How to Cite | Publication History | PlumX Article Matrix
Akshaya Mohan, Sasikala Shanmugam and Nithyalakshmi V.
Department of Food Process Engineering , SRM University ,Chennai.
Corresponding Author E-mail: sasishan.shiva@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2140
ABSTRACT: The preservation of food by drying is one of the most commonly used methods in the food processing industry. Watermelon belongs to the family of Cucurbitaceae .The processing of watermelon generates a large amount of waste in the form of rind, peel and seeds. The watermelon rind is an under- utilised waste generated after the consumption of the fruit. Watermelon rind contains vitamin C, dietary fiber, citrulline, potassium, a small amount of vitamin B-6. It is also known to contain a variety of bioactive compounds like cucurbitacin, triterpenes, sterols and alkaloid. The citrulline in watermelon rind gives it antioxidant effects that protect the body from free-radical damage. In this study efforts have been made to dry and preserve the watermelon rind by hot air drying and freeze drying technique. The water absorption capacity, rehydration ratio and the solubility of the freeze dried watermelon rind powder were found to be 11.42±0.6 (g/g), 9±0.8 (g/g) and 8±0.5% respectively. Hot air drying significantly reduced the water absorption capacity of the watermelon rind while it increased the water solubility and bulk density. The tannins ,alkaloids and saponin content were found to be lesser in hot iar dried samples compared to freeze dried.
KEYWORDS: Watermelon Rind; Hot air drying; Freeze Drying; Anti-nutrients
Download this article as:| Copy the following to cite this article: Mohan A, Shanmugam S, Nithyalakshmi V. Comparison of the Nutritional, Physico-Chemical and Anti-Nutrient Properties of Freeze and Hot Air Dried Watermelon (Citrullus Lanatus) Rind. Biosci Biotech Res Asia 2016;13(2) |
| Copy the following to cite this URL: Mohan A, Shanmugam S, Nithyalakshmi V. Comparison of the Nutritional, Physico-Chemical and Anti-Nutrient Properties of Freeze and Hot Air Dried Watermelon (Citrullus Lanatus) Rind. Biosci Biotech Res Asia 2016;13(2). Available from: https://www.biotech-asia.org/?p=13332 |
Introduction
Fruits and vegetables contain a variety of nutrients like carbohydrate, proteins, fats, fibres and phytochemicals. A regular consumption of these will significantly reduce the risk of chronic diseases and also help in meeting the nutrient requirement for optimum health.
Consumption of a large variety of fruits and vegetables is commonly practised all around the world, but it is only the pulp of the fruits that is consumed. The rind and the seeds are usually discarded. Consuming a diet rich in fruits and vegetables has shown to have significantly lower rates of occurrence of many types of cancers [1]. Despite this fact, not much importance has been given to the vitamins, minerals, fibres, phytochemicals and antioxidant in the pulps, seeds and rinds of fruits and vegetables.
Agro-wastes are the large volumes of solids waste resulting from the production, preparation and consumption of fruit and vegetable pose potential disposal and pollution problems along with loss of valuable biomass and nutrients. There is a potential for conversion of agro-wastes into useful products or even as raw material for other industries. The utilization of wastes of fruit and vegetable processing as a source of functional ingredients is a promising field [2] .
Watermelon belongs to the Cucurbitaceae family, which include melons, cucumbers and pumpkins. Watermelon is generally grown in the warmer regions of the world.
3 main cultivar groups of watermelon have been identified
Citroidesgroup
Lanatusgroup
Vulgarisgroup
Watermelon rinds are avoided by most people due to their unappealing flavour but they are found to contain many hidden nutrients [3]. Only half of the watermelon fruit is edible while the other half, consisting of the rind and peel goes for a waste. The skin of the watermelon is smooth, with dark green or pale green stripes that turn yellowish green when ripe [4] .
The rind is not as juicy as the flesh of the watermelon, but is edible. A 1-inch cube of watermelon rind contains approximately 1.8 calories. One serving provides 2% of the recommended intake of vitamin C and 1 percent of the vitamin B-6 required by the body every day. Watermelon rinds also offer a high dose of L-citruline – an amino acid – which helps in dilating blood vessels, improve blood circulation and gives watermelon its antioxidant effects [5].
Drying of food products is a very challenging process of food industry as most food products are subjected to some form of drying during their production[6].
Hot air-drying is more commonly used in food industry as it is relatively cheaper but the prolonged drying time usually results in inferior product quality [7].
Freeze-drying, also known as lyophilisation or cryodesiccation is a process of removal of water and is used to preserve a material or make the material more convenient for transport. The principal of freeze-drying involves freezing the material and then reducing the surrounding pressure so as to allow the frozen water to sublimate directly from the solid phase to the gaseous phase. Due to absence of liquid water and low temperature processing, most of the biochemical and microbiological reactions are inhibited which provides very good quality of the final product [8].
Among the various drying processes, freeze-drying provides highest product quality but the relatively high production cost is a major drawback of the process.
Materials and Methods
Collection of raw materials
Fresh watermelon with dark and light green stripes was chosen. Watermelon rinds were collected from a local market and were used fresh.
Preparation of watermelon rind for drying
The whole fruit was first washed and the rind was separated from the flesh. The rind was then grated to reduce its size.
Hot air drying
100g of the grated watermelon rind samples were subjected to drying at 50°C. The oven was preheated to the required temperature and then the single layer loaded trays were kept. The temperature was maintained at 50°C and the process was carried out until the rind was completely dried. The loss in weight of the samples was noted every half hour. Drying was carried out until the samples reached constant weight.
Freeze drying
200g of watermelon rind was ground in a mixer and poured into plates and kept for pre-freezing at 4C for 2 days. The plates were then freeze dried at -40°C at a pressure of -3mbar for 3 days to obtain freeze dried watermelon rind powder.
Determination of proximate analysis
The proximate analysis of the watermelon rind was carried out according to Association of Official Analytical Chemists [9]. All the analysis were done in triplicates.
Determination of ash content
The inorganic matter left over after the organic matter has been completely destroyed is represented by ash content. 2g of the sample was kept in muffle furnace at 525°C for 4-6 hours. Weight of ash was determined using the formula,
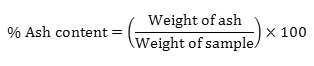
Determination of Fat Content
2g of dried sample was weighed and taken in a thimble and extraction was carried out for 4 hours in soxhlet apparatus. After extraction, the solvent (n- hexane) was completely evaporated and the residue is weighed.
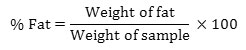
Determination of Moisture content
The moisture content was determined using the air oven method. The samples were dried in the oven at 105 °C. The moisture content of the samples was calculated as:

Determination of carbohydrate content
The carbohydrate content of the samples was determined using Anthrone method. The carbohydrate content of the samples is determined from the standard glucose plot.
Determination of protein content
The protein content of the samples was determined using Lowry’s method. BSA (Bovine serum Albumin) is used as standard and the protein content of samples is determined from standard plot.
Determination of Physical Properties
Determination of colour
The colour index of the dried watermelon rind samples were measured using a –Colour Quest XE Hunter Colour Meter, based on the L* a* b* colour system. L* represents the lightness on a 0 – 100 scale from black to white while a* and b* denote the hues which represent two colour axes with a* denoting redness (+) or greenness (–) and b* denoting yellowness (+) or blueness (–). The equipment is calibrated using a white tile. The whiteness index (WI) is used to indicate the overall whiteness of the food product and also the extent of colour loss during the drying process. The whiteness index is calculated as:
![]()
Determination of Rehydration ratio (RR)
RR was determined according to the method described by [10]. Rehydration ratio is an important factor for dehydrated products.5 g of dried sample was mixed with 150ml of water and poured into a beaker and kept for 50 or 60 min for soaking. The mixture is then boiled and the liquid portion is drained off. The solid material is placed on a filter paper and excess water was removed. The solid material is then weighed and rehydration ratio is calculated as(RR)
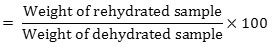
Determination of Water absorption capacity (WAC)
The WAC of the samples was determined using the method described by [11]. 0.5g of sample was weighed into a tube and vortexed with 20ml of distilled water. The tubes were then heated on a water bath at 60°C for 30minutes and then were cooled to room temperature and centrifuged at 2200 rpm for 15min.The pellet is weighed and water absorption capacity of the sample is measured using the formula:
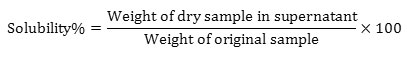
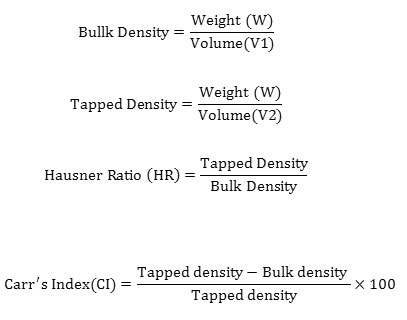
Determination of Solubility
1g of watermelon rind powder is mixed with 10ml distilled water in a centrifuge tube. The centrifuge tube is heated to 80˚C for 30 minutes with continuous shaking. The tube is removed from the bath, wiped dry, cooled to room temperature and centrifuged for 15 minutes at 2200 rpm. The supernatant is evaporated, and the residue is weighed to determine the solubility. Solubility is determined using the formula.
