How to Cite | Publication History | PlumX Article Matrix
V. Padhmavathi and Radhakrishnan Preetha*
Department of Food Process Engineering, SRM University, Kattankulathur, 603203
Corresponding Author E- Mail: preetha.r@ktr.srmuniv.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2148
ABSTRACT: Probiotics are microorganisms which, when taken orally, provide beneficial effects on human gut health. Microencapsulation of probiotics is a technique that is currently receiving considerable interest as it helps probiotics to survive against adverse environmental conditions in the human body. In this project , a study to determine the stronger encapsulating material is done by encapsulating Lactobacillus sp. using alginate and skim milk alginate. The encapsulation was done by extruding into 100mM CaCl2 solution. Viability test, bile salt tolerance test and storage stability was performed. On analyzing the results it was found that, the skim milk alginate beads had the efficiency of 96.48%. The bacteria encapsulated using skim milk alginate was more viable and more tolerant towards bile salt. The storage stability test was carried out for a period of 28 days and it was found that the probiotic encapsulated using skim milk alginate were more viable. From the results it was concluded that skim milk alginate was a stronger encapsulating material than alginate because of the strong network forming nature of milk proteins.
KEYWORDS: Probiotics; Lactobacillus sp; microencapsulation; alginate; skim milk
Download this article as:| Copy the following to cite this article: Padhmavathi V, Preetha R. Microencapsulation of Lactobacillus Sp. Using Two Different Materials and Comparison for Encapsulation Efficiency. Biosci Biotech Res Asia 2016;13(2) |
| Copy the following to cite this URL: Padhmavathi V, Preetha R. Microencapsulation of Lactobacillus Sp. Using Two Different Materials and Comparison for Encapsulation Efficiency. Biosci Biotech Res Asia 2016;13(2).Available from: https://www.biotech-asia.org/?p=13939 |
Introduction
Probiotics are defined as “Live microorganisms which when administered in adequate amounts confer a health benefit on the host” [1]. When consumed in adequate amounts, probiotics are live microorganisms that provide a health benefit to the host [2]. Interest in the consumption of probiotic food products is increasing and a many functional foods have been developed [3]. They confer many health benefits such as suppressing growth of pathogens, preventing diarrhoea, constipation, and food allergies, synthesizing nutrients and enhancing their bioavailability, and anti-neoplastic activity [4, 5]. The survivability of the probiotics is to be maintained during manufacture, storage, and delivery to gastrointestinal tract to provide their health benefit [3]. The most common probiotic-containing foods are fermented dairy products that contain lactic acid bacteria (LAB). Probiotics are highly stable in dairy products than in non-dairy products. Various solutions to this problem, such as durable strain selection in adverse environments [6] and addition of prebiotics [7] have been evaluated.
Encapsulation has been investigated for protecting probiotics in gastrointestinal tract [8]. The advantages of encapsulation are prevention of interfacial inactivation, stimulation of production and excretion of secondary metabolites, and continuous utilization. During fermentation it also enhances microbial survival and operating efficiency [9]. Several studies have shown successful microencapsulation and coating of bacteria using various encapsulating materials and methods [10, 11, 12, 13, 14]. A process in which the cells are retained within an encapsulating matrix or membrane is known as microencapsulation.
Extrusion technique of microencapsulation has been used exclusively for encapsulating volatile and unstable flavors in glassy carbohydrate matrices and probiotic microorganisms [15, 16, 17, 18, 19, 20, 21]. The main advantage of this process is that, the matrix is strong enough and protects the microbes in very low pH conditions too.
Alginates are natural anionic polysaccharides made up of D-mannuronic and L-guluronic acid residues [22]. Alginate, a polymer extracted from seaweed, is a commonly used encapsulation agent because it is non-toxic, biocompatible, and inexpensive. The additional benefits of using alginate is the ease of solubilizing alginate gel (by Ca++ sequestration) and its release of entrapped cells within the human intestine [23,24]. There are few studies on the effect of whey protein and skim milk powder on encapsulation efficiency of probiotic microorganisms [25]. Milk proteins have good immobilization properties.
Strains of Lactobacillus and Bifidobacterium species , which are lactic acid bacteria, are the most common microbes employed as probiotics. Other species considered as probiotic may include lactococci, some enterococci and some streptococci. Curd and other dairy products are a very good source for Lactobacillus species.
Materials and Methods
Subculturing and maintenance
The probiotic organism, Lactobacillus sp, was obtained from MTCC, Chandigarh. The lyophilised powder was sub-cultured by inoculating into MRS broth and incubating at 37oC for 24 hours. After 24hours, the culture from the broth was further sub-cultured by streak plating onto MRS agar and incubated for 24hours at 37oC. These plates were then stored at 4oC for a period of one month before sub-culturing again.
