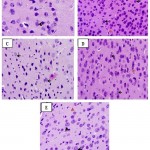 |
Figure 2: Photomicrograph of the prefrontal cortex of sleep deprived mice |
Slide A: Non-sleep deprived group, VEH 10 mL/kg.
Slide B: Sleep deprived group, VEH 10 mL/kg +SD.
Slide C: Low-dose quercetin group, QCT 25 mg/kg +SD.
Slide D: High-dose quercetin group, QCT 50 mg/kg +SD.
Slide E: Astaxanthin group, AXT 50 mg/kg +SD.
Black arrows: Normal neuronal cells.
Red arrows: Neuronal cells undergoing necrosis.
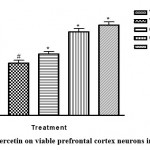 |
Figure 3: Effect of quercetin on viable prefrontal cortex neurons in sleep-deprived mice |
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
Also from this study, recognition memory impairment caused by sleep deprivation was accompanied by increased brain oxidative stress, as indicated by elevated levels of nitrite and decreased antioxidant defence systems in the brain (Table 1). In the brain tissue of the sleep deprived group, SOD and CAT activity were significantly (p<0.05) lower, whereas nitrite levels was significantly increased (p <0.05) when compared to the control group. This effect was however attenuated by quercetin pre-treatment as effectively as astaxanthin. This is in line with previous studies19, 20. Thus, the capability of quercetin to reverse sleep deprivation-induced memory impairment in mice suggests an action that may involve the inhibition of central oxidative stress. This action possibly resulted from its ability to replenish antioxidant defence systems (SOD and catalase) and decrease NO levels. .
Table 1: Effect of quercetin on brain oxidative stress parameters in sleep deprived mice.
| Treatment | CAT
(units/mg protein) |
SOD
(units/mg protein) |
NO
(µ M) |
| VEH 10 mL/kg | 33.47+1.93 | 16.91+0.62 | 47.37+4.55 |
| VEH 10 mL/kg +SD | 15.87+1.94# | 10.32+0.95# | 76.59+6.37# |
| QCT 25 mg/kg +SD | 28.88+3.81* | 16.93+0.86* | 51.65+3.01* |
| QCT 50 mg/kg +SD | 32.60+3.71* | 20.67+0.94* | 39.77+4.45* |
| AXT 50 mg/kg +SD | 27.10+1.88* | 15.61+1.07* | 42.34+5.14* |
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
Furthermore, other mood-related behaviours (depression and anxiety) were assessed in this study. Sleep deprived mice were more active in the open field test (Table 2), signalling anxiety; and increased duration of immobile state in the TST (Figure 4), signalling depression. Sleep deprivation significantly (p <0.05) diminished the motor activity of mice as shown in less number of lines crossed and duration of ambulation and significantly (p<0.05) increased the immobility time of mice in the TST when compared to the non-sleep deprived group. However, quercetin-treated groups exhibited improved mood recorded as increased number of lines crossed in the open field test and decreased duration of immobility in the TST. This agrees with previous studies which have outlined the beneficial role of quercetin on anxiety- and depressive-like symptoms22.
Table 2: Effect of quercetin on spontaneous motor activity in sleep deprived mice
|
Treatment |
Number of lines crossed |
Ambulation (min) |
| VEH 10 mL/kg | 143.70±8.19 | 3.52±0.36 |
| VEH 10 mL/kg + SD | 213.50±10.22# | 6.88±0.39# |
| QCT 25 mg/kg + SD | 175.50±13.18* | 4.80±0.38* |
| QCT 50 mg/kg + SD | 165.70±7.66* | 4.34±0.46* |
| AXT 50 mg/kg+ SD | 185.50±4.87* | 5.09±0.28* |
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
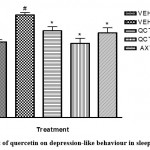 |
Figure 4: Effect of quercetin on depression-like behaviour in sleep-deprived mice |
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
Also in this study, triglyceride level was significantly (p<0.05) elevated while high-density lipoprotein was significantly (p<0.05) diminished in the sleep deprived group (Table 3). Literary evidence has associated sleep disorders with serious complications like type 2 diabetes mellitus23 and glucose intolerance or insulin resistance24.The liver which is the site of metabolism is particularly vulnerable to oxidative stress thus predisposing the organism to further damage25, 26. Results from this study also support this evidence with sleep deprived mice showing a significant (p<0.05) elevation in nitrite levels and decrease in antioxidant levels (Table 4). The administration of quercetin in sleep deprived mice was able to attenuate these deleterious effects of sleep deprivation.
