Manuscript accepted on :
Published online on: --
Effect of Bryophyllum pinnatum Lam. on N-diethylnitrosamine Induced Hepatic Injury in Rats
Muhammad Afzal1-2, Kamran Ahmad3, Shakir Saleem3, Imran Kazmi1 and Firoz Anwar1
1Pacific University, Udaipur, Rajasthan, India.
2Siddhartha Institute of Pharmacy, Dehra Dun, Uttarakhand, India.
3Department of Pharmacology, Luqman College of Pharmacy, Gulbarga, Karnataka, India.
DOI : http://dx.doi.org/http://dx.doi.org/10.13005/bbra/1060
ABSTRACT:
Present study designed to evaluate the effect of Bryophyllum pinnatum Lam. on n-diethylnitrosamine (DENA) induced hepatic injury in rats. The aerial part of B. pinnatum aqueous and ethanolic extract was prepared in the doses of 250mg/kg and 500mg/kg. Hepatic injury was induced by DENA. Acute toxicity was also carried out. Treatment with different doses of ethanolic extract of B. Pinnatum (250 mg/kg, p.o.) not significantly able to treat the liver injury induced by DENA, but 500 mg/kg dose of ethanolic extract of B. Pinnatum slightly protect the liver. Treatment with different doses of aqueous extract of B. Pinnatum (250 and 500 mg/kg, p.o.) significantly (p*<0.05; p**<0.01 and p***<0.001) treat the liver injury induced by DENA. It may be inferred from the present study that the hepatoprotective activities of the aqueous extract of B. Pinnatum leaves in DENA-induced hepatotoxicity may involve its antioxidant or oxidative free radical scavenging activities by alleviating lipid peroxidation through scavenging of free radicals, or by enhancing the activity of antioxidants.
KEYWORDS: Bryophyllum pinnatum; N-diethylnitrosamine; Histopathology; Rats.
| Copy the following to cite this article: Afzal M, Ahmad K, Saleem S, Kazmi I, Anwar F. Effect of Bryophyllum pinnatum Lam. on N-diethylnitrosamine Induced Hepatic Injury in Rats. Biosci Biotech Res Asia 2012;9(2) |
| Copy the following to cite this URL: Afzal M, Ahmad K, Saleem S, Kazmi I, Anwar F. Effect of Bryophyllum pinnatum Lam. on N-diethylnitrosamine Induced Hepatic Injury in Rats. Biosci Biotech Res Asia 2012;9(2). Available from: https://www.biotech-asia.org/?p=10090 |
Introduction
DENA (N-diethylnitrosamine) a hepatocarcinogen, is known to cause perturbations in the nuclear enzymes involved in DNA repair/replication and is normally used as a carcinogen to induce liver cancer in animal models (Newell et al., 2008). Investigations have provided evidence that N-nitrosamines cause a wide range of tumors in all animal species and these compounds are considered to be effective health hazards to man (Akintonwa, 1985). Nitrosamines are a class of mutagenic, teratogenic and carcinogenic chemicals in the environment as by-products of various manufacturing, agricultural and natural processes (Brunnemann and Hoffmann, 1978). DENA has been found in a variety of products that would result in human exposure, including mainstream and sidestream tobacco smoke, meat and whiskey (Hoffmann et al., 1980). Metabolism of certain therapeutic drugs is also reported to produce DENA. The International Agency for Research on Cancer (IARC) has classified DENA as a probable human carcinogen, despite the lack of epidemiologic data (Hoffmann et al., 1980). Administration of DENA to experimental animals has been shown to cause cancer in liver and at lower incidences, in other organs as well (Bansal et al., 2005). It is also reported to be a hepatotoxic agent causing hepatocellular necrosis in experimental animals (Loeppky, 1994). The formation of reactive oxygen species (ROS) is apparent during the metabolic biotransformation of DENA resulting in oxidative stress. Oxidative stress leads to carcinogenesis by several mechanisms including DNA, lipid and protein damage, change in intracellular signaling pathways and even changes in gene expression. Together, these oxidative modifications promote abnormal cell growth and carcinogenesis. Hence the model of DENA-induced HCC is considered as one of the most accepted and widely used experimental models to study hepatocarcinogenesis (Pitot et al., 1989). Considering the above factors, it is likely that human exposure to DENA is inevitable. Hence, the development of an effective hepatoprotective agent against DENA induced hepatotoxicity has become the need of the day.
