Manuscript accepted on : May 03, 2009
Published online on: 28-06-2009
Sanjay Mohan Shrivastava*, Sanjeev Kumar Shukla and Manu Chaudhary
Venus Medicine Research Center, Hill Top Industrial Estate, Jharmajri EPIP, Phase I (Extn) Bhatoli Kalan, Baddi India.
Corresponding Author E-mail: dgmtechnical@venusremedies.com
ABSTRACT: Ceftazidime belongs to cephalosporin class of antibiotics with broad spectrum activity. Tobramycin is an aminoglycoside antibiotic used to treat various types of bacterial infections, particularly gram negative infections. This study was aimed at evaluating microbial efficacy of Tobracef, a Fixed Dose Combination (FDC) of ceftazidime and tobramycin in comparison with ceftazidime and tobramycin alone. Efficacy was evaluated on the basis of Minimum Inhibitory Concentration (MIC) and time kill curve analysis in Acinetobacter baumanii, Mycobacterium smegmatis, Citrobacter braaki and Pseudomonas aeruginosa. In case of A. baumanii, M. smegmatis, C. braaki and P. aeruginosa, MIC were found to be 0.0625 mg/l, 0.125 mg/l, 0.03125mg/l and 0.25 mg/l in Tobracef respectively. In ceftazidime alone the MIC were found to be 0.25mg/l, 0.25mg/l, 1mg/l and 1mg/l respectively. For tobramycin alone the MIC were found to be 0.125mg/l, 0.25mg/l, 0.0625mg/l and 0.5mg/l respectively. In all organisms under study, time-kill curve analysis demonstrated bacterial maximum killing at 4 hours. In conclusion, Tobracef, a FDC of ceftazidime and tobramycin was found to have more bacterial inhibiting properties than ceftazidime and tobramycin alone.
KEYWORDS: Minimum Inhibitory Concentration (MIC); ceftazidime; tobramycin; tobracef
Download this article as:| Copy the following to cite this article: Shrivastava S. M, Shukla S. K, Chaudhar M. Minimum Inhibitory Concentration and Time Kill Curve Studies of Tobracef, A Fixed Dose Combination of Ceftazidime and Tobramycin. Biosci Biotechnol Res Asia 2009;6(1) |
| Copy the following to cite this URL: Shrivastava S. M, Shukla S. K, Chaudhar M. Minimum Inhibitory Concentration and Time Kill Curve Studies of Tobracef, A Fixed Dose Combination of Ceftazidime and Tobramycin. Biosci Biotechnol Res Asia 2009;6(1). Available from: https://www.biotech-asia.org/?p=8224 |
Introduction
Ceftazidime belongs to cephalosporin class of antibiotics with broad spectrum activity.1, 2 It is stable to both plasmid and chromosomal β – lactamase resistance then other cephalosporins.3, 4 Ceftazidime is a third generation cephalosporin and is resistant to hydrolysis. It is effective against a broad range of gram positive and gram negative bacteria and also against bacteria resistant to cephalosporins.
Tobramycin is an aminoglycoside antibiotic used to treat various types of bacterial infections, particularly gram negative infections. It is often used concomitantly with other antibacterials to extend its spectrum of efficacy or increases its effectiveness. Treatment with a combination of an aminoglycoside with a β – lactam has showed increased efficacy.
Ceftazidime and tobramycin combination therapy is considered by some clinicians to be the clinical standard.5 Antibacterial drugs have been highly successful in controlling the morbidity and mortality that accompany serious bacterial infections. Some of the exiting antibiotics may cause adverse effects in some patients. Some of these side effect may be significant enough to require that therapy should be discontinued.6, 7
Combination therapy of cephalosporins and aminoglycosides is also used to broaden the antimicrobial spectrum in critically ill patients while awaiting a bacteriological diagnosis or proven polymicrobial infection. Synergism appears to be maintained even at very high MIC with drug combinations within achievable therapeutic ranges.8, 9, 10, 11
The fight against bacterial infection represents one of the highest point of the modern medicine. Since the development of antibiotics, this powerful tool has saved millions of lives. However, because of inappropriate and large use of antibiotics, many antibiotic resistant strains are growing in number. The resistant bacteria pose a significant threat to human health and a challenge to researches.12, 13 Keeping this in the view, the present study was planned to evaluate efficacy of Tobracef, FDC of ceftazidime and tobramycin against some clinically significant microorganisms.
Materials and Methods
Bacterial Strains
Following strains obtained from Microbial Type Collection Center of Institute of Microbial Technology, Chandigarh, India were used for the study, Acinetobacter baumanii (MTCC No. – 1425), Mycobacterium smegmatis (MTCC No. – 995), Citrobacter braaki (MTCC No. – 2690) and Pseudomonas aeruginosa (MTCC No. – 1688)
Antibiotic
Tobracef, ceftazidime and tobramycin used in study were provided by manufacturer, Venus Remedies Limited, India.
Medium
Mueller Hinton (MH) media supplemented with Calcium (25 mg/l) and Magnesium (1.25 mg/l) was used for MIC and susceptibility tests experiments. Colony counts were determined with MH agar plates.
Susceptibility Testing
The MIC of ceftazidime and tobramycin alone and in a Tobracef against A. baumanii, M. smegmatis, C. braaki and P. aeruginosa were determined by broth micro dilution method as per the standard National Committee for Clinical Laboratory Standards.14 Overnight MH broth cultures were used to prepare inocula of 105 CFU/ml. The MIC was defined as the lowest concentration of antimicrobial agent that prevented turbidity after 24 hours of incubation at 37 0C.
Results
MIC studies
In case of A. baumanii, M. smegmatis, C. braaki and P. aeruginosa MIC were found to be 0.25 mg/l, 0.25 mg/l, 1 mg/l and 1 mg/l for ceftazidime respectively and in tobramycin alone the MIC were found to be 0.125 mg/l, 0.25 mg/l, 0.0625 mg/l and 0.5 mg/l respectively. In a tobracef MIC were found to be 0.0625 mg/l, 0.125 mg/l, 0.03125mg/l and 0.25 mg/l respectively.
The MIC of all microbial strains under study resulted in significant reduction in ceftazidime, tobramycin alone and tobracef. (Table -1)
Table 1: Minimum Inhibitory Concentrations determination of ceftazidime, tobramycin and Tobracef with A. baumanii, M. smegmatis, C. braaki and P. aeruginosa.
| S. No. | Microorganism | MICs(mg/L) | ||
| Ceftazidime | Tobramycin | Tobracef | ||
| 1 | A. baumanii | 0.25 | 0.125 | 0.0625 |
| 2 | M. smegmatis | 0.25 | 0.25 | 0.125 |
| 3 | C. braaki | 1 | 0.0625 | 0.03125 |
| 4 | P. aeruginosa | 1 | 0.5 | 0.25 |
Time kill curve analysis
Bactericidal effect, with 2X the MIC of tobracef, ceftazidime and tobramycin achieved the earliest killing at 4 hours. Bacterial killing rate in toracef were distinctly higher than ceftazidime and tobramycin alone in all the strains under study. (Fig – 1, Fig – 2, Fig – 3 and Fig – 4).
In A. baumanii time kill curve analysis demonstrated bacterial killing in 0 hrs, 2 hrs, 4 hrs, 6 hrs, 8 hrs, 10 hrs and 12 hrs for ceftazidime alone 6.31 log10CFU/ml, 5.94 log10CFU/ml, 4.95 log10CFU/ml, 5.40 log10CFU/ml, 5.62 log10CFU/ml, 6.27 log10CFU/ml and 6.46 log10CFU/ml, for a tobramycin alone 6.15 log10CFU/ml, 5.81 log10CFU/ml, 4.70 log10CFU/ml, 5.32 log10CFU/ml, 5.76 log10CFU/ml, 6.26 log10CFU/ml and 6.40 log10CFU/ml, and tobracef were 6.09 log10CFU/ml, 5.76 log10CFU/ml, 4.30 log10CFU/ml, 5.08 log10CFU/ml, 5.59 log10CFU/ml, 6.09 log10CFU/ml and 6.33 log10CFU/ml. (Fig – 1)
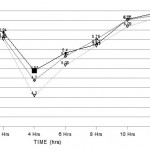 |
Figure 1
|
In M. smegmatis time kill curve analysis demonstrated bacterial killing in 0 hrs, 2 hrs, 4 hrs, 6 hrs, 8 hrs, 10 hrs and 12 hrs for tobracef were 5.99 log10CFU/ml, 5.43 log10CFU/ml, 4.30 log10CFU/ml, 5.08 log10CFU/ml, 5.48 log10CFU/ml, 5.92 log10CFU/ml and 6.10 log10CFU/ml respectively, for ceftazidime alone 6.25 log10CFU/ml, 5.49 log10CFU/ml, 4.48 log10CFU/ml, 5.18 log10CFU/ml, 5.48 log10CFU/ml, 5.96 log10CFU/ml and 6.16 log10CFU/ml receptively and tobramycin alone 6.01 log10CFU/ml, 5.49 log10CFU/ml, 4.48 log10CFU/ml, 5.18 log10CFU/ml, 5.48 log10CFU/ml, 5.96 log10CFU/ml and 6.10 log10CFU/ml. (Fig – 2)
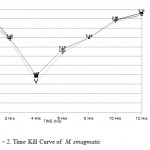 |
Figure 2: Time Kill Curve of M. smagmatis.
|
In C. braaki time kill curve analysis demonstrated bacterial killing in 0 hrs, 2 hrs, 4 hrs, 6 hrs, 8 hrs, 10 hrs and 12 hrs for ceftazidime alone 6.33 log10CFU/ml, 5.99 log10CFU/ml, 4.90 log10CFU/ml, 5.46 log10CFU/ml, 5.83 log10CFU/ml, 6.04 log10CFU/ml and 6.29 log10CFU/ml, for a tobramycin alone 6.22 log10CFU/ml, 6.00 log10CFU/ml, 4.85 log10CFU/ml, 5.54 log10CFU/ml, 5.77 log10CFU/ml, 6.07 log10CFU/ml and 6.32 log10CFU/ml, and tobracef were 6.11 log10CFU/ml, 5.98 log10CFU/ml, 4.70 log10CFU/ml, 5.45 log10CFU/ml, 5.65 log10CFU/ml, 6.02 log10CFU/ml and 6.27 log10CFU/ml. (Fig – 3)
 |
Figure 3: Time Kill Curve of C. braakii.
|
In P. aeruginosa time kill curve analysis demonstrated bacterial killing in 0 hrs, 2 hrs, 4 hrs, 6 hrs, 8 hrs, 10 hrs and 12 hrs for tobracef were 6.46 log10CFU/ml, 6.26 log10CFU/ml, 4.78 log10CFU/ml, 5.96 log10CFU/ml, 6.26 log10CFU/ml, 6.46 log10CFU/ml and 6.55 log10CFU/ml respectively, for ceftazidime alone 6.47 log10CFU/ml, 6.28 log10CFU/ml, 5.00 log10CFU/ml, 5.80 log10CFU/ml, 6.12 log10CFU/ml, 6.48 log10CFU/ml and 6.60 log10CFU/ml receptively and tobramycin alone 6.45 log10CFU/ml, 6.27 log10CFU/ml, 4.95 log10CFU/ml, 6.02 log10CFU/ml, 6.28 log10CFU/ml, 6.47 log10CFU/ml and 6.55 log10CFU/ml. (Fig – 4).
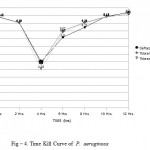 |
Figure 4: Time Kill Curve of P. aeruginosa.
|
Discussion
A further indication for antibiotic combinations is to prevent emergence of resistance.15 A synergistic interaction between the two antibiotics is one reason for using this combination.16 Antibiotic combinations have long been used to provide antibacterial activity against multiple potential pathogen for initial empirical treatment of critically ill patients. Several studies of antibiotic combination therapy for gram negative infection conducted from the 1970s to the 1990s. The consensus is that combination therapy is probably more effective than mono therapy only for infections. Gram negative bacterial species typically have a higher degree of antibiotic resistance than gram positive bacteria. This is largely in part due to the presence of a selectively permeable outer membrane which restricts the entrance of small hydrophobic molecules, including many available antibiotics.17 Aminoglycoside class antibiotic exert a killing effect by binding to bacterial ribosomes and inhibiting bacterial protein synthesis.
Ceftazidime is a third generation cephalosporins, with good antibacterial activity.2, 18 Clinically administration of two or more antibiotics in the treatment of infections is usually rationalized with the knowledge that multiple antibiotics often exert additive or synergistic effects, increasing the likelihood of pathogen eradication. In comparison with older cephalosporins, it crosses the bacterial outer cell membrane faster and has advantages of rapid penetration in periplasmic space as well as of extended spectrum of the activity that include gram positive and gram negative organisms.
Useful antibiotic classes based on a β – lactam structure include broad penicillins, cephalosporins, carbapenems, all of which inhibit bacterial cell wall synthesis. β – lactamase enzymes that rapidly degrade the cephalosporins β – lactam ring have a primary bacterial resistance mechanism against this class of drug since the commencement of clinical cephalosporins use in the 1940s. Because of this, most cephalosporins derived antibiotics still clinically used are formulated to include a β – lactamase inhibitor in order to increase the drug’s effectiveness. Cephalosporins also contain a β – lactam ring, but are structurally more resistance to β – lactamase degradation.
Tobracef has lower MIC than ceftazidime and tobramycin alone against A. baumanii, M. smegmatis, C. braaki and P. aeruginosa.
This investigation indicated that Tobracef has better efficacy as compared to ceftazidime and tobramycin alone in organisms under study.
Acknowledgments
Authors are greatful to acknowledge the CGM, Domestic operations of Venus Remedies Limited for providing the samples of ceftazidime, tobramycin and Tobraceffor the study and financial support for this investigation.
References
- Seibert G. Limbert M. Winkler I. and Dick T., The Antibacterial activity in – vitro and β – lactamase stability of the new cephalosporin HR 810 in comparison with five other cephalosporins and two aminoglycosides, Infection., 11 [5], 275 – 279 (1983).
- Jone R. N. Pfaller M. A. Allen S. D. Gerlach E. H. Fuchs P. C. and Aldridge K. E., Antimicrobial activity of Ceftazidime : an update compared to five third – generation cephalosporins against nearly 6000 recent clinical isolates from five medical centers, Diagn. Microbiol. Infect. Dis., 14, 361 – 364 (1991).
- Jacoby G. A. and Carreras I., Activities of β – lactam antibiotics against Escherichia coli strains producing extended spectrum β – lactamases, Antimicrob. Agents Chemother., 34 [5], 858 – 862 (1990).
- Stobberingh E. E. and Houbesn A. W., Including capacity and selection of resistance varients of Ceftazidime [HR 810] in comparison with other β – lactam compounds, Chemother., 34, 490 – 496 (1988).
- Master V. Roberts G. W. Coulthard K. P. Baghurst P. A. Martin A. Roberts M. E. Onishko C. R. Martin A. J. Linke R. J. Holmes M. Jarvinen A. Kennedy D. Colebatch K. A. Hansman D. and Parsons D. W., Efficacy of once – daily tobramycin monotherapy for acute pulmonary exacerbations of cystic fibrosis : a preliminary study, Pediatr. Pulmonol., 31 (5), 367 – 376 (2001).
- Dancer S. J., How antibiotics can make us sick : the less obvious adverse effects of antimicrobial chemotherapy, Lancet. Infect. Dis., 4, (10), 611 – 9 (2004).
- Gleckman R. A. and Czachor J. S., Antibiotic side effects, Semin. Respir. Crit. Care. Med., 21, 53 – 60 (2000).
- Baltch A. L. Bassey C. Hammer M. C. Smith R. P. Conroy J. V. and Michelsen P. B., Synergy with cefsulodin or piperacillin and three aminoglycosides or aztreonam against aminoglycoside resistant strains of Pseudomonas aeruginosa, J. of Antimicrob. Chemother., 27, 801 – 808 (1991).
- Cappelletty D. M. and Rybak M. J., Comparison of methodologies for synergism testing of drug combinations against resistant strains of Pseudomonas aeruginosa, Antimicrob. Agents Chemother., 40, 677 – 683 (1996).
- Chin N. X. and Neu H. C., Synergy of new C-3 substituted cephalosporinsand Tobramycin against Pseudomonas aeruginosa and Pseudomonas cepacia, Diagn. Microbiol. Infect. Dis.,12, 343 – 349 (1989).
- Den Hollander J. G. Horrevorts A. M. Van Goor M. L. Verbrugh H. A. and Mouton J. W., Synergism between Tobramycin and Ceftazidime against a resistant Pseudomonas aeruginosa strain, tested in an in – vitro pharmacokinetic model, Antimicrob. Agents Chemother., 41, 95 – 100 (1997).
- French G. L., Clinical impect and relevance of antibiotic resistance, Adv. Drug. Deliv. Rev., 57 , 1514 – 27 (2005).
- Harbarth S. Nobre V. and Pittet D., Dose antibiotic selection impact patient outcome ?, Clin. Infect. Dis., 44, 87 – 93 (2007).
- National Committee for Clinical Laboratory Standards, Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7 – A4 (1997) National Committee for Clinical Laboratory Standards., (1997).
- Moellering R. C. Allsop A. Bush K. Quinn J. Baquero F. and Philipps I., Antibiotic resistance: lessons for the future, Clin. Infectious. Dis., 27, 135 – 40 (1998).
- Farrell W. Wilks M. Drasar F. A., Synergy between aminoglycosides and semi synthetic penicillins against gentamicin resistant gram negative rods, J. of Antimicro. Chemother., 5, 23 – 9 (1979).
- Zhanel G. G. Hoban D. J. Schurek K. and Karlowsky J. A., Role of efflux mechanisms on fluoroquinolone resistance in Streptococcus pneumoniae and Pseudomonas aeruginosa, Inter. J. of Antimicro. Agent., 24 [6], 259 – 535 (2004).
- Banerjee D. and Stableforth D., The Treatment of Respiratory Pseudomonas Infection in Cystic Fibrosis : What Drug and & Which Way ?, Drugs., 60 [5], 1053 – 1064 (2000).

This work is licensed under a Creative Commons Attribution 4.0 International License.