Table 3: Effect of quercetin on triglyceride and high-density lipoprotein levels in sleep deprived mice
| Treatment | HDL (mg/dL) | Triglycerides(mg/dL) |
| VEH 10 mL/kg | 118.3+2.10 | 110.0+2.68 |
| VEH 10 mL/kg +SD | 88.00+4.02# | 166.3+5.76# |
| QCT 25 mg/kg +SD | 106.8+2.48* | 141.4+6.41* |
| QCT 50 mg/kg +SD | 113.3+3.45* | 134.9+3.42* |
| AXT 50 mg/kg +SD | 104.0+2.36* | 149.9+3.40* |
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
Table 4: Effect of quercetin on liver oxidative stress parameters in sleep deprived mice
| Treatment | CAT
(units/mg protein) |
SOD
(units/mg protein) |
NO
(µ M) |
| VEH 10 mL/kg | 65.80+5.15 | 58.14+3.71 | 34.62+5.71 |
| VEH 10 mL/kg +SD | 26.28+3.21# | 27.64+2.91# | 81.13+4.85# |
| QCT 25 mg/kg +SD | 49.60+5.49* | 43.10+4.70* | 51.81+6.87* |
| QCT 50 mg/kg +SD | 58.03+5.42* | 52.44+3.62* | 40.28+3.00* |
| AXT 50 mg/kg +SD | 48.23+4.31* | 45.35+3.90* | 49.41+5.15* |
# depicts significance (p<0.05) compared to non sleep-deprived group.
* depicts significance (p<0.05) compared to vehicle + SD group.
VEH: Vehicle. AXT: Astaxanthin. QCT: Quercetin. SD: Sleep deprivation.
Conclusions
Based on the antioxidant and protective effects of quercetin on the brain and liver of mice in this study, we believe that this compound stands a chance of being a potential therapeutic agent for the treatment of neuronal degeneration resulting from sleep deprivation. Nevertheless, more reliable methods such as immunocytochemistry are recommended for further investigation of the neuroprotective effect of quercetin.
Acknowledgement
We appreciate the technical support of the laboratory personnel of Pharmacology department. Also, we give special gratitude to Mr.AdedamolaFafure for the histology.
Conflict of Interest
The authors declare the absence of conflicts of interest
Funding support
The authors received no external funding for this research
References
- Carley D. W. and Farabi S. S. Physiology of sleep. Diabetes Spectr. 2016; 29(1): 5-9
CrossRef - Zhai B, Wu C, Wang L, Sachs M. S. and Lin X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob Agents Chemother 2012; 56(7):3758-3766
CrossRef - Jones E. A. and Weissenborn K. Neurology and the liver. Journal of Neurology, Neurosurgery and Psychiatry 1997; 63:279-293
CrossRef - Ossola B, Kaariaiinen T. M. and Mannisto P. T. The multiple faces of quercetin in neuroprotection. Expert Opinion on Drug Safety 2009; 8(4):397-409
CrossRef - Ishisaka A, Ichikawa S, Sakakibara H, et al. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radical Biology and Medicine 2011; 51(7); 1329-1336
CrossRef - Das S, Mandal A. K, Ghosh A, Panda S, Das N. and Sarkar S. Nanoparticulatedquercetin in combating age related cerebral oxidative injury. Current Aging Science 2008; 1(3); 169-174
CrossRef - Bona S, Filippin L. I, Di Naso F. C, et al. Effect of antioxidant treatment on fibrogenesis in rats with carbon tetrachloride-induced cirrhosis. ISRN Gastroenterol 2012; 762920
CrossRef - Shinomiya K, Shigemoto Y, Okuma C, Mio M. and Kamei C. Effects of short-acting hypnotics on sleep latency in rats placed on grid suspended over water. European Journal of Pharmacology 2003; 460:139-144.
CrossRef - Adeoluwa O. A, Aderibigbe A. O, Agu G. O, Adewole F. A. and Eduviere A. T. Neurobehavioral and analgesic properties of ethanol bark extract of Terminaliaivorensis A Chev. (Combrataceae) in mice. Drug Res 2015; 65;545-551
CrossRef - Annafi O. S, Aluko O. M, Eduviere A. T, Omorogbe O. and Umukoro S. Probable mechanisms involved in the antipsychotic-like activity of methyl jasmonate in mice. Naunyn-Schmiedeberg’s Arch Pharmacol 2017; 390:883–892
CrossRef - Adebesin A, Adeoluwa O. A, Eduviere A. T. and Umukoro S. Methyl jasmonate attenuated lipopolysaccharide-induced depressive-like behaviour in mice. Journal of Psychiatric Research 2017; 94; 29-35
CrossRef - Eduviere A. T, Umukoro S, Adeoluwa O. A, Omogbiya I. A. and Aluko O. M. Possible mechanisms involved in attenuation of lipopolysaccharide-induced memory deficits by methyl jasmonate in mice. Neurochem Res 2016; 41:3239–3249
CrossRef - Bucolo G. and David H. Quantitative determination of serum triglycerides by use of enzymes. Clinical chemistry 1973; 19: 476-482.
CrossRef - Umukoro S, Aluko OM, Eduviere AT, Owoeye O. Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brain Res Bull. 2016;121:105-114.
CrossRef - New method for assessment of serum catalase activity. Indian Journal of Science and Technology2016; 9(4):1
CrossRef - Green L. C, Wagner D. A, Glogowski J, Skipper P. L, Wishnok J. S. and Tannenbaum S. R. Analysis of nitrate, nitrite, and [15N] nitrate in biological fluids. Anal Biochem 1982; 126(1): 131-138
CrosssRef - Garman R. H, Li A. A, Kaufmann W, Auer R.N. and Bolon B. Recommended methods for brain processing and quantitative analysis in rodent developmental neurotoxicity studies. Toxicologic Pathology 2016; 44(1): 14-42
CrossRef - Keller D, Ero C. and Markram H. Cell denisities in the mouse brain: a systematic review. Front. Neuroanat. 2018. Accessed from https://doi.org/10.3389/fnana.2018.00083
CrossRef - Umukoro S. and Eduviere A. T. Methyl jasmonate attenuates memory dysfunction and decreases brain levels of biomarkers of neuroinflammation induced by lipopolysaccharide in mice. Brain Research Bulletin 2017; 131; 133–141
CrossRef - Reimund E. The free radical flux theory of sleep. Medical Hypotheses 1994; 43(4);231-233
CrossRef - Villafuerte G, Miguel-Puga A, Rodriguez E. M, Machado S, Manjarrez E. and Arias-Carrion O. Sleep deprivation and oxidative stress in animal models: a systematic review. Oxidative Medicine and Cellular Longevity 2015; 234952
CrossRef - Samad N, Saleem A, Yasmin F. and Shehzad M. A. Quercetinprotect against stress-induced anxiety- and depression-like behaviour and improve memory in male mice. Res. 2018; 67: 795-808
CrossRef - Khandelwal D, Dutta D, Chittawar S. and Kalra S. Sleep disorders in type 2 diabetes. Indian J EndocrinolMetab 2017; 21(5):758-761
CrossRef - Hargens T. A, Kaleth A. S, Edwards E. S. and Butner K. L. Association between sleep disorders, obesity, and exercise: a review. Nat Sci Sleep 2013; 5:27-35
CrossRef - Umukoro S, Oluwole O, Olamijowon H, Omogbiya A. I. and Eduviere A. T. Effect of MSG on behavioural phenotypes, biomarkers of oxidative stress in brain tissues and liver enzymes in mice. World Journal of Neuroscience 2015; 5:339-349
CrossRef - Omogbiya A. I, Ben-Azu B, Eduviere A. T, Eneni A. O, Nwokoye P. O, Ajayi A. M and Umukoro S. Monosodium glutamate induces memory and hepatic dysfunctions in mice: ameliorative role of Jobelyn® through the augmentation of cellular antioxidant defence machineries. Toxicol. Res 2020; 37(2):1-15.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





