How to Cite | Publication History | PlumX Article Matrix
Lamya Ahmed Al-Keridis
Department of Biology, Faculty of Science, Princess Nourah Bint Abdulrahman University, 11474 Riyadh, Saudi Arabia.
DOI : http://dx.doi.org/10.13005/bbra/2165
ABSTRACT: Environmental concern issues controlled the chemical pesticides usage since residue might cause a health problem. An alternative safe way for pest control is the main objective of this investigation. The recent study was conducted to evaluate the effect of some Entomopathogenic Fungi (Penicillium sp) to control the rust red beetle (Tribolium castaneum). different concentrationsof fungal extract (10, 30, 50%) were appliedbeside,40 % application of the whole fungal organ against insects.Results from this investigation showed that, the treatment withEntomopathogenic fungus Penicilliumsp. affected significantly (P <0.05) the numberof living insects. Higher effect on the number of the living treated insects was observed by the third concentrations (50%)compared with other concentration (10% and 30%) in the first treatment.The treatment with the whole fungus organs showed high effect on the number of the live insects where after 7 days all the insects were dead. Generally the concentration of the extract, the days of exposture and the interaction between days and concentration had significant effect on the live number of insects.Furthermore, metabolomics study of extracts by the means of GC-MS must be studied for determination of the active compounds by means of chromatographic techniques.
KEYWORDS: Entomopathogenic fungi; Penicillium sp; Red Bettel Rust
Download this article as:| Copy the following to cite this article: Al-Keridis L. A. Application of Penicillium sp as Entomopathogenic Fungi to Control the Red Rust Beetle Tribolium castaneum (Hbst.) (Coleoptera:Tenebrionidae). Biosci Biotech Res Asia 2015;12(spl.edn.2) |
| Copy the following to cite this URL: Al-Keridis L. A. Application of Penicillium sp as Entomopathogenic Fungi to Control the Red Rust Beetle Tribolium castaneum (Hbst.) (Coleoptera:Tenebrionidae). Biosci Biotech Res Asia 2015;12(spl.edn.2). Available from:https://www.biotech-asia.org/?p=12751 |
Introduction
Radical decrease in Agricultural production resulting from numerous pests like fungiand insects is considered as global problem that sustain economic and environmental system. Chemical pesticides use for plant protection strategy is a well-known method applied in integrated pest management system of plants. Although, chemical application may lead to effective result in pest control but, different environmental hazards and mankind toxicity beside development of pesticide resistance species had led to look for an eco-friendly alternative strategy in best management. This leads to increased development of compounds based on the models of naturally occurring toxins of biological origin, having various biological activities1. biochemical pesticides might be a good alternative for best management without any harmful effect on the environment.2characterized the bio-pesticides as 1- Microbial pesticidesthat contain a microorganism (bacterium, fungus, virus, protozoan or alga) as the active ingredients, 2- plant- Incorporated-Protectants(PIPs)which are pesticidal substances that plants produce from genetic material that has been added to the plantand 3-Biochemical pesticideswhich are naturally occurring substances that control pests by non-toxic mechanisms. In the present investigation microbial pesticides was applied in a form of Entomopathogenic fungi. 3reported that fungal disease in insect are common and widespread, fungi infect the host by breaking the host cuticle, avoid insect immune system response by utilizing nutrient present in the haemocoel and producing toxin.The efficacy of seven strains of entomopathogenic fungi against Ceratitis capitata adults was assessed in vitro 4. A recent study5demonstrated the ability of entomopathogenic fungiMetarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri (Hemiptera: Psyllidae)in Persian lime under field conditions. Entomopathogen fungus Metarhizium anisopliae against adult Aedes aegypti and Aedes albopictus mosquitoes also has been stated by6 who indicated a positive effect and higly suscepatbility to infection with this pathogenic fungus. 7documented a high pathogenicity level isolated entomopathogenic fungion Heliothus armigera and cotton aphids in virto.Successful infection of entomopathogenic fungidependsprimarily on the adherence and penetration ability of a fungus to the insect integuments by a variety of degradable extracellular enzymes that hydrolyze the epidermis of the insect such as lipases, proteases and chitinases 8-9. Furthermore, entomopathogenic fungi can infect non-feeding stages such as eggs and pupae since fungi do not have to be ingested and can invade their hosts directly through the exoskeleton or cuticle10. The current investigation focused on Red flour beetle, T. castaneum(Col.; Tenebrionidae) which is diverse pest in flour mills and wherever dried foods processed or stored, 11found different virulence ability among Nine isolates of entomopathogenic fungi B. bassianaagainest adults of Tribolium castaneum (Herbst). Lecanicillium muscarium¡Beauveria bassianaandCalvatia carniiformis were studied against rust red beetle (Tribolium castaneum) with different fungal concentration and showed different response in the ability to reduce the growth of insect12.
Despite the information that Penicillium sp. is one of the entomopathogenic fungi that have the potential to be developed as biological control agent of pests there was few report about Penicillium sp as Entomopathogenic fungi. Therefore, the aim of current investigation focused onPenicillium sp and considered as Entomopathogenic fungi model against Red flour beetle, T. castaneum. Fungal enzymes, toxins, spores and hyphae were applied and the live insectsnumber were calculated after the treatment.
Materials and methods
The evaluation was conducted in the Biology section laboratory, Faculty of Science, Princess Nourah Bent Abdul-Rahman university, Riyadh, Saudi Arabia.
Entomopathogenic Fungi
Penicillium sp was as entomopathogenic fungi and deposited in the entomopathogenic fungi collection of PNU. The strain was successively sub-cultured on Potato Dextrose Agar (PDA) at 25ºC, in complete darkness. Fungal strains maintenance was Identified. In general, 14-day-old spores of studied strainwere cultivated on PDA and used as inoculum in the growth media.
The Bioassay
The experiment was performed on white flour where the biological control was tested using the fungus Penicillium sp. The treatments were as follows: control anddifferent fungus concentrations (10%, 30% and 50%). For each concentration was represented in six replicates where the control was represented 2 times. The flour was distributed evenly in all cans.
Methodology
Fungi were grown on liquied Sabouraud dextrose, enriched with yeast extract and incubated for a period of 14 days at 25ºC. After that the medium was filtrated using filter paper in sterilized volumetric flask (bio-control using fungal enzymes and toxins). Furthermore, the different concentrations (10, 30, 50%) were prepared from the original extract using distilled water. For each concentration treatment (10, 30 and 50%) 120 red rust beetle distributed in six cans and treated. Each can was tightly closed using sterilized tissue paper and incubated in electric incubator in 25- 27ºC. data and observation was taken every 5 days for two weeks and the number of live beetle was recorded.
The Second Treatment
In a second experiment Penicillium sp. was cultivated in solid Sabouraud dextrose agar for 14 daysat 25ºC (bio-control using fungus enzymes, toxins, spores and hyphae). A sample from the fungus was taken using sterile needle and placing in sterile water in special water spryer system and well shaken to insure homogeneity. Thereafter the solution was used to spray on the red rust beetle in 2 flour cans each contain 20 beetles. Then all cans were tightly closed using sterilized tissue paper and incubated in electric incubator at25- 27ºC for one week. data and observation was taken on daily bases and the number of live beetle was recorded.
Statistical Analysis
All results were computed and expressed as mean ± standard deviation (SD) for six replicates. Statistical analysis was performed using JMP software (version18.0) with analysis of variance (One-Way ANOVA).
Results
Penicillium spwas cultured and identified as Phialides produced singly, in groups or from branched metulae, giving a brush-like appearance known as a penicillus (Figure 1).All cells between the metulae and the stipes of the conidiophores are referred to as branches, fungal description was approved using Mycology on line(http://www.mycology.adelaide.edu.au/).
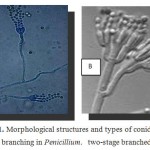 |
Figure 1: Morphological structures and types of conidiophore branching in Penicillium. two-stage branched13. |
The ability of Penicillium spasentomopathogenic fungi against Red flour beetle, T. castaneum was investigated in the present study. Results revealed thatthe application of different concentrations of fungal extract showed different responses of the Red flour beetle as a number of live insects. Table 1 below showed that the application of higher fungal concentrations (50%)on Red flour beetle resulted in low number of live insects compared with other concentration (10% and 30%). Furthermore, the longer the exposure days to the fungal extract, the lower the live insect number detected, in day 15 the number of the live insects was lower than the other days (Table 1). Significant effect of the treatment (different fungal concentration), exposure days and the interaction between the fungal concentrations and exposure days on the live insect number was well documented. Significant negative correlations.
Table 1: The effect of different fungal extract concentrations treatment on the rate of the number of Red rust beetle (Tribolium castaneu).
Treatment(Con%) Insects Numbers
Exposture duration (Days)
1 5 10 15 Means
|
. |
20 ± 0 a |
18.6 ± 0.4ab |
16.3 ± 0.5ab |
14.5 ±0.4b |
17.41667 |
| Fungal Extract(30%) | 18.6 ± 0.4a | 16.6 ± 0.5ab | 14.0 ± 0.3ab | 13 ± 0b | 15.66667 |
| Fungal Extract(50%) | 17.4 ± 0.2a | 15 ± 0.3ab | 11.3 ± 0.5b | 9 ± 0c | 13.16667 |
Treatment ** Exposure duration** Treatment x date ***
** represent the significant effect in the Pd”0.05.Different letter expressed significant variation in the insect live number during the experimental period for one concentration.
between the number of the insectsand the fungal extractconcentration (- 0.6**) and between the number of the insectsand exposure days (-0. 8 **) were noticed.
Furthermore, the application of fungal extract with 40% concentration as a fungal, enzyme, toxin, hyphae and spores was investigated as second treatment against Red flour beetle, T. castaneum. Observed reductions in the insect live number after the treatment were observed. From a number of 20 insects in the 7th day of the treatment only one insect was alive. Moreover, significant effect was observed for the treatment, the exposure duration and the interaction between them. On the other hand, negative correlation (-0.99***) was detected the number of the insect and the exposure days to the fungal extract. Effective results in suppression of insect growth treated with fungal extracts was observed when the insect treated with the whole fungus organs extract, since after day 7 there was no live insects. On the other hand, the number of the untreated insects was constant in both treatments.
Discussion
The ever continuous useof conventional agrochemical insecticides may develop insecticide resistance organisms besides the chemical residue that may affect the human health and environment. Therefore an eco-friendly alternative is needed to reduce expected negative impacts for these materials. Using of living organisms as bio- pesticides which pose less threat to the environment and to human health might be a good alternative. Entomopathogenic fungi as bio- pesticides are important natural regulators of insect populations and have potential as myco-insecticide agents against diverse insect pests in agriculture1.Special attention has been given to entomopathogenic fungal research because they have the ability to penetrate through insect cuticle14. The present study used Penicilliumsp as entomopathogenic fungi against red rust beetle (Tribolium castaneum). The ability of different fungal extract concentrations to kill the red rust beetle was approved positively, Same line of observation was documented by15 when heisolated Quinolactacide compounds from Penicillium citrinum which showed 88% mortality against green beach aphids. Furthermore, Positive reduction in the Tribolium castaneum living number when treated with different concentrations of pathogenic FungiBeauveria bassiana, Lecanicillium muscarium and Calvatia carniiformswas observed12.On the other hand, positive correlation between the concentration and dead number and between the concentration and exposure days to the fungal extract might be due to increase number of fungal spores per spacesince16mentioned that the death of the insect is due to the fact that fungi might infect the insect or the spores contacted the insect. When the spores contacted the insect cuticle it will grow and spread throughout the insect obtaining nutrients,leading to the death of the host by physiological starvation 3 – 7 days after infection9-Furthermore, whole fungus organ treatment showed good results since secretion of cuticle degrading enzymes such as chitinases,lipases, N- acetylglucosaminidases and esterases, is the mode by which Entomopathogenic fungi infect insects17. Pathogenicity in the target host is well correlated by the production of thevirulence protease Pr1 which plays asignificant role in the success of entomopathogenic fungi in insect infection18- 19.Furthermore, Entomopathogenic fungi may produce secondary intermediates and toxins, some of which have insecticidal activities afterinvasion of the fungus organ inside the insect which lead to rapid host death20-21.
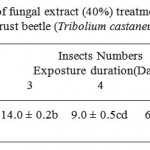 |
Table 2: The effect of fungal extract (40%) treatment on the rate of the number of Red rust beetle (Tribolium castaneu) for one week. |
Conclusion
In conclusion of this study a significant role of Penicillium spas biotic factors regulating Red rust beetle populations is well documented. Furthermore, the higher the concentration of the entomopathogenic fungal extract the higher effect was observed.
Acknowledgment
The author is grateful to Dr. Afrah E. Mohammed, department of biology, Faculty of Science, Princess Nourah Bent Abdul-Rahmn University, for her valuable comments and suggestions during writing of this work.
Refrences
- Mazid, S., Rajkhowa, R.C., Kalita, J.C. A review on the use of biopesticides in insect pest management Inter J Sci Adva Tech,2011; 1: 169 – 178.
- Gupta, S., Dikshit, A. K. Biopesticides: An ecofriendly approach for pest control. JBiopest, 2010; 3: 186 – 188.
- Hajeck, A. E.,St Leger, R. J. Interactions between fungal pathogens and insect hosts, Annual Review of Entomology, 1994; 39:293 – 322.
- Castillo,M.A., Moya,P., Hernandez, E., Yufera,E.P. Susceptibility of Ceratitis capitata Wiedemann (Diptera: Tephritidae) to Entomopathogenic Fungi and Their Extracts, Bio Cont, 2000; 19:274- 282.
