How to Cite | Publication History | PlumX Article Matrix
Follow-Up Alterations of Catecholamine Hormones after an Intensive Physical Activity
Ali Reza Shahsavar and Mohammad Javad Pourvaghar
Department of Physical Education, West Tehran Branch, Islamic Azad University, Tehran Iran.
Corresponding Auhtor E-mail: alirezashahsavar@yahoo.com
ABSTRACT: Catecholamines play an important role in a number of bodily functions such as metabolism of carbohydrates and lipids. Any change in the quantity of the hormones has negative effect on the performance of athletes. Fourteen young athletes participated in this study. The participants took Bruce Test and ran on the treadmill with an average of 16.05 minutes and were totally exhausted. Four blood samples were taken. The first sample served as a pretest and the second sample was taken after Bruce test. The third and fourth samples were taken one day and two days after the Bruce test, respectively. The results of the sample tests were analyzed by using paired sample t-test and repeated measures. The results showed that extreme exhaustive aerobic exercises significantly increase Epinephrine (E) (P = 0.0001) and Norepinephrine (NE) (P = 0.01). The results also showed that the increase for norepinephrine continued two days after physical exercise. It seems that Catecholamines react differently to physical exercises and might be influenced by the psychological state of the athlete before the exercise and the duration and intensity of the exercise. The amount of increase in the epinephrine and norepinephrine seem to contribute to the better athletic performance because these two hormones perhaps have a positive effect on cardiovascular system and metabolism.
KEYWORDS: Catecholamines; Physical exercise; Hormone
Download this article as:| Copy the following to cite this article: Shahsavar A. R, Pourvaghar M. J. Follow-Up Alterations of Catecholamine Hormones after an Intensive Physical Activity. Biosci Biotech Res Asia 2011;8(2) |
| Copy the following to cite this URL: Shahsavar A. R, Pourvaghar M. J. Follow-Up Alterations of Catecholamine Hormones after an Intensive Physical Activity. Biosci Biotech Res Asia 2011;8(2). Available from: https://www.biotech-asia.org/?p=9444/ |
Introduction
The term ‘catecholamines’ is composed of several components that are all derived from an amino acid, e.g. tyrosine. The principal components are adrenalin (epinephrine) and noradrenalin (nore-pinephrine) (1, 2). Catecholamines play a fundamental role in the physiology of exercise in all animal species, since they regulate many biochemical processes involved in energy metabolism as well as the adaptation of organism homeostasis to physical activity (3, 4). The action of these molecules is reflected in many physiological functions, such as increased heart rate, blood pressure, sweating, mobilization of energetic substrates and respiratory rate (5, 6, 7, 8, 9).
Catecholamines are both neurotransmitters and hormones, and play a dominant role in helping the individual respond to the stress of exercise (4). Among these responses are the capacity to control cardiac function and metabolism, to control blood flow in the working muscles, and substrate mobilization and utilization (6, 7, 8, 4).
Catecholamines have been shown to stimulate respiratory, cardiac, metabolic and thermoregulatory functions (2). Catecholamines act well simultaneously at several levels to permit the realization and/or the prolongation of physical exercise. For example, during prolonged exercise, catecholamines play a major role in oxygen and energetic substrates transportation to active muscles (1).
The changes in plasma catecholamines following various forms of exercise training have been characterized. The magnitude of the plasma catecholamine response to an acute bout of endurance exercise is dependent on the exercise duration and the relative stress of physical activity, i.e. the exercise intensity at a certain percentage of maximal oxygen consumption (10, 11).
In adult men, it has been shown that plasma adrenalin and noradrenalin concentrations increase markedly in response to intense exercise (12, 13). At rest, several studies did not observe any differences between pre- and post-training results (1). Even later when comparing endurance-trained and untrained subjects, researchers failed to demonstrate a significant training effect on adrenalin and noradrenalin resting values (1).