The use of natural products with therapeutic properties is as ancient as human civilisation and for a long time, mineral, plant and animal products were the main sources of drugs Furthermore, throughout the development of human culture, the use of natural products has had magical religious significance and different points of view regarding the concepts of health and disease existed within each culture. In recent years, there has been growing interest in alternative therapies and the therapeutic use of natural products, especially those derived from plants (Bose and Gupta, 1999; Goldfrank et al., 1982).
pinnatum commonly known as life plant, air plant, love plant, miracle leaf, Canterbury bells is widely distributed in tropical Africa, America, Hawaii, India, China, Australia and Madagascar, and has been used in folk medicine (Lans, 2006) . In Europe, its use is limited almost exclusively to anthroposophic medicine (Simoes-Wust and Rist, 2007). The leaves are used as astringent, refrigerant, emollient, mucilaginous, haemostatic, vulnerary, depurative, constipating, anodyne, carminative, disinfectant and tonic. It is also useful in vitiated conditions haematemesis, haemorrrhoids, menorrhagia, cuts and wounds, discolorations of the skin, boils, sloughing ulcers, ophthalmia, burns, scalds, corn, diarrhea, dysentery, vomiting (Khare, 2004). A number of active compounds, including alkaloids, triterpenes, lipids (Kirtikar and Basu, 2000), flavonoids (Muzitano et al., 2006), glycosides (Muzitano et al., 2006), bufadienolides (Gaind and Gupta, 1973), phenols (Gaind and Gupta, 1973) and organic acids (Marriage and Wilson, 1971) have been isolated. The leaves of this plant have been reported to possess anti-diabetic (Ojewole, 2005) antihypertensive (Ojewole, 2002), antimicrobial (Akinpelu, 2000) antifungal (Misra and Dixit, 1979), anti-inflammatory and analgesic (Pal and Nag, 1992) and anti-ulcer (Pal and Nag, 1991) activities. The plant is used for a variety of purposes in the Ayurvedic system of medicine. The root extracts are used as a laxative, as a diuretic, for liver troubles, for tuberculosis and for mental disorders. On the basis of it’s traditionally uses to treat the liver disease and as hepatoprotective activity, this study was conducted to explore the effect of aqueous and ethanolic extracts of aerial parts of B. pinnatum on DENA induced hepatic injury in rats.
Materials and Methods
Animals
Wistar albino male rats of 150-200 g were used for the study. The inbred colonies of rats were obtained from Siddhartha institute of Pharmacy, Dehradun (Uttarakhand). They were maintained in the animal house of Siddhartha institute of Pharmacy, Dehradun (Uttarakhand) for experimental purpose. The animals were maintained under controlled conditions of temperature (23 ± 2°C), humidity (50 ± 5%) and 12-h light-dark cycles. The animals were randomized into experimental and control groups and housed individually in sanitized polypropylene cages containing sterile paddy husk as bedding. They had free assessed to standard pellets as basal diet and water ad libitum. All the studies conducted were approved by the Institutional Animal Ethical Committee (IAEC) of Siddhartha institute of Pharmacy, Dehradun (Uttarakhand).
Chemicals
Diethylnitrosamine (DENA) was purchased from Sigma Chemical, USA. All other chemicals used were of analytical grade and were purchased locally.
Collection of plant material
Aerial parts of B. pinnatum were collected in the month of September 2010 from the local region of Pauri, Garhwal (Uttarakhand, India) and identified by Dr. S. B. Singh, Scientist, NISCAIR, New Delhi. A voucher specimen (NISCAIR/RHMD/consult/-04-10-11/1573/236) was deposited in the herbarium of NISCAIR, India.
