Manuscript accepted on : 16-08-2022
Published online on: 19-09-2022
Plagiarism Check: Yes
Reviewed by: Dr. Sonal Mahajan
Second Review by: Dr. Tayebe Bagheri Lotfabad
Final Approval by: Prof. Imran Ali
Preparation of Collagen Particles from Fish Waste Infused with Cassia auriculata
Department of Biotechnology, Aarupadai Veedu Institute of Technology, Vinayaka Missions Research Foundation, Paiyanoor, Chengalpatt (Dt), Tamilnadu Pin-603 104. India.
Corresponding Author E-mail: drnirmala81@gamil.com
DOI : http://dx.doi.org/10.13005/bbra/3014
ABSTRACT: The human skin encompasses a characteristic capacity to advance the self-regeneration after harm; this capacity can be compromised beneath particular conditions, like extensive skin loss, constant wounds, deep burns, non-healing ulcers and diabetes. Improper healing can lead the wound to enter in a constant state, which increments the chance of disease to chronic state; it affects the patient health and his/her quality of life. Due to growing concerns about unhealthy consequences of chemicals in the health care system, the interest towards natural and herbal substances has been growing every day. The plant Cassia auriculata is widely used medicinal plant in India and also popular in Indigenous system of medicines like Ayurveda and siddha. The collagen was successfully extracted from the Cattla Cattla fish scales using acid solubilizing collagen extraction method and the collagen was infused with plant extract of Cassia auriculata. The characterisation such as protein estimation (Lowry method), solubility (pH 1, 3, 5, 7, 9, 11 and 13), UV visible and FTIR was performed for collagen and phytochemical analysis (Alkaloids, Flavonoids, Saponins, Tannins, Phenol, Terpenoids, Glycosides and Steroids) anti-bacterial (E. coli and S. aureus) and anti-inflammatory test in different concentration (25mg, 50mg, 100mg / ml) was performed for the plant extracts and collagen infused with plant extracts. The result of the present study was confirmed that final product prepared using collagen with plant extract can be used to prepare the dressing material such as gels, ointments, bio-films, powdered flakes etc.
KEYWORDS: Collagen; Cassia auriculata; Diabetic Foot Ulcer; Marine Source; Phytochemicals
Download this article as:| Copy the following to cite this article: Nirmala A, Karthika M. Preparation of Collagen Particles from Fish Waste Infused with Cassia auriculata. Biosci Biotech Res Asia 2022;19(3). |
| Copy the following to cite this URL: Nirmala A, Karthika M. Preparation of Collagen Particles from Fish Waste Infused with Cassia auriculata. Biosci Biotech Res Asia 2022;19(3). Available from: https://bit.ly/3UeQE3T |
Introduction
Wound healing is a complex progression that takes place in skin after it has been damaged. Hemostasis, inflammation, proliferation and maturation are the four stages of wound healing1. In hemostasis phase, platelets aggregate and stick to the epithelial wall of blood vessels, where fibrin strands begin to adhere to the blood and transformed for the release of prothrombin. Platelets and blood cells are trapped in the wound area and forming a clots. In damaged cells, germs and bacteria are evacuated from the wound region during the inflammatory phase. Swelling, heat, discomfort and redness are frequent during this stage of wound healing and can be caused by white blood cells, growth hormones, nutrients and enzymes. In proliferation phase, new collagen and extracellular matrix-based tissues are formed in the injured area. A new network of blood vessels is being built to ensure that the damaged area receives enough oxygen and nutrients. The maturation phase of wound healing is last stage, when collagen is transformed from type III to type I, the wound is completely healed. Healed wound have a lower tensile strength than normal skin, with just 80% of the tensile strength of unwounded skin 2.
Physiological changes in tissues and cell induced wound healing get delayed and difficult to manage in diabetics, as well as having their impact. Diabetes affects the healing of both acute and chronic wounds3. Factors both external and internal to the wound and its biology impede wound healing in diabetes mellitus. Thickening of the capillary and arteriole membranes is common in diabetics, leading to slow wound healing and the formation of persistent ulcers4.