- Lezama-Gutie´rrez, R., Molina-Ochoa, J., Cha´vez-Flores, O., A.,´ ngel-Sahagu´n, C. A.,Skoda, S.R., Reyes-Martý´nez, G., Barba- Reynoso, M.,Rebolledo-Domý´nguez, O.,Ruý´z-Aguilar, G.M.L., Foster, J.E. Use of the entomopathogenic fungi Metarhizium anisopliae, Cordyceps bassiana and Isaria fumosorosea to control Diaphorina citri(Hemiptera: Psyllidae) in Persian lime under field conditions. Inter J Trop Insect Sci, 2012; 32: 39– 44.
- Scholte,E.J., Takken,W., Knols,G.J. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae, Acta Tropica, 2007; 102: 151 – 158.
- Smith, R. J., Pekrul, S. Grula, E. A. Requirement for sequential enzymatic activities for penetration of the integument of the corn earworm. J invert path,1981; 38: 335 – 344.
- Shahid, A. A., Sattar, A., Chaudhry, B., Riazuddin, S.Determination of protein virulence factors and pathogenicity of entomopathogenic fungi. Pak. J. Biochem and Mol. Biology, 2003; 36: 100 – 107.
- Shahid, A.A., Rao, A., Bakhsh, A., Husnain, T. Entomopathogenic fungi as biological controllers: New insights into their virulence and pathogenicity. Arch. Biol. Sci., Belgrade, 2012; 64: 21 – 42.
- Hussain,A., Tian, M., Ahmed, S. Shahid, M.Current Status of Entomopathogenic Fungi as Mycoinecticides and Their Inexpensive Development in Liquid Cultures, from Zoology, edited by Dr. María-Dolores García (Ed.), InTech 2012: 103 – 122.
- Golshan, H., Saber, M., Majidi-Shilsar, F., Karimi, F., Ebadi, A.A.Laboratory Evaluation of Beauveria bassiana Isolates on Red Flour Beetle Tribolium castaneum and Their Characterization by Random Amplified Polymorphic DNA. J. Agr. Sci. Tech. 2014; 16: 747 – 758.
- Hussein, A. M., Kamass, N. A., Sultan, A., Al Saidy, H. A. Using some entomopathogenic fungi to control the red rust beetle Tribolium castaneum (Hbst.) (Coleoptera : Tenebrionidae). Diali J for agri sci, 2013; 2: 275 – 282.
- Samson, R.A., Hoekstra, E.S.,Van Oorschot, C.A.N. Introduction to food-borne fungi. Centraalbureau voor Schimmelcultures, Baarn (Netherlands) 1984; 248 p.
- Gouli, V., Gouli, S., Marcelino, J.A.P., Skinner, M., Parker, B.L. Entomopathogenic Fungi Associated with Exotic Invasive Insect Pests in Northeastern Forests of the USA. Insects, 2013; 4: 631 – 645.
- Abe, M., Ima, T., Ishii, N., Usu, M., Okuda, T., Oki, T. Quinolactacide, a new quinolone insecticide from Penicillium citrinum Thom F 1539. Biosci Biotechnol Biochem, 2005; 69: 1202 -1205.
- Long, D.W., Drummond,G.A., Groden,E. Horizontal transmission of Beauveria bassiana.Agri Forest Ento, 2000; 2: 11 – 17.
- St. Leger, R.J., Charnley, A.K., Cooper, R.M.Cuticle degrading enzymes of entomopathogenic fungi: Synthesis in culture on cuticle. J Invert Path, 1986; 48: 85 – 95.
- Shah, F.A., Butt, T.M., Influence of nutrition on the production and physiology of sectors produced by the insect pathogenic fungus Metarhizium anisopliae. FEMSMicro Lett, 2010; 250: 201 – 207.
- Hussain, A., Tian, M.Y., He, Y.R., Lin, R., In vitro and in vivo culturing impacts on thevirulence characteristics of serially passed entomopathogenic fungi. J Food Agri & Envir, 2010; 8: 481 – 487.
- McCauley, V.J.E., Zacharuk, R.Y., Tinline, R.D., Histopathology of the greenmuscardine in larvae of four species of Elateridae (Coleoptera). J Inverteb Path, 1968; 12: 444 – 459.
- Vey, A., Hoagland, R.E., Butt, T.M., Toxic metabolites of fungal control agents. In: Fungi as Biocontrol Agents. Edited by Butt TM, Jackson C &Magan N, CABInternational (New York) 2001; 311 – 346.

This work is licensed under a Creative Commons Attribution 4.0 International License.