Preparation of the extract
Aerial parts of B. pinnatum were powdered. 150 g plant was soaked in cold ethanol (99.99 %) for 48 h. The macerate was filtered and ethanol was evaporated in vacuum using a rotary evaporator. A final residue (13.90 g) was obtained after lyophilization. Before each pharmacological test, the lyophilized extract was freshly suspended in 1% Tween 80. For the aqueous extract, 1 L water was added to 150-g plant material, soaked for 48 hours, and percolation was performed until the solvent became colorless. The extract was then concentrated in a vacuum to the desired volume (90% solvent out). It was dried completely and final residue (20.0 g) was obtained.
Acute (Oral) Toxicity Study (Fixed Dose Procedure)
Method
Acute toxicity studies for ethanolic and aqueous extracts of B. pinnatum were conducted as per OECD guidelines 420 using Albino Swiss mice. Each animal was administered ethanolic and aqueous extracts solution of B. pinnatum by oral route. The test procedure minimizes the number of animals required to estimate the oral acute toxicity of a chemical and in addition estimation of LD50, confidence intervals. The test also allows the observation of signs of toxicity and can also be used to identify chemicals that are likely to have low toxicity.
Principle of the Fixed Dose Procedure
The fixed dose procedure is method for assessing acute oral toxicity that involve the identification of a dose level that cause evidence of non-lethal toxicity (termed evident toxicity) rather than a dose level that cause lethality. Evident toxicity is a term describing clear signs of toxicity following administration of test substance, such that an increase to the next highest fixed dose would result in the development of severe toxic signs and probably mortality.
Procedure
As suggested, after acclimatization of animals for 4-5 days, study was carried out as follows:
Healthy, young adult Albino Swiss mice (20-25gms), nulliporous and non pregnant were used for this study. Food, but not water was with held for 3-4 hours and further 1-2 hours post administration of sample under study.
Fixed dose level of 5, 50, 500 mg/kg were initially chosen as dose level that would be expected to allow the identification of dose producing evident toxicity.
During the validation procedure, a fixed dose of 2000 mg/kg was added to provide more information on substance of low acute toxicity.
Dosed one animal at the test dose by oral route.
Since, this first test animal survived, four other animals were dosed (orally) on subsequent days, so that a total of five animals were tested.
Observation
Animals were observed individually at least every 5 minutes once during first 30 minutes after dosing, periodically at 2 hrs during the first 24 hours (with special attention during the first four hours) and daily thereafter, for a total of 14 days.
An attempt was made to identify LD50 of ethanolic and aqueous extracts of B. pinnatum. Since no mortality was observed at 2000 mg/kg, it was thought that 2000 mg/kg was the cut off dose. Therefore 1/8th and 1/4th dose (i.e. 250 mg/kg and 500 mg/kg) were selected for all further in-vivo studies.
Experimental design
Albino wistar rats of either sex weighing between 150-200gms were divided into six groups of six animals each.
Group-I – Negative Control.
Group-II- DENA (200 mg/kg, i.p.)
Group-III– DENA (200 mg/kg, i.p.) + Ethanolic extract of B. Pinnatum (250 mg/kg, p.o.)
Group-IV – DENA (200 mg/kg, i.p.) + Ethanolic extract of B. Pinnatum (500 mg/kg, p.o.)
Group-V – DENA (200 mg/kg, i.p.) + Aqueous extract of B. Pinnatum (250 mg/kg, p.o.)
Group-VI– DENA (200 mg/kg, i.p.) + Aqueous extract of B. Pinnatum (500 mg/kg, p.o.)
At the end of experimental period, animals were subjected to ether anaesthesia, blood was collected from retro orbital plexus and serum was separated by centrifugation. Animals were sacrificed by cervical decapitation and the liver was excised, washed in ice-cold saline and blotted to dryness. A 1% homogenate of the liver tissue sue was prepared in Tris–HCl buffer (0.1 M; pH 7.4), centrifuged and the clear supernatant used for further biochemical assays.
The activities of serum SGPT, SGOT and ALP were determined. Cholesterol, triglyceride and HDL were also determined in serum to assess the acute hepatic injuries by using standard enzyme kits.
For lipid peroxidation (LPO) liver homogenate was prepared in cold 50mM potassium phosphate buffer (pH 7.4), but for Superoxide dismutase (SOD) and Catalase (CAT) 1mM EDTA was added in it, using REMI homogenizer. The unbroken cells and debris were removed by centrifugation at 10,000 rpm for 15 min at 4oc using a REMI cooling centrifuge and the supernatant was used for the estimation of LPO, SOD and CAT by using standard enzyme kit.
Histopathology
The animals used in the curative study were sacrificed and liver tissue was examined grossly. A small portion of liver tissue of each animal was fixed in 10% neutral buffered formalin processed and embedded in paraffin wax to obtain 5–6 µm thick hematoxylin and eosin stained sections (Krajian, 1963).
Statistical analysis
All data were represented as mean ± SD. Significant difference between the mean values were statistically analyzed using one-way analysis of variance (ANOVA) using Prism 5 Graphpad software. The DENA alone treated group and the extract plus DENA treated groups were further analyzed by Tukey’s test. P values less than 0.05 were considered as significant.
Results
Acute toxicity studies for ethanolic and aqueous extracts of B. Pinnatum belonging to the family “Gentianaceae” were conducted as per OECD guidelines 420 using albino Swiss mice. Each animal was administered ethanolic and aqueous extracts by oral route. The animals were observed for any changes continuously for the first 2 hrs and up to 24 hrs for mortality. There were no mortality and noticeable behavioral changes in all the groups tested. The extracts were found to be safe up to 2000 mg/kg body weight.
An attempt was made to identify LD50 of ethanolic and aqueous extracts of aerial parts of B. pinnatum. Since no mortality was observed at 2000 mg/kg. It was thought that 2000 mg/kg was the cut off dose. Therefore, 1/8 and 1/4 dose i.e. 250 mg/kg. and 500 mg/kg. were selected for all further in vivo studies.
Administration of DENA (200 mg/kg, i.p.) to rats caused significant liver damage, as evidenced by the altered serum biochemical parameters. DENA significantly (p*<0.05) increased cholesterol, triglyceride, LOP, SGPT, SGOT and ALP. It also decreased HDL, SOD and CAT significantly (p*<0.05 and p***<0.001). Treatment with different doses of ethanolic extract of B. Pinnatum (250 and 500 mg/kg, p.o.) not significantly reversed these parameters, but 500 mg/kg dose of ethanolic extract significantly (p*<0.05 and p**<0.01) increased SGPT and ALP level, whereas treatment with different doses of aqueous extract of B. Pinnatum (250 and 500 mg/kg, p.o.) significantly (p*<0.05, p**<0.01 and p***<0.001) reversed these parameters dose dependently.(Table 1,2 and 3) (Figure 1,2 and 3).
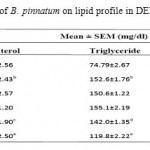 |
Table 1: Effect of aerial part of B. pinnatum on lipid profile in DENA induced hepatotoxicity in rats.
|
Values are mean±SEM expressed as (n=6)
Whereas Group I as Negative control; Group II as DENA (200mg/kg); Group III as DENA (200mg/kg) + Ethanolic extract (250mg/kg); Group IV as DENA (200mg/kg) + Ethanolic extract (500 mg/kg); Group V as DENA (200mg/kg) + Aqueous extract (250 mg/kg); and Group VI as DENA (200mg/kg) + Aqueous extract (500 mg/kg).
a)P*<0.05 as compared with Group I, b) P***<0.001 as compared with Group I, c) P*<0.05 as compared
with Group II, d) P**<0.01 as compared with Group II, e) P***<0.001 as compared with Group II.
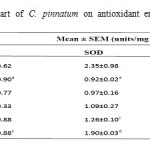 |
Table 2: Effect of aerial part of C. pinnatum on antioxidant enzymes in DENA induced hepatotoxicity in rats.
|
Values are mean±SEM expressed as (n=6)
Whereas Group I as Negative control; Group II as DENA (200mg/kg); Group III as DENA (200mg/kg) + Ethanolic extract (250mg/kg); Group IV as DENA (200mg/kg) + Ethanolic extract (500 mg/kg); Group V as DENA (200mg/kg) + Aqueous extract (250 mg/kg); and Group VI as DENA (200mg/kg) + Aqueous extract (500 mg/kg).
a)P***<0.001 as compared with Group I, b) P*<0.05 as compared with Group II, c) P**<0.01 as compared
with Group II, d) P***<0.001 as compared with Group II.
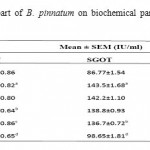 |
Table 3: Effect of aerial part of B. pinnatum on biochemical parameters in DENA induced hepatotoxicity in rats.
|
Values are mean±SEM expressed as (n=6)
Whereas Group I as Negative control; Group II as DENA (200mg/kg); Group III as DENA (200mg/kg) + Ethanolic extract (250mg/kg); Group IV as DENA (200mg/kg) + Ethanolic extract (500 mg/kg); Group V as DENA (200mg/kg) + Aqueous extract (250 mg/kg); and Group VI as DENA (200mg/kg) + Aqueous extract (500 mg/kg).
a)P***<0.001 as compared with Group I, b) P*<0.05 as compared with Group II, c) P**<0.01 as compared
with Group II, d) P***<0.001 as compared with Group I
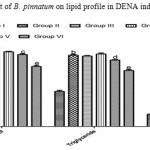 |
Figure 1: Effect of aerial part of B. pinnatum on lipid profile in DENA induced hepatotoxicity in rats.
|
 |
Figure 2: Effect of aerial part of B. pinnatum on antioxidant enzymes in DENA induced hepatotoxicity in rats.
|
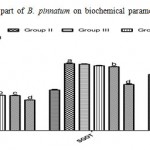 |
Figure 3: Effect of aerial part of B. pinnatum on biochemical parameters in DENA induced hepatotoxicity in rats.
|
The histopathological studies also supported the protective properties of aerial parts of B. pinnatum. The areas of necrosis and ballooning degeneration of hepatocytes were observed in the DENA control group. The group of animals pre-treated with aqueous extract of aerial parts of B. pinnatum showed a dose dependent marked protective effect with decreased necrotic zones and hepatocellular degeneration whereas ethanolic extract at dose of 250 mg/kg not shown any protective effect and ethanolic extract at 500 mg/kg dose shown minor decrease in necrotic zone. The photomicrographs of the liver sections were given in Figure 4.
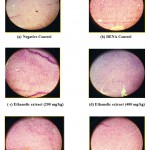 |
Figure 4: Histopathological architecture of the rat liver in DENA induced hepatotoxicity.
|
Discussion
In this present study it was noted that a significant increase in the levels of triglycerides and cholesterol in the group II rats clearly indicate the hyperlipidaemic conditions caused by exposure to DENA. These parameters were brought back to the normal levels in the group V and group VI rats indicate the beneficial effects of administration of aqueous extract of B. Pinnatum
during DENA –induced hepatocarcinogenesis in rats. It is believed that it may be due to the antioxidant and antiperoxidative effects coupled with an ability to correct the abnormalities in lipid and lipoprotein metabolism through an increase in the activities of few lipid metabolizing enzymes Viz lecithin cholesterol acyl transferase, lipoprotein lipase and hepatic triglyceride lipase, but the clear mechanism of action for the observed hypolipidaemic effects is not well understood at this stage of study. DENA is a major environmental hepatocarcinogen. Since liver is the main site of DENA metabolism, the production of ROS in the liver may be responsible for its carcinogenic effects (Klaunig and Kamendulis, 2004). DENA is well known to generate free radicals, disturbing the antioxidant status and ultimately leading to oxidative stress and carcinogenesis (Landsman et al., 2007). In group II, DENA (200 mg/kg) treated rats were shown an decrease in SOD along with catalase. The group II rats also showed increase in liver lipid peroxidation, which indicate a cellular damage caused by free radicals. In group V and group VI, aqueous extract of B. Pinnatum treated rats in the present study showed an extremely significant rise in SOD along with catalase. The extract also showed extreme significant decrease in liver lipid peroxidation, which signifies the antioxidant activity on liver of the treated animals. ALT is more selectively a liver paranchymal enzyme than AST. ALT is a sensitive indicator of acute liver damage and elevation of this enzyme in non hepatic diseases is unusual. Normally, AST and ALP are present in high concentration in liver. Due to hepatocyte necrosis or abnormal membrane permeability, these enzymes are released from the cells and their levels in the blood increases. In the present study, the activities of these enzymes were found to increase in the group II, DENA treated rats, and were significantly reduced in groups of aqueous extract of B. Pinnatum administered rats as compared to that of hepatotoxic rats. This confirms the protective effect of aqueous extract of B. Pinnatum against DENA induced hepatic damage. The effect was more pronounced with 400 mg/kg extract. A possible mechanism of the B. Pinnatum extract as hepatoprotective may be due to its anti-oxidant effect. This might be due to the higher contents of xanthones present in the extract which could have reduced the accumulation of toxic DENA derived metabolites. Histopathological examination of the liver section of the group II, DENA treated rats showed intense centrilobular necrosis and vacuolization. The rats treated with aqueous extract of B. Pinnatum extracts along with DENA showed sign of protection against these toxicants to considerable extent as evident from formation of normal hepatic cards and absence of necrosis and vacuoles, dose dependently respectively.
Conclusion
It may be inferred from the present study that the hepatoprotective activities of the aqueous extract of B. Pinnatum leaves in DENA-induced hepatotoxicity may involve its antioxidant or oxidative free radical scavenging activities by alleviating lipid peroxidation through scavenging of free radicals, or by enhancing the activity of antioxidants. The mechanism of action is yet to be investigated but may be due to the antioxidant effects of xanthones and free radical scavenging properties found to be present in the Aerial parts of B. pinnatum.
References
- Bansal, A.K., Bansal, M., Soni, G., Bhatnagar, D., 2005. Protective role of Vitamin E pre- treatment on Nnitrosodiethylamine induced oxidative stress in rat liver. Chemico- Biological Interactions, 156(2-3): 101- 11.
- Verna, L., Whysner, J., Williams, G.M. 1996. N-Nitrosodiethylamine mechanistic data and risk assessment: bioactivation,DNA-adduct formation, mutagenicity and tumor initiation, Pharmacol. Ther. 71, 57–81.
- Hoffmann, D., Adams, J. D., Piade, J. J. and Hecht, S. S. (1980) Chemical studies on tobacco smoke LXVIII. Analysis of volatile and tocacco-specific nitrosamines in tobacco products. IARC Sci. Publ. 31: 507-5 14.
- Brunnemann, K. D., Hoffmann, D. 1978. Chemical studies on tobacco smoke LIX. Analysis of volatile nitrosamines in tobacco smoke and polluted indoor environments. In: Environmental Aspects of N-Nitroso Compounds Scientific Publication No. 19, pp. 343- 356, Walker, E. A., Griciute, L., Castegnaro, M., Lyle, R. E. and Davis, W. (eds.) IARC, Lyon.
- Akintonwa, D.A., 1985. The derivation of nitrosamines from some therapeutic amines in the human environment, Ecotoxicol. Environ. Safe. 9, 64–70.
- Lans, C.A., 2006. Ethnomedicines used in Trinidad and Tobago for urinary problems and diabetes mellitus. J Ethnobiol Ethnomed 2, 45-55
- Simoes-Wust, A.P., Rist L., 2007. Bryophyllum in der präklinischen und klinischen Forschung. Der Merkurstab 5, 415-420.
- Khare, C.P., 2004. Encyclopedia of Indian Medicinal Plants. New York: Springer 4, 276.
- Kirtikar, K.R., Basu, B.D., 2000. Indian Medicinal Plants. 2nd edn. vol. 2. Allahabad :Oriental Enterprises 2, 1511.