Collagen is a selective organic macromolecule with a triple helix structures that make up the larger part of the extracellular dermal network (ECM). In both vertebrate and invertebrate creatures, collagen is the foremost plenteous mammalian protein and the major basic strong protein of the extracellular matrix. Collagen is found in sinewy connective tissues, the dermis of the skin, corneas, cartilages, bones and sclera, where it plays a vital basic part in tissues and organs by giving malleable quality and flexibility5, 6. Collagen type I accounts for 70% of collagen in the skin, with type III accounting for 10% and collagen types IV, V, VI, and VII accounting for trace amounts. Collagen’s main purpose in connective tissue is to act as a scaffold, which it does primarily in its type I, II, and III forms. Type III formed first during the early stages of wound healing, with the amount of type I increasing as scar formation develops and it is rebuilt7,6. Diabetes patients have poor biomechanical qualities, such as increased skin stiffness and decreased skin elasticity. Collagen synthesis and breakdown are out of balance, resulting in these characteristics5. External collagen sources, such as plant-based collagen, marine collagen and so on, can be used to boost tissue regeneration potency when collagen production is lacking. The abundance of structurally and physiologically active chemicals found in marine species is surprising and plentiful. Fish, seaweeds, sponges and jellyfish are all sources of marine collagen. It has several advantages over mammalian collagen, including as it can be extracted easily, the fact that it is water-soluble, it is free of animal disease and infections, it has better chemical and physical durability and also it is readily available. It has a wide range of applications due to its biocompatibility, biodegradability, ease of supply and adaptability 8.
In 21st century, home grown drugs are being favoured generally compared to cutting edge medication due to their security, social adequacy, viability and less antagonistic impacts. Conventional herbs have a long history of practice and are considered to be more secure than engineered drugs. Plant and plant based compounds have been utilized with changing victory to diminish, avoid illnesses and diseases in all the time 9 . One such a plant Cassia auriculata commonly known as “avaram” is a shrub that belongs to the Caesalpiniaceous family. The individual parts of the plant can be used for the treatment of various disease and disorders in humans. The plant contains several biological properties like hepato protective, anticancer, antioxidant, antidiabetic, anti-inflammatory and antimicrobial properties10. It has phytochemicals like alkaloids, tannins, saponins, phenolics and terpenoids exhibit excellent anti-inflammatory activity. Restorative properties of its flower have antioxidant and antimicrobial activity was contributed by nearness of its phytoconstituents. The flavonoids shown in it act as cancer prevention agents which give assurance against free radicals that harm cells, tissues additionally the tannins progress the wound recuperating .11
Wound dressing derived from common body components may be beneficial to progress biocompatibility and to maintain a strategic distance from conditions like aggravation or rejections. The most objective of employing a natural wound dressing is to improve wound patching by avoiding bacterial infection and to speed up the tissue recovery. Wound dressing materials should have basic properties like adaptability, durability, gas penetrability and capacity to avoid water loss. Collagen as base fabric offers a few points of interest, which has well recorded basic structural, physical and chemical properties. Hence, the present work focussed on the preparation of collagen from marine source and it can be incorporated with Cassia auriculata, which can be used as dressing material for diabetic wound.
Materials and Methods
Preparation of Fish sample for collagen extraction
Waste parts of Cattla Cattla fish (scales) was washed in water and shade dried for 6-7 days until they are completely dried. Then the samples were ground into small pieces by using a blender. Pre-treatment was conducted, to make sure all the unwanted materials like unground scales were removed. Therefore there will be no unwanted materials that will affect the collagen and make sure that the collagen was completed extracted without any remote materials.