Plasma catecholamine concentrations are known to increase markedly in men during dynamic exercise in various sporting specialties, such as running, (4) cycling (14, 15, 16) and swimming (17).
In fact, while Kjaer et al. (1986) reported greater adrenalin responses in endurance-trained subjects compared to untrained ones in response to 2 min at 110% of VO 2max, Zouhal et al. (2001) did not find any significant differences between endurance-trained and untrained subjects in response to a Wingate test, but reported significant higher plasma adrenalin concentrations in sprint-trained subjects. Thus, these authors suggested that endurance-trained subjects tested in the study by Kjaer et al. (1986) may have been more intensively trained (18, 19). The main purpose of present study was to find out the changes in hormones secretions of adrenaline gland in order to know whether strenuous aerobic exercises can change catecholamines hormones or not.
Material and method
Fourteen male young athletes with no impairment medical history participated in the study. The research design used was Quasi-experimental. The participants’ height, weight, and blood pressure were measured (Table 1). Bruce test was used and the formula below was used to measure the maximum volume of oxygen consumption (VO 2max) of the participants. The results are reported in table 1.
Men: VO 2max (ml/kg/min) = (2.94) T+7.65
Table 1. The Characteristics of Subjects in the Day of Experiment
| Variables | Mean | Standard Deviation |
| Age (Y) | 21.87 | 3.65 |
| Height (Cm) | 175.33 | 4.84 |
| Weight (Kg) | 75.43 | 2.65 |
| BMI (Cm2) | 22.21 | 3.03 |
| VO 2max ml. kg-1. min-1 | 58.09 | 2.87 |
| Systolic Blood Pressure(mm Hg) | 118.65 | 3.52 |
| Diastolic Blood Pressure(mm Hg) | 89.23 | 4.23 |
The participants attended the physiology lab before breakfast at 8:00 am. Four blood samples form elbow vein were taken. The first sample was taken as a pre-test in rest situation, that is, before any physical exercise. The participants, then, took Bruce test on Germany made treadmill-hp/cosmos.
The second blood sample was taken after Bruce test. The time average for Bruce test was measured 16.05 minutes. The participants were allowed to rest for two days. The third blood sample was taken the day after and finally, the fourth sample was taken two days later. The athletes were advised not to take any medicine or supplements. The blood samples were later taken to a pathology lab for analysis. Chemiluminescence’s method was used for analysis of the blood samples. In this method serum adrenalin was measured on a scale of (ng/ml) and noradrenalin was measured on a scale of (pg/ml). SPSS 14 was used for comparison of the means and repeated measures.
Results
Table 2 reports the results of serum adrenalin which include the mean, standard deviation, P-value and t-value. The mean of adrenalin of the first sample was 59.2 ng/ml and for the second sample 67.6 ng/ml which shows an increase of 8.4 ng/ml. This increase is statistically significant at (p = 0.001). This means that extreme aerobic exercise can influence the level of adrenalin. This effect diminished 24 hours later and the adrenalin level was back to normal and the difference was not significant (P = 0.733). After 48 hours rest, the mean differences of the first sample and fourth sample were not statistically significant (P = 0.007). Based on these findings we can argue that exhaustive exercise can significantly increase adrenalin of the blood but this increase will go away becomes normal after 24 and 48 hours Table 2.
Comparison of the mean value of noradrenalin serum of rest time and after aerobic exercise shows that intense exhaustive aerobic exercise with 16 minutes mean can significantly increase the level of noradrenalin serum (p = 0.01). The results showed an increase in noradrenalin. It changed from 109.6 pg/ml to 119.5 pg/ml.
As stated earlier, the participants had a 24 rest and during this period the noradrenalin of the third sample compared to the second sample had a decrease of 59.9 pg/ml. It was significant at P-value of 0.003. Compared with the first sample, the difference was significant at P = 0.013 (Table 2). The maximal oxygen consumption of the participants was measured by Bruce test. The value obtained was 54.83 ml/kg per minute.