Microencapsulation of probiotic
Microencapsulation of the organism was done using two materials mentioned below by extrusion technique.
Alginate
Alginate solution was sterilised at 121oC for 15min. 14 ml sodium alginate was added to a beaker.1 ml culture was added to the beaker. This mixture was extruded in 100mM CaCl2 solution and stirred for 30 min at 100 rpm. The beads obtained were washed with distilled water and sealed in sterile conical flasks.
Skim milk-alginate
Alginate solution was sterilised at 121oC for 15min. Skim milk was sterilised at 110oC for 15 min. 13 ml sodium alginate was added to a beaker along with 6 ml skim milk. 1 ml culture was added to the beaker. This mixture was extruded in 100mM CaCl2 solution and stirred for 30 min. The beads obtained were washed with distilled water and sealed in sterile conical flasks.
Encapsulation efficiency
The microencapsulated bacterial sample was solubilized in sodium citrate solution. The sample was serially diluted up to 10 fold and plated onto MRS agar by spread plating. It was incubated at 37oC for 24 hours. Number of colonies were counted and the encapsulation efficiency (EE) was calculated as described by [26].
EE = N / No × 100
Where, N – Number of colonies from beads
No- Number of colonies from free cell suspension.
Bile salt tolerance test of free and encapsulated probiotic
Free cell suspension (0.5ml) was added to 4.5ml of bile salt solution (2%) and kept at 37oC for 1 hour and 2 hours. Solution from each flask was then serially diluted up to 10 fold using saline solution. 1ml from last test tube was plated onto MRS agar plates by spread plate method. These plates were incubated at 37oC for 24 hours and number of colonies were counted.
0.5g beads were added to 4.5ml of bile salt solution (2%) and kept at 37oC for 1 hour and 2 hours. The capsules were removed from bile salt solution at specified time intervals and added to 4.5ml of sodium citrate solution to be solubilised. Once released completely these solutions were serially diluted upto 10 fold using saline solution. 1ml from last test tube was plated onto MRS agar plates by spread plate method. These plates were incubated at 37oC for 24 hours and number of colonies were counted.
Storage stability of free and encapsulated cells
Encapsulated beads and free cell suspension are stored at 4oC for a period of 1 month. At regular intervals such as 1, 3, 5, 7, 14, 21, 28 days the encapsulated cultures were revived by using sodium citrate solution and serially diluted. These were then plated onto MRS agar. While, the free cells were serially diluted up to 10 fold and plated onto MRS agar. The colonies were counted to determine the survivability.
Statistical analysis
All the experiments were repeated three times. The data was subjected to analysis of variance (ANOVA) and the significance of difference between means was determined by Duncan’s multiple range tests (p < 0.05). The results were presented in mean value ±standard deviation (SD).
Results and Discussion
Size and Encapsulation efficiency
Extrusion method is the most commonly used method for encapsulating probiotic. In extrusion method the size and encapsulation yield of microspheres is affected by several factors such as nozzle size, polymer concentration and composition [27]. Their results showed that diameters of alginate milk microspheres, prepared using nozzle 0.45 and 0.20 mm were 830 ± 10 and 381 ± 8 µm (figures not shown), respectively. The size of microspheres should be below 100 µm to avoid negative sensory impact in food products [28]. Due to the limitation of nozzle size (minimum nozzle size provided was 0.20mm) ,the minimum size of microspheres obtained by Voo et al., 2011 was around 381 ± 8 µm.
In this study it was seen that polymer composition influenced the slight variation in diameter of microspheres. Alginate and skim milk alginate were the two different compositions used. The microspheres were prepared with a 0.20mm nozzle. Alginate microspheres had a diameter of 321 ± 10 µm and skim milk alginate microspheres had a diameter of 367 ± 6 µm.
Studies show that high encapsulation efficiency (close to 100%) were easily obtained through extrusion method [29,30]. In the present study, 94.44% efficiency was obtained when alginate was used as encapsulating material. On the other hand, a significantly different (p<0.05) efficiency of 96.48 % was obtained when skim milk alginate was used. The encapsulation efficiency of skim milk-alginate was higher than that of alginate.