The fish scales were soaked in 0.1 M sodium hydroxide (NaOH) preparation with proportion of 1:8 (w/v) for 6 hrs and kept in stirrer for the expulsion of non-collagenous protein. The NaOH solution was changed for each 3 hrs washed completely with cold refined water until the flushed water ended up neutral (pH 7) as measured by using pH meter12. Fish scales were soaked in 0.5 M Ethylene Diamine Tetra Acetic acid (EDTA) at pH 7.4 for 24 hrs with persistent mixing. The solution was changed for each 12 hrs. Then, it was washed with refined water for 3 times 13.
The collagen was extracted using acid soluble collagen extraction method.
The samples were undergone acid soluble collagen (ASC) extraction process by using 0.5 M acetic acid with the sample in solution ratio 1:10 (w/v) for 24 hrs with continuous stirring.
The extracts were centrifuged at 10, 000 x g for 30 mins at 4ºC and the supernatant was separated. The residues then were re-extracted with 0.5 M acetic acid with sample to solution ratio of 1:10 (w/v) for 12 hrs and centrifuged at 10,000 x g for 30 mins at 4ºC.
Both supernatants were combined and sodium chloride (NaCl) was included to salt out until the ultimate concentration of the supernatant was 0.7 M for the precipitation to happen.
The supernatant was centrifuged once more at 2,500 x g to get the precipitate. The precipitates were lyophilised14
Preparation of aqueous extract of Cassia auriculata:
The Cassia auriculata flowers were collected and shade dried for 3-4 days. From this the unwanted parts like stems and leaves are removed.
The dried flowers were made into partial pieces using a blender.
One gram (1g) of blended flowers were mixed with 20 mL of water and kept in shaking incubator at 50-60 rpm for 24 hrs.
The mixture was then filtered through the Whatman No.1filter paper to ensure that no particles were present in the solution and the extract was collected (Fig -1).
The filtered solution was again filtered using syringe
 |
Figure 1: Preparation of aqueous extract of Cassia auriculata. |
Infusion of collagen with Cassia auriculata
The prepared collagen from fish scales and flower extract from Cassia auriculata was combined together using cross linker.
Using 0.05M acetic acid (in the ratio 1:10) collagen solution was prepared
The solution was agitated using magnetic stirrer for 1h and kept for 24 h incubation at 4° C.
The solution was then filtered and homogenized.
25µg/ml, 50µg/ml, 100µg/ml of plant extracts were added to 20ml of collagen solution and mixed well.
Varying quantities of ethylene glycol (cross linker) were added (0.5ml-2.5ml).
Solution was poured in plates and it was air dried or freeze dried (Fig-2).
The prepared collagen samples were stored for further analysis 11
 |
Figure 2: Infusion of collagen with Cassia auriculata. |
Estimation of protein by Lowry’s method
Reagent A
It consists of aqueous solution of 0.4% potassium-sodium tartrate, 10% Na2CO3, and 0.5 M NaOH.
Reagent B
It consist of an aqueous solution of 2% potassium-sodium tartrate, 3% CuSO4, 5 H2O and 0.1 M NaOH. Reagents A and B are stable for 1 month at room temperature. The Folin-Ciocalteu reagent was diluted with water (1:15), the resulting solution being 0.15 N.
Incubation of collagen samples in alkaline solution at high temperature (50°C) leads to the unfolding of the collagen rigid triple helix (the melting temperature of collagens being – 40-43°C). In this way, the collagen polypeptide spines can frame complexes with Cu’+ more promptly and decrease Folin’s reagent. 200 µl of collagen solutions were incubated with 180 µl of reagent A and 20 µl of reagent B at 50°C for 20 min. After cooling at room temperature, 600 µl of diluted Folin-Ciocalteu reagents were included and forcefully shaken and the samples were incubated at 50°C for 20 min. The absorbance of the samples was read at 650 nm against the reference after cooling in room temperature. BSA was utilized as standard.