Table 2. The Alteration of Serum Adrenalin in Rest and After Training.
| Variables(M±SD)
Adrenalin (ng/ml) |
Variables(M±SD)
Adrenalin (ng/ml) |
Paired S. D | T Value | P Value |
| a S 1, 59.2±25.57 | S. 2, 67.6±25.6 | 5.059 | 5.25 | 0.001 |
| S. 1, 59.2±25.57 | S. 3, 64.6±34.89 | 48.6 | 0.35 | 0.733 |
| S. 1, 59.2±25.57 | S. 4, 83.8±21.9 | 37.81 | 2.057 | 0.07 |
| S. 2, 67.6±25.6 | S. 3, 64.6±34.89 | 49.57 | 0.191 | 0.852 |
| S. 2, 67.6±25.6 | S. 4, 83.8±21.9 | 37.32 | 1.372 | 0.203 |
| S. 3, 64.6±34.89 | S. 4, 83.8±21.9 | 20.622 | 2.944 | 0.016 |
a Stage,
Table 3: The Alteration of Serum Noradrenalin in Rest and After Training
| Variables(M±SD) Noradrenalin (pg/ml) | Variables(M±SD) Noradrenalin (pg/ml) | Paired S. D. | T Value | P Value |
| a S1, 109.6±30.73 | S. 2, 119.5±32.35 | 9.58 | 0.001 | 0.01 |
| S. 1, 109.6±30.73 | S. 3, 83.8±22.52 | 26.48 | 0.733 | 0.013 |
| S. 1, 109.6±30.73 | S. 4, 69.9±20.25 | 34.52 | 0.07 | 0.005 |
| S. 2, 119.5±32.35 | S. 3, 83.8±22.52 | 28.44 | 0.852 | 0.003 |
| S. 2, 119.5±32.35 | S. 4, 69.9±20.25 | 39.12 | 0.203 | 0.003 |
| S. 3, 83.8±22.52 | S. 4, 69.9±20.25 | 32.82 | 0.016 | 0.213 |
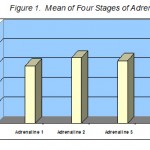 |
Figure 1
|
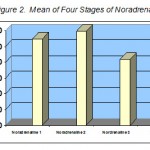 |
Figure 2
|
Discussion
The focus of this study was investigating the immediate and delayed effect of serum adrenalin and noradrenalin after Bruce aerobic exercise. Minor changes in the level of these hormones triggered major changes in processes of energy metabolism (17, 2), increase in heart beat, blood pressure, sweat, and breathing (10, 14, 7). However, some have argued a small change in the level of adrenalin and noradrenalin can negatively influence the athlete’s performance (10, 14, 7).
Four blood samples were taken from the participants. The level of adrenalin and noradrenalin were followed in the samples for comparison. Comparison of the results showed that intense aerobic exercise significantly influences the adrenalin and noradrenalin level (P = 0.001). Adrenalin increased 8.4 ng/ml. This equals 14.9% ng/ml. This increase can in turn influence the metabolism of carbohydrates through Cyclic Adenosine Monophosphate (cAPM). In addition, an increase in epinephrine leads to the release of the free fatty acids form the body fat reserves. This mechanism is achieved through activation of lipase by cyclic adenosine monophosphate (cAPM). In this study, adrenalin was measured 48 after the aerobic activity. Results of the third sample after 48 hours rest the serum adrenalin level returned to normal. This was more evident in the case noradrenalin though these two hormones are complementary (5). The level of noradrenalin in pre-and post test change by 9.03%. It was 109.6 pg/ml in the pre-test and 119.5 pg/ml in the post-test.
The participants had 48 hours rest but the level of noradrenalin did change as noradrenalin significantly decreased after 24 and 48 hours with the P value of 0.003.
All in all, the results are not compatible with Steinberg et al. (2000) and Jacob et al. (2004). In these studies, the level of adrenalin and noradrenalin after cycling exercise had changed. The results are also different form Zouhal (1998). This can be attributed to intensity of exercise. In Zouhal study, Wingate test was used as an anaerobic exercise.
Conclusion
Based on the results, it can be argued the observed increase in the adrenalin and noradrenalin level can improve the athlete’s performance as they both contribute to heart activity and metabolism. Also, the decrease in adrenalin and noradrenalin after the aerobic exercise is due to the decrease in heart beat which results from physical exercise. It can be also argued that intensity of exercise and duration of physical activity can cause different responses in serum adrenalin and noradrenalin hormones. In addition, intensity of exercise can lead to psychological changes which in turn influence these hormones. In sum, further research is necessary to delve into some possible factors that may influence these two hormones.
Acknowledgment
The researchers should sincerely acknowledge the students for giving blood samples. The authors are also grateful to the Vice- Chancellor of research at west Tehran branch, Islamic Azad University, Tehran, Iran.
References
- Zouhal H. Jacob C. Delamarche P. and Gratas- Delamarche, A., Article Sports Med., 38 (5), 401-423 (2008).
- Guyton, Arthur C., Hall, John E. Textbook of Medical Physiology, Elsevier Inc. (2006).
- Pritzlaff CJ. Wideman L. Blumer J. Jensen M. Abbott RD. Glenn AG. Veldhuis JD, Weltman A., J Appl Physiol., 89, 937-46, (2000).
- Steinberg L.L. Lauro F.A.A. Sposito M.M.M. Tufik S. Mello M.T. Naffah-Mazzacoratti M.G. Cavalheiro E.A. and Silva A.C., Brazilian Journal of Medical and Biological Research., 33, 913-918, (2000).
- Baragli P. Pacchini S. Gatta D. Ducci M. and Sighieri C., Ann Ist Super Sanità., Vol. 46, No. 1: 96-100, (2010).
- Botcazou M. Zouhal H. Jacob C. Gratas-Delamarche A. Berthon P. M. Bentue´ -Ferrer D. Delamarche P., Eur J Appl Physiol., 97: 68–75, (2006).
- Hooker SP. Wells CL. Manore MM. Philip SA. & Martin N., Medicine and Science in Sports and Exercise., 22: 779-784, (1990).
- Mazzeo RS., Medicine and Science in Sports and Exercise., 23: 839-845, (1991).
- Savard GK. Richter EA. Strange S. Kiens B. Christensen NJ. & Saltin B., American Journal of Physiology., 257: 812-818, (1989).
- Kjaer M., Eur J Appl Physiol., 77:195–199, (1998).
- Michael Bracken R., Springer-Verlag, August, 343 347, (2009).
- Moussa E. Zouhal H. Gratas-Delamarche A. Bentue-Ferrer D. Delamarche P., J Sports med Phys Fitn., 43:546–553, (2003).
- Zouhal H. Rannou F. Gratas-Delamarche A. Monnier M. Bentue- Ferrer D. Delamarche P., Int J Sports Med., 19:172–176, (1998).
- Jacob C. Zouhal H. Prioux J. et al., Eur J Appl Physiol., 91 (1): 35-40, (20040.
- Vincent S. Gratas-Delamarche A. Berthon PM. et al., Can J Appl Physiol., 28, (2003).
- Zouhal H. Jacob C. Rannou F. et al., J Sports Med Phys Fitness., 41 (3) 330-6, (2001).
- Hickson RC. Hagberg JM. Conlee RK. et al., Eur J Appl Physiol., 41: 211-9, (1979).
- Kjaer M. Farrell PA. Christensen NJ. Galbo H., J Appl Physiol., 61:1693–1700, (1986).
- Zouhal H. Jacob C. Gratas-Delamarche A. Rannou F. Bentue´ – Ferrer D. Delamarche P., J Sports Med Phys Fitness., 41:330–336, (2001).

This work is licensed under a Creative Commons Attribution 4.0 International License.