- Muzitano, M.F., Cruz, E.A., Almeida, A.P., Da Silva, S.A., Kaiser, C.R., Guette, C., Rossi-Bergmann, B., Costa, S.S., 2006. Quercitrin: an antileishmanial flavonoid glycoside from Kalanchoe pinnata. Planta Med 72, 81-83.
- Muzitano, M.F., Tinoco, L.W., Guette, C., Kaiser, C.R., Rossi-Bergmann, B., Costa, S.S.,2006. The antileishmanial activity assessment of unusual flavonoids from Kalanchoe pinnata. Phytochemistry 67, 2071–2077.
- Supratman, U., Fujita, T., Akiyama, K., Hayashi, H., Murakami, A., Sakai, H., Koshimizu, K., Ohigashi, H., 2001. Anti-tumor promoting activity of bufadienolides from Kalanchoe pinnata and K. daigremontiana×tubiflora. Biosci Biotechnol Biochem 65, 947-949.
- Gaind, K.N., Gupta, R.L., 1973. Phenolic components from the leaves of Kalanchoe pinnata. Planta Med 23, 149-153.
- Marriage, P.B., Wilson, D.G.,1971. Analysis of the organic acids of Bryophyllum calycinum. Can J Biochem 49, 282-296.
- Ojewole, J.A.O., 2005. Antinociceptive, anti-inflammatory and antidiabetic effects of Bryophyllum pinnatum (Crassulaceae) leaf aqueous extract. Ethnopharmacol 99, 13-19.
- Ojewole, J.A.O., 2002. Antihypertensive properties of Bryophyllum pinnatum (Clam ) Oken) leaf extracts. Am J Hypert 15, 34-39.
- Akinpelu, D.A., 2000. Anti-microbial activity of Bryophyllum pinnatum leaves. Fitoterapia 71, 193-194.
- Misra, S., Dixit, S.N., 1979. Anitfungal activity of leaf extract of some higher plants. Acta Botanica Indica 7, 147-150.
- Pal, S., Nag, C.A.K., 1992. Further studies on the anti-inflammatory profile of the methanolic fraction of the fresh leaf extract of Bryophyllum pinnatum. Fitoterapia 63, 451-459.
- Pal, S., Nag, C.A.K., 1991. Studies on the antitulcer activity of a Bryphyllum pinnatum leaf extract in experimental animals. J Elhnopharmacol 33, 97-102.
- Newell P, Villanueva A, Friedman SL, Koike K, Llovet JM. Experimental models of hepatocellular carcinoma. J Hepatol. 2008; 48(5): 858–879.
- Loeppky, R.N., 1994. Nitrosamine and nitroso compound chemistry and biochemistry, in: ACS Symposium Series, American Chemical Society, Washington, DC, vol. 553, pp. 1–12.
- Pitot, H., Campbell, H., Maronpot, R., Bawa, N., Rizvi, T., Xu, Y.H., Sargent, L., Dragan, Y., Pyron, M., 1989. Critical parameters in the quantitation of the stages of initiation promotion and progression in one model of hepatocarcinogenesis in rats, Toxicol. Pathol. 17, 594–612.
- Bose, S., Gupta, Y.K., 1999. Effect of CNS active herbal drugs on swim test in mice. Ind J Pharm 31, 75.
- Goldfrank, L., et al., 1982. The Pernicious Panacea: Herbal Medicine. Hospital Physician 10, 64–86.
- Krajian AA (1963). Tissue cutting and staining. In: Frankel, S., Reitman, S.( Eds.), Gradwohl’s Clinical Laboratory Method and Diagnosis. The CV. Mosby Co., Saint Louis, USA, p. 1639.
- Klaunig, J.E., Kamendulis, L.M., 2004. The role of oxidative stress in carcinogenesis, Annu. Rev. Pharmacol. Toxicol. 44, 239–267.
- Landsman, N.A., Swancutt, K.L., Branford, C.N., Cox, C.R., Kidle, J.J., Mezyk, S.P. 2007. Free radical chemistry of advanced oxidation process removal of nitrosamines in water, Environ. Sci. Technol. 41, 5818–5823.

This work is licensed under a Creative Commons Attribution 4.0 International License.