Solubility of collagen at different pH
The dissolvability of the collagens at the diverse pH and NaCl concentrations were decided by the method of Montero et al., 16 with a slight alteration. Crude collagen samples were broken down using 0.5 M acidic acid to get a final concentration of 3 mg/mL and the blends were mixed at 4°C for 24 h. At that point, the blends were centrifuged at 5000 g for 15 min at 4°C. The crude solutions (8 mL) were transferred to 50 mL centrifuge tube. The pH was balanced with either 6 M NaOH or 6 M HCl to get the final pH extending from 1, 3, 5, 7, 9, 11 and 13. At that point, the volume of solution was made up to 10 mL by deionized water and the solution pH was balanced to the same pH as the collagen solution. The solution was centrifuged at 15000 g for 30 min at 4°C. Finally the protein substance within the supernatant was measured.
*Percentage of Relative solubility = Estimated protein content / 3 mg (Initial volume taken) × 100.
Phytochemical analysis of plant extract
Alkaloids
In a test tube 0.5ml of extract, few drops of Meyer’s reagent were added. White precipitate indicates the presence of Alkaloids.
Flavonoids
In a test tube 0.5ml of extract few drops of 20%NaOH was added. Intense yellow colour indicates the presence of Flavonoids.
Saponins
In a small test tube, 0.5ml of extract, 2ml of distilled water was added and shaken vigorously. Formation of bubbles shows the presence of saponins.
Tannins
In a test tube, 0.5ml of extract few drops of alcoholic FeCl3 was added. Formation of brownish blue or black colour indicates the presence Tannins.
Phenol
In a test tube, 0.5ml of extract few drops of 5% FeCl3 was added. Formation of blue-black colour indicates the presence of phenol.
Terpenoids
In a test tube 0.5ml of extract, 0.5ml chloroform and few drops of H2SO4 was added. Reddish brown colour indicates the presence of terpenoids.
Glycosides
0.5ml of extract, 0.5ml glacial acetic acid and few drops of FeCl3 was added. Formation of brown ring indicates the presence of glycosides.
Steroids
In a test tube 0.5ml of extract, 0.5ml chloroform and a few drops of conc.H2SO4 were added. Chloroform layer appears red while acid shows Fluorescence.
Antibacterial activity
The anti- bacterial assay was carried out for both plant extract and collagen infused plant extract. The bacterial strains of one gram negative bacteria (Escherichia coli )and one gram positive bacteria (Staphylococcus aureus) were standardized to 0.5 McFarland to obtain 108 cells/ml. Amoxicillin was used as a positive control whereas water was used as negative controls.
Anti-inflammatory assay: BSA Inhibition Method
The anti-inflammatory activity was carried out by Bovine Serum album method. The percentage inhibition was calculated for both plant extract and plant extract infused collagen.
*% Antidenaturation Activity = % Inhibition of protein Denaturation = % Anti-inflammatory Activity
UV visible spectrum
UV visible spectroscopy was performed to estimate the purity of the collagen particles extracted using acid solubilizing method.
FTIR spectrum
FTIR (Fourier Transform Infrared Spectroscopy) was performed to identify and to detect the peak of the active components present and newly formed compounds by bonding and stretching in the collagen, plant extract and collagen infused plant extract.
Results and Discussion
Estimation of protein
The protein content present in crude collagen was estimated using Lowry method with some modifications. The presented graph showed the protein content to be 81.5 mg/100 mg of sample. (Abs of sample = 0.035). The result in graph-1 shows higher amount of protein content, higher the protein concentration influenced by the high amount of amino acids content in the collagen. Concurring to Veeruraj et al., 17 glycine is a major amino acid present in the collagen. This is basic for the arrangement of super helical structures of collagen that makes a difference in tissue. Furthermore, this amino acid content also has an influence on the stability of collagen fibre, shrinkage and denaturation temperature of the collagen 18, 19.
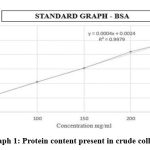 |
Graph 1: Protein content present in crude collagen. |
Solubility of collagen at different pH
The most elevated dissolvability was watched at pH 1, pH 3 and pH 5 separately (Table-1, Graph-2). Agreeing to Wu et al., 20 collagen ought to be more soluble in acidic pH, within the range of pH 1 to 4 and less soluble in isoelectric point. Since within the acidic pH, the charge will be positive which lead to a solid electrostatic repulsion between collagen molecules. This study compared with findings of Veeruraj et al. 17 higher solubilisation occurred in acidic pH from 1 to 5.
Table 1: Solubility of collagen at different pH
| pH | Protein Content mg/ml | Percent of Relative solubility |
| pH 1 | 2.94 | 98 |
| pH 3 | 2.5 | 83.33 |
| pH 5 | 2.32 | 77.33 |
| pH 7 | 2.1 | 70 |
| pH 9 | 1.4 | 46.67 |
| pH 11 | 1.1 | 36.67 |
| pH 13 | 0.75 | 25 |
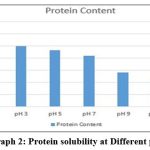 |
Graph 2: Protein solubility at Different pH. |
Phytochemical analysis of plant extract
The phytoconstituents were identified by chemical tests, which showed the presence of various phytoconstituents in plant extract of Cassia auriculata, reveals the presence of flavonoids, phenols, tannins, terpenoids in maximum amount (Table -2)
Table 2: Phytoconstituents present in plant extracts.
| S.No | Phytochemical | (Crude extracts) |
| 1 | Alkaloids | + |
| 2 | Flavonoids | ++ |
| 3 | Saponins | + |
| 4 | Tannins | +++ |
| 5 | Phenol |
+++ |
| 6 | Terpenoids | ++ |
| 7 | Glycosides | + |
| 8 | Steroids | – |
Phenols
Phytochemical screening of the aqueous extract revealed the existence of phenolic compound in the extracts (Graph-3). By using Folin Ciocalteu method the total phenolic content was determined in terms of the Gallic acid equivalent (GAE) in mg/g of the extract. With the help of graph, the total phenolic content was determined and the standard curve equation was y = 0.0048x – 0.0732, where R² = 0.972. The total phenolic content (Gallic acid equivalents, mg/g) in the aqueous extracts were calculated to be 150.66 μg/ml.
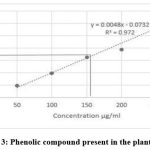 |
Graph 3: Phenolic compound present in the plant extract. |
Flavonoids content
Aqueous extract of the plant shows plentiful amount of phenolic group of phytochemicals (Graph-4), one of the most bioactive ingredient present in it. Hence quantitative assessment of flavonoids, a sub class of phenol was also performed for the sample. The total contents of flavonoids were determined by colorimetric method. The solution was mixed well, and the absorbance was measured at 510nm. To measure the standard curve, Quecertin was used (y=0.0007x+0.054, R2 = 0.9759) and the results were expressed as 210 μg of Quecertin equivalents (CEs) per g of extract.
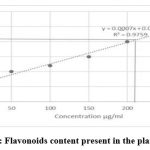 |
Graph 4: Flavonoids content present in the plant extract Click here to view graph |
Phenols, alkaloids, flavonoids and terpenoids exhibit excellent anti-inflammatory activity, through any of the several cellular mechanisms like, altering the activities of immune cells involved in inflammatory reaction like the macrophages and neutrophil, modulating the proinflammatory gene expression, or by regulating the functional expression enzymes, as mentioned by Kolapo AL et al,.21.
Antibacterial activity
The results of antibacterial activity of plant extract and collagen infused plant extract of Cassia auriculata against Gram negative bacteria E. coli and Gram positive bacteria S. aureus are reported in table-3 and 4. The concentration showed higher average zone of inhibition was 100mg/ml as mentioned by Sudharsana et al 22. Our study results in accordance with Hosamani et al 2011 and Scalbert, 1991. In their study observed that antimicrobial activity of Cassia auriculata may be due to the presence of phytochemical constituents like flavonoids and phenolic compounds present in the plant as secondary metabolites. In our study we found that the high amount phenol, Tannins and Flavonoids content 23, 24.
Table 3: Antibacterial activity of Cassia auriculata
| Sample code | Compound | Zone of Inhibition in mm (E.coli) | Zone of Inhibition in mm (S.aureus) |
| 01 | Positive standard | 22 | 17 |
| 02 | Plant extract – 100 mg | 15 | 13 |
| 03 | Plant extract – 50 mg | 14 | – |
| 04 | Plant extract – 25 mg | – | – |
| 05 | Negative control | – | – |
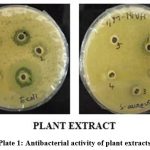 |
Plate 1: Antibacterial activity of plant extracts Click here to view plate |
Table 4: Collagen infused plant extract against Gram negative (E. coli) and Gram positive bacteria (S. aureus).
| Sample code | Compound | Zone of Inhibition in mm (E.coli) | Zone of Inhibition in mm (S.aureus) |
| R1 | Positive standard | 23 | 19 |
| R2 | Plant extract – 100 mg infused collagen | 15 | 13 |
| R3 | Plant extract – 50 mg infused collagen | 14 | 12 |
| R4 | Plant extract – 25 mg infused collagen | 13 | 11 |
| R5 | Negative control | – | – |
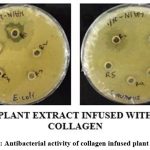 |
Plate 2: Antibacterial activity of collagen infused plant extract. |
Plant extract showed greater ZOI towards gram positive bacteria (Plate-1). Whereas collagen infused plant extract showed greater ZOI towards both gram positive and gram negative bacteria (Plate-2).
Anti-inflammatory assay
Anti-inflammatory test was done at 660nm, with absorbance values. OD value can be obtained to calculate inhibition percentage. Here plants extract showed percentage inhibition for 100mg / ml and the collagen infused plant extract showed slightly higher percentage of inhibition for 100mg / ml (Table-5). Our study in accordance with the Lilian et al, 1998. They have reported that methanolic extract of C. auriculata leaves showed potent anti-inflammatory activity compared to aqueous, hydroalcoholic and ethyl acetate extracts 25. As the anti-inflammatory effect was more significant during later phase of inflammation, it can be concluded that there might be inhibition of inflammatory mediators such as prostaglandins, leukotrienes, polymorphonuclear cells or bradykinins etc., This anti-inflammatory activity due to presence of active phytoconstituents like steroids, flavonoids, alkaloids, terpenoids, tannins and other phytochemicals.
Table 5: Anti-inflammatory activity of plant extracts and plant extracts infused with collagen.
| Plant extract | Absorption @660nm | Percentage inhibition (%) | Plant extract infused with collagen | Absorption @
660nm |
Percentage inhibition (%) |
| Positive standard | 0.412 | 20.17 | Positive standard | 0.412 | 20.17 |
| Plant extract-100mg | 0.565 | 16.27 | Plant extract-100mg infused with collagen | 0.510 | 17.25 |
| Plant extract-50mg | 0.542 | 13.00 | Plant extract-50 mg infused with collagen | 0.595 | 13.42 |
| Plant extract-25mg | 0.654 | 7.32 | Plant extract-25 mg infused with collagen | 0.641 | 9.24 |
| Negative control | 0.689 | 2.18 | Negative control | 0.689 | 2.18 |
UV visible spectrum
The UV spectrum showed maximum absorbance around 277nm. This defines that the acid solubilizing method was successful in extraction of collagen with high purity of protein content (Graph-5).
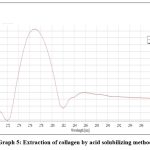 |
Graph 5: Extraction of collagen by acid solubilizing method. |
FTIR spectrum
The FTIR result in disappearance of the peaks at 1500 cm-1 and 2250 cm-1 on comparing between graph 6 and graph 7 indicates formation of new group thereby bonding between collagen and plant extract. As mentioned by Manuel J. seixas 26 the result showed that some frequency of stretching in the collagen samples which refers to new formation of compounds.
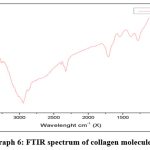 |
Graph 6: FTIR spectrum of collagen molecule. |
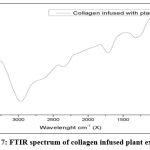 |
Graph 7: FTIR spectrum of collagen infused plant extract. |
Conclusion
In conclusion, the collagen was successfully extracted from the Cattla Cattla fish scales using acid solubilizing collagen extraction method and collagen was infused with plant extract of Cassia auriculata. The results obtained from this study confirmed that final product prepared using collagen and plant extract can be used to prepare the dressing material such as gels, ointments, bio-films, powdered flakes etc. This combination can helps in effective wound healing and can act as great alternative dressing material for those who are having diabetic wound. Since diabetic person have a low potential in wound healing mechanism, this can enhance the wound healing process. It can be used to treat in various stages and types of wound in medical field. In short, the characteristics of the isolated collagens obtained from this study are similar to those of commercial collagen, indicating that a value-added products could be produced from Cattla Cattla fish. Nevertheless, the use of collagen substitutes to fasten wound healing processes has attracted by many researchers and manufacturers in industry.
Acknowledgment
The authors are thankful to Aarupadai Veedu Institute of Technology, Vinayaka Missions Research Foundation for providing laboratory facility and their support.
Conflict of Intrerest
Conflict of interest: The authors declare no conflict of interest.
Funding Sources
No specific grant was received from any funding agency.
References
- Rúben F, Pereira and Paulo Bártolo J. Traditional Therapies for Skin Wound Healing. Wound. Care (New Rochelle). 2016, 5(5), 208–229.
CrossRef - Esraa Mohamed Abdullzaher, Physiopedia. Diabetes and its effect on wound healing and patient care, Nurs Time.2003, 99(42), 73-4.
- Maria Mousley. Diabetes and its effect on wound healing and patient care, Nurs Times.2003, 99(42), 73-4.
- Mellitus Elena Tsourdi, Andreas Barthel, Hannes Rietzsch, Andreas Reichel, Stefan R. Bornstein. Current Aspects in the Pathophysiology and Treatment of Chronic Wounds in Diabetes. Biomed Res Int.2013, 6.
CrossRef - Vanessa Goulding. The effects of diabetes on collagen within wound healing. The Diabetic Foot Journal. 2015, Vol 18 (2): 201.
- Ye-Seon Lim, Ye-Jin Ok, Seon-Yeong Hwang, Jong-Young Kwak and Sik Yoon. Marine Collagen as A Promising Biomaterial for Biomedical Applications, Mar Drugs. 2019, 17(8), 467.
CrossRef - Harsha MP, Brundha. Role of collagen in wound healing. Drug Invention Today, 2020, 13 (1), 55-57.
- Ye-Seon Lim, Ye-Jin Ok, Seon-Yeong Hwang, Jong-Young Kwak and Sik Yoon. Marine Collagen as A Promising Biomaterial for Biomedical Applications, Mar drugs. 2019, 17(8), 467.
CrossRef - Salma B, Muthukumar S.P, Avinasha S and Manjula S.N. Review in ethnobotany phytochemistry and pharmacological properties of cassia auriculata, Pharmacy pharmacology International Journal. 2020, Volume 8 (2), 106-111.
CrossRef - Lalitha Vaidyanathan, Devi Thanikachalam, Lokeswari Sivaswamy T.S. Evaluation of Wound Healing Potency of Cassia auriculata Flower Extracts Using Chick Embryo Wound Model. J. Pharm. Sci. Rev. Res.2014, 27(2), 222-227.
- Viji Chandran S, Trikkurmadom Seetharaman Amritha, Rajalekshmi, G, Sujatha S and Pandimadevi M.Collagen – Azadirachta indica (Neem) Leaves Extract Hybrid Film as a Novel Wound Dressing: In vitro Studies , J. Pharm. Sci. Rev. Res.2015, 32(2), 193-199.
CrossRef - Hukmi N.M.M, Sarbon N.M. Isolation and characterization of acid soluble collagen (ASC) and pepsin soluble collagen (PSC) extracted from silver catfish (Pangasius sp.) skin. International Food Research Journal, 2018, 25(5), 1785-1791.
- Hamdan F.S and Sarbon N.M. Isolation and characterisation of collagen from fringescale sardinella (Sardinella fimbriata) waste materials. International Food Research Journal.2019, 26(1), 133 – 140.
- Nurtasha A, Baderi and Sarbon N.M .Microstructure, extractability and physicochemical properties of short fin scads (Decapterus macrosoma) bone collagen as influenced by acetic acid concentration. International Food Research Journal, 2019, 26(2), 451-458.
- Monisha M, Sowmiya R, Ragunathan, Jesteena Johney. Extraction of Bio Active Compounds from Cassia Auriculata Pods and Leaves and its Medicinal Uses. Int.J.Curr.Microbiol.App.Sci. 2017, 6(8), 425-434.
CrossRef - Montero P, Alvarez C, Martí M.A and Borderías.A.J. Plaice Skin Collagen Extraction and Functional Properties, Journal of Food Science. 2006, 60(1), 1 – 3.
CrossRef - Veeruraj A, Arumugam M and Balasubramanian T. Isolation and characterization of thermostable collagen from the marine eel-fish (Evenchelys macrura). Process biochemistry 2013; 48(10):1592-1602.
CrossRef - Pati F, Adhikari B and Dhara S. 2010. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresource Technology 101: 3737– 3742.
CrossRef - Hashim P, Ridzwan M. S. M, Bakar J and Mat Hashim D. 2015. Collagen in food and beverage industries (mini review). International Food Research Journal 22: 1–8.
- Wu G.P, Wang X.M, Lin L.P, Chen S.H and Wu Q.Q. Isolation and characterization of pepsin-solubilized collagen from the skin of black carp (Mylopharyngdon piceus). Advances in Bioscience and Biotechnology, 2014, 642-650.
CrossRef - Kolapo A.L, Okunade M.B, Adejumobi J.A and Ogundiya M.O. Phytochemical composition and antimicrobial activity of Prosopis Africana against some selected oral pathogens, World Journal of Agricultural Sciences, World Journal of Agricultural Sciences.2009, 5 (1), 90-93.
- Sudharsana A and Sankari R. Antimicrobial Activity of Cassia auriculata Flower Extract on Periodontal Pathogens: An In Vitro Study. J.Pharm. Sci. & Res. Vol.2017, 9(3), 267-268.
- Hosamani P.A, Lakshman H.C, Sandeepkumar K and Hosamani R.C. Antimicrobial activity of leaf extract of Andrographis paniculata Wall. Sci Res Rep. 2011,1, 92‑5.
- Scalbert A. Antimicrobial properties of tannins. Phytochemistry,1991, 30, 3875‑83.
CrossRef - Lilian E.P, Teresita G, Americo OJ and Eduardo G. Acute and chronic anti-inflammatory effects of plant flavonoids, Il Farmaco. 1998, 53, 421-424.
CrossRef - Manuel J, Seixas, Evaa Martins, Rui L, Reis and Tiago Silva H. Extraction and characterization of collagen from elasmobranch byproducts for potential biomaterial use. Mar Drug.2010, 18(12):617.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