Bile salt tolerance of free and encapsulated cells
In this study, as result of action of bile salts , there was deterioration in cell wall intergrity and free Lactobacillus sp totally lost its viability in bile salt. Many references mentioned probiotics were sensitive to bile salt solution. There are observations that a decrease of 5 logCFU/ml in viable cell counts of Bifidobacterium adolescentis (B. adolescentis) occurs in 2% bile salt solution at 37̊C after 12 h incubation [31]. It has been found that B.adolescentis reduced by about 2 logCFU/mL after 2 h incubation in 0.5% bile salt at 37̊C [28]. However, the results shown in Table 1 clearly indicate that encapsulated microspheres could provide a good protection against the damage of the bile salt solution compared to free Lactobacillus sp cells and there was a significant difference (p<0.05) in the results.
Storage stability of free and encapsulated cells
Different researchers have used different concentrations and sources of bile salts. So, making a comparison was difficult. It was also found that encapsulated probiotic bacteria could survived better than free probiotic cells in 1–3% bile salts solution [32, 26].
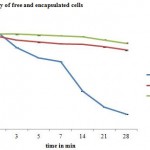 |
Figure 1: Storage stability of free and encapsulated cells |
In the present study it was seen that the number of viable cells reduced drastically when left without encapsulation. Encapsulated cells were protected and hence the cells were viable and survived a storage period of 28 days. Special treatments, such as coating of beads with polymers, blending with other polymers, improves the storage stability of probiotic [33]. The protective effect by whey protein encapsulation was evaluated at 5̊C on storing for 180 days [34]. Fig 1, below, clearly indicates that microencapsulation using skim milk alginate, improved the viability of cells on storage.
Conclusion
Lactobacillus sp. was successfully encapsulated in alginate and skim milk- alginate microspheres prepared by extrusion method. The cells of Lactobacillus sp. encapsulated in microspheres showed better survival ability than that of free cells in high bile salt concentrations (2.0%) and long time storage (28 days). It was also found that skim milk-alginate was a stronger material when compared to alginate as all results indicated more viability in skim milk- alginate microspheres. Encapsulation has once again proved to be a good method to protect probiotics in gastrointestinal environments. Skim milk- alginate microspheres show the potential as a new encapsulating material for preserving the viability of probiotics during oral administration. Further studies are to be carried out in gastro-intestinal environments.
Acknowledgement
We express our thanks to Prof. C. Muthamizchelvan, Director, Engineering Technology and Dr. M. Vairamani, Dean School of Bioengineering, SRM University and Dr. K. A. Athmaselvi, Head of the department, Department of Food Process Engineering, for their help and encouragement.
References
- Joint FAO/WHO Working Group. Guidelines for the evaluation of probiotics in food. London: World Health Organization, ON, Canada: Food and Agriculture Organization. 2002.
- Saarela M, Lähteenmäki L, Crittenden R, Salminen S, Mattila-Sandholm T. Gut bacteria and health foods—the European perspective. International journal of food microbiology. 2002 Sep 15;78(1):99-117.
- Ying DY, Phoon MC, Sanguansri L, Weerakkody R, Burgar I, Augustin MA. Microencapsulated Lactobacillus rhamnosus GG powders: relationship of powder physical properties to probiotic survival during storage. Journal of Food Science. 2010 Nov 1;75(9):E588-95.
- Rafter J. Probiotics and colon cancer. Best Practice & Research Clinical Gastroenterology. 2003 Oct 31;17(5):849-59.
- Salminen S, von Wright A, Morelli L, Marteau P, Brassart D, de Vos WM, Fondén R, Saxelin M, Collins K, Mogensen G, Birkeland SE. Demonstration of safety of probiotics—a review. International journal of food microbiology. 1998 Oct 20;44(1):93-106.
- Chou LS, Weimer B. Isolation and characterization of acid-and bile-tolerant isolates from strains of Lactobacillus acidophilus. Journal of Dairy Science. 1999 Jan 31;82(1):23-31.
- Topping DL, Fukushima M, Bird AR. Resistant starch as a prebiotic and synbiotic: state of the art. Proceedings of the Nutrition Society. 2003 Feb 1;62(01):171-6.
- Favaro-Trindade CS, Grosso CR. Microencapsulation of L. acidophilus (La-05) and B. lactis (Bb-12) and evaluation of their survival at the pH values of the stomach and in bile. Journal of microencapsulation. 2002 Jan 1;19(4):485-94.
- Champagne CP, Lacroix C, Sodini-Gallot I. Immobilized cell technologies for the dairy industry. Critical Reviews in Biotechnology. 1994 Jan 1;14(2):109-34.
- Annan NT, Borza A, Moreau DL, Allan-Wojtas PM, Hansen LT. Effect of process variables on particle size and viability of Bifidobacterium lactis Bb-12 in genipin-gelatin microspheres. Journal of microencapsulation. 2007 Jan 1;24(2):152-62.