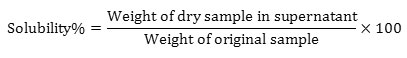
Determination of bulk density, tap density Hausner Ratio (HR) and Carr’s Index (CI)
The parameters were determined according to [12]. Bulk density- 2g of watermelon rind powder (W) was added to a pre-weighed volumetric cylinder and the volume was read as V1.
Tapped density- 2g of sample is placed into a measuring cylinder and tapped until a consistent volume (V2) is reached which corresponds to the maximum packing density of the material.
By measuring both the untapped volume and the tapped volume the following parameters were be determined:
Determination of Anti-nutrients
Determination of alkaloid content
The alkaloid content of the samples was determined as per the method described by [13] .5 g of the sample was weighed into a 250 ml beaker and 200 ml of 10% acetic acid in ethanol was added and covered and allowed to stand for 4 hours. This was filtered and the extract was concentrated on a water bath to one-quarter of the original volume. Concentrated ammonium hydroxide was added dropwise to the extract until the precipitation was complete. The whole solution was allowed to settle and the precipitated was collected and washed with dilute ammonium hydroxide and then filtered. The residue is the alkaloid, which was dried and weighed.
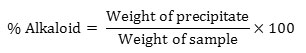
Determination of Tannin Content
Tannin content was determined by the method described by [14]. About 0.2 g of finely ground samples was weighed into a 500 ml sample bottle.10 ml of 70 % aqueous acetone was added and properly covered. The bottles were put in an ice bath shaker for 2 hours at 30ºC. Each solution was then centrifuged and the supernatant stored in ice. 0.2 cm3 of each solution was pipette into the test tubes and 0.8ml of distilled water was added. Standard tannin solutions were prepared from a 0.5 mg/ml stock and the solution made up to 1 ml with distilled water. 0.5ml Folin-ciocalteu’s reagent was added to both sample and Standard followed by 2.5 ml of 20 % Na2CO3. The solutions were then vortexed and allowed to incubate for 40 minutes at room temperature. Its absorbance was read at 725 nm against a reagent blank concentration of the same solution from a standard tannic acid curve.
Determination of Saponin content
The method used was that of [15]. 20 g of samples powder was put into a conical flask and 100 ml of 20% aqueous ethanol were added. The samples were heated over a hot water bath for 4 h with continuous stirring at about 55°C. The mixture was filtered and the residue re-extracted with another 200 ml 20% ethanol. The combined extracts were reduced to 40 ml over water bath at about 90°C. The concentrate was transferred into a 250 ml separating funnel and 20 ml of diethyl ether was added and shaken vigorously. The aqueous layer was recovered while the ether layer was discarded. The purification process was repeated. 60 ml of n-butanol was added. The combined extracts were washed twice with 10 ml of 5% aqueous sodium chloride. The remaining solution was heated in a water bath. After evaporation the samples were dried in the oven to a constant weight and the saponin content was calculated as percentage.
Statistical analysis
Data were expressed as means ± standard deviation (SD) of three replications and statistical analysis was done using SPSS program (version 19.0 SPSS Inc.).The values were considered to be significantly different when p<0.05.
Results and Discussion
Proximate analysis
Carbohydrate content of the samples did not vary significantly between the different drying techniques. The moisture content of freeze dried sample was found to be lower than the hot air dried samples. The fresh watermelon rind was found to contain 0.15% protein. Freeze drying was found to protect the native protein structure in comparison to the hot air drying which brought about denaturation of the protein structure. Ash content of the freeze dried sample was found to be higher in comparison to the hot air dried samples. The results obtained were in accordance with [7]. The results are tabulated in table 1.
Table 1: Proximate Analysis of Watermelon Rind Dried by Hot Air Drying and Freeze Drying.
| Proximate analysis | Sample | ||
| Fresh | FD | HAD | |
| Carbohydrate (%) | 4.36±0.6b | 78.45±0.89a | 78.3.1±1.2a |
| Moisture (%) | 91.3±2.3a | 3.7±0.56c | 4.9±0.11b |
| Fat (%) | 0.09±0.01c | 2.9±0.12a | 2.63±0.21b |
| Protein (%) | 0.15±0.04c | 2.067±0.08a | 1.83±0.1b |
| Ash (%) | 3.84±0.19b | 12.92±0.11a | 12.78±0.17a |
*Data are expressed as mean±standard deviation. Data in the same row bearing different superscripts are statistically different at 5% level of significance.
Determination of Density parameters
Watermelon rind dried by freeze drying and hot air drying showed significant difference in bulk density values. The density of the samples was found to vary linearly with moisture content. The results obtained were in accordance with [7]. The results are tabulated in table 2.
Table 2: Density Analysis of Watermelon Rind Dried by Hot Air Drying and Freeze Drying.
| Sample | Bulk Density(g/ml) | Tapped Density(g/ml) | Hausner Ratio(HR) | Carr’s Index(CI) |
| FD | 0.09±0.02b | 0.11±0.04b | 1.22±0.1b | 18.18±0.08b |
| HAD | 0.40±0.12a | 0.56±0.01a | 1.4±0.02a | 28.57±0.02a |
*Data are expressed as mean ± standard deviation. Data in the same column bearing different superscripts are statistically different at 5% level of significance(p<0.05)
Determination of Water Absorption Capacity and Rehydration Ratio
Water absorption capacity is an index of starch gelatinisation. The WAC value of Hot air dried sample was higher when compared to that of Freeze dried sample. The value of Hot air dried sample at 50°C was 8.27 ± 0.41 (g/g), which indicates that at this drying temperature tissue damage occurs due to which the watermelon rind dried at this temperature could retain a greater amount of water than samples dried at higher temperatures.
The rehydration ratio and water absorption capacity of the freeze dried sample was found to be greater than the hot air dried sample which makes freeze dried foods more suitable for the production of ready to eat meals. The results obtained were as stated by [8].
Water absorption capacity and rehydration ratio were found to vary significantly with changes in temperature, thus indicating that temperature is a major factor affecting the 2 parameters. The results are tabulated in table 3.
Determination of solubility
Water solubility indicates the extent of starch degradation. A higher solubility was found in hot air dried samples as compared to the freeze dried samples. This increase in the solubility can be attributed to the increased degradation of starch during hot air drying. Similar results were obtained by [16] in the drying of pumpkin. The solubility of the samples was found to vary significantly. The results are tabulated in table 3.
Table 3: Rr, Wac, And Solubility of Watermelon Rind Dried by Hot Air Drying and Freeze Drying
| Sample | RR(g/g) | WAC (g/g) | Solubility (%) |
| FD | 7.02±0.8a | 11.40±0.6a | 5.12±0.5b |
| HAD | 4.4±0.7b | 8.27±0.41b | 11.29±0.6a |
*Data are expressed as mean ± standard deviation. Data in the same column bearing different superscripts are statistically different at 5% level of significance (p<0.05).
Determination of Colour
Freeze drying results in lighter coloured samples as shown by the higher L values of the freeze dried sample. The results indicated that freeze drying was found to prevent the occurrence of browning and produced high quality of freeze dried watermelon rind powder. The whiteness index (WI) represents the overall extent of discolouration of the samples and was found vary significantly with change in temperature. Similar results were obtained by [17]. The results are tabulated in table 4.
Table 4: Colour Attributes of Watermelon Rind Dried by Hot Air Drying and Freeze Drying.
| Sample | L* | a* | b* | dE | WI |
| Fresh | 49.94 ± 0.1c | -3.42 ±0.1c | 10.69 ± 0.3c | 45.05 ± 0.12a | 48.69 ± 0.2c |
| FD | 77.22 ± 0.2a | -0.38 ± 0.2a | 19.8 ± 0.2b | 25.1 ± 0.18c | 69.8 ± 0.31a |
| HAD | 62.51± 0.1b | -1.67 ± 0.1b | 21.2 ± 0.1a | 36.89 ± 0.19b | 56.89 ± 0.24b |
*Data are expressed as mean ± standard deviation. Data in the same row bearing different superscripts are statistically different at 5% level of significance (p<0.05).
Determination of Anti-nutrients
The tannin, saponin and alkaloid content of the watermelon rind were found to be higher in the freeze dried samples as compared to the hot air dried samples. Hot air dying brings about oxidation of bioactive compounds and enzymatic degradation due to higher temperatures and longer drying time which accounts for the reduction in the tannin, saponin and alkaloid contents in the watermelon rind. The results obtained were in accordance with [18] . The results are tabulated in table 5.
Table 5: Anti-Nutrients in Watermelon Rind Dried by Hot Air Drying and Freeze Drying
| Sample | Saponins (%) | Tannins (%) | Alkaloids (%) |
| FRESH | 0.34±0.02b | 0.12±0.01c | 0.62±0.05b |
| FD | 2.03±0.17a | 0.43±0.12a | 1.42±0.17a |
| HAD | 1.95±0.21a | 0.31±0.09b | 1.37±0.16a |
Conclusion
Current findings revealed that freeze drying was the preferred method for drying of watermelon rind. Nutritional properties of the watermelon rind are better preserved by freeze drying in comparison to hot air drying. Freeze drying resulted in concentration of the nutrients and may be used as a technique for the preservation and utilisation of watermelon rind.
References
- L.E. Voorrips, R.A Goldbohm, G.V. Poppel, F.Sturmans, R. J. J. Hermus, and P.A. van den Brandt. Vegetables and fruits consumption and risk of colon and rectal cancer in a prospective cohort study: the Netherlands cohort study on diet and cancer. American Journal epidemiology, 2000; 152: 1081-1092.
- Schieber, A., Stintzing, F. C., Carle, R. By-products of plant food processing as a source of functional compounds-recent developments. Trends in Food Science & Technology, 2001;12: 401–413.
- Rattray and Diana. Southern U.S. Cuisine: Judy’s Pickled Watermelon Rind. Southernfood.about.com.2012
- M.M Hoque and A.Iqbal.Drying of watermelon rind and development of cakes from rind powder. International journal of novel research in life sciences, 2015; 2(1): 14-21.
- A.M. Rimando and P.Perkins-veazie. Determination of citrulline in watermelon rind. Journal of Chromatography A, 2005; 196–200.
- P.Wankhade, R.Sapkal and V.Sapkal. Drying characteristics of okra slices on drying in hot air dryer. Procedia Engineering, 2013; 371-374.
- C.L. Hsua, W. Chenb, Y.M. Wenga and C.Y. Tsenga. Chemical composition, physical properties, and antioxidant activities of yam flours as affected by different drying methods. Journal of food chemistry, 2003; 85-92.
- C.Ratti. Hot air and freeze-drying of high-value foods: A review. Journal of Food Engineering, 2001; 49: 311–319.
- AOAC. 2002. Official Methods of Analysis -17th ed. Association of Official Analytical Chemist, Maryland.
- S. Pervin, M. S. Islam and M. N. Islam. Study on rehydration characteristics of dried lablab bean (Lablab Purpureus) Seeds. Journal of Agriculture and rural Development, 2008; 6: 157-163.
- E. Elaveniya1 and J. Jayamuthunagai. Functional, physicochemical and anti-oxidant properties of dehydrated banana blossom powder and its incorporation in biscuits. International Journal of ChemTech Research, 2014; 6: 4446-4454.
- C.W. Lee, H.J. Oh, S.H. Han and S.B. Lim. Effects of hot air and freeze drying methods on physicochemical properties of citrus ‘hallabong’ powders. Food Science and Biotechnology, 2012; 21: 1633-1639.
- Oseni, O. A & Okoye, V. I._Studies of Phytochemical and Antioxidant properties of theFruit of Watermelon (Citrullus lanatus). Journal of pharmaceutical and biomedical sciences ,2013; 27(27): 508-514.
- Makker AOS, Godchild AV. Quantifying of tannins. A laboratory manual. Internationalcentre for agricultural research in Dry Areas. (ICARDA), 1996;4:35.
- J. A. V. Famurewa, J. B. Olatujoye and A. Ajibode. Drying Phenomenon and Influence on the Anti-Nutritional and Pasting Properties of Cocoayam (Taro). Journal of Scientific Research & Reports, 2014; 3(2): 275-283.
- F.Que, L.Mao, X.Fanq and T.Wu. Comparison of hot air-drying and freeze-drying on the physicochemical properties and antioxidant activities of pumpkin (Cucurbita moschata Duch.) flours. International Journal of Food Science and Technology,2008; 43: 1195-1201.
- M.K. Youssef and S.M. Mokhtar. Effect of drying methods on the antioxidant capacity, colour and phytochemicals of Portulaca oleracea L. leaves. Journal of Nutrition and Food Sciences, 2014; 4.
- A.E.Irondi ,K.K.Anokam and U.S.Ndidi. Effect of drying method on phytochemicals compostition and antioxidant activities of Carica papaya seeds. International Journal of Biosciences,2013;3(11) :154-163.

This work is licensed under a Creative Commons Attribution 4.0 International License.