- Jankowski T, Zielinska M, Wysakowska A. Encapsulation of lactic acid bacteria with alginate/starch capsules. Biotechnology Techniques. 1997 Jan 1;11(1):31-4.
- Kebary KM, Hussein SA, Badawi RM. Improving viability of bifidobacterium and their effect on frozen ice milk.
- Khalil AH, Mansour EH. Alginate encapsulated bifidobacteria survival in mayonnaise. Journal of Food Science. 1998 Jul 1;63(4):702-5.
- Lee KY, Heo TR. Survival of Bifidobacterium longumImmobilized in Calcium Alginate Beads in Simulated Gastric Juices and Bile Salt Solution. Applied and Environmental Microbiology. 2000 Feb 1;66(2):869-73.
- Benczedi D, Blake A. Encapsulation and the controlled release of flavours. Leatherhead Food RA Food Industry Journal. 1999;2:36-48.
- Benczedi D, Bouquerand PE. Process for the preparation of granules for the controlled release of volatile compounds. PCT WO. 2001;1(17372):A1.
- Blake A. Flavor encapsulation with carbohydrate glasses. International Food Ingredient. 1994;3:30-4.
- Gunning YM, Gunning PA, Kemsley EK, Parker R, Ring SG, Wilson RH, Blake A. Factors affecting the release of flavor encapsulated in carbohydrate matrixes. Journal of agricultural and food chemistry. 1999 Dec 20;47(12):5198-205.
- Qi ZH, Xu A. Starch-based ingredients for flavor encapsulation. Cereal Foods World. 1999.
- Reineccius GA. Carbohydrates for flavor encapsulation. Food Technology. 1991;45(3):144-6.
- Saleeb FZ, Pickup JG, inventors; General Foods Corporation, assignee. Fixation of volatiles in extruded glass substrates. United States patent US 4,820,534. 1989 Apr 11.
- Thu B, Bruheim P, Espevik T, Smidsrød O, Soon-Shiong P, Skjåk-Bræk G. Alginate polycation microcapsules: I. Interaction between alginate and polycation. Biomaterials. 1996 Dec 31;17(10):1031-40.
- Adhikari K, Mustapha A, Grün IU. Survival and metabolic activity of microencapsulated Bifidobacterium longum in stirred yogurt. Journal of Food Science. 2003 Jan 1;68(1):275-80.
- Sultana K, Godward G, Reynolds N, Arumugaswamy R, Peiris P, Kailasapathy K. Encapsulation of probiotic bacteria with alginate–starch and evaluation of survival in simulated gastrointestinal conditions and in yoghurt. International journal of food microbiology. 2000 Dec 5;62(1):47-55.
- Andic SE, Zorba O, Tuncturk Y. Effect of whey powder, skim milk powder and their combination on yield and textural properties of meat patties. International Journal Agricultural Biology. 2010 Nov 1;1:871-6.
- Kailasapathy K. Survival of free and encapsulated probiotic bacteria and their effect on the sensory properties of yoghurt. LWT-Food Science and Technology. 2006 Dec 31;39(10):1221-7.
- Voo WP, Ravindra P, Tey BT, Chan ES. Comparison of alginate and pectin based beads for production of poultry probiotic cells. Journal of bioscience and bioengineering. 2011 Mar 31;111(3):294-9.
- Hansen LT, Allan-Wojtas PM, Jin YL, Paulson AT. Survival of Ca-alginate microencapsulated Bifidobacterium spp. in milk and simulated gastrointestinal conditions. Food Microbiology. 2002 Feb 28;19(1):35-45.
- Ma Y, Pacan JC, Wang Q, Xu Y, Huang X, Korenevsky A, Sabour PM. Microencapsulation of bacteriophage felix O1 into chitosan-alginate microspheres for oral delivery. Applied and environmental microbiology. 2008 Aug 1;74(15):4799-805.
- Ma Y, Pacan JC, Wang Q, Sabour PM, Huang X, Xu Y. Enhanced alginate microspheres as means of oral delivery of bacteriophage for reducing Staphylococcus aureus intestinal carriage. Food hydrocolloids. 2012 Mar 31;26(2):434-40.
- Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunology and Cell Biology. 2000 Feb 1;78(1):80-8.
- Chandramouli V, Kailasapathy K, Peiris P, Jones M. An improved method of microencapsulation and its evaluation to protect Lactobacillus spp. in simulated gastric conditions. Journal of microbiological methods. 2004 Jan 31;56(1):27-35.
- Krasaekoopt W, Bhandari B, Deeth H. Evaluation of encapsulation techniques of probiotics for yoghurt. International Dairy Journal. 2003 Dec 31;13(1):3-13.
- Rodrigues D, Sousa S, Rocha-Santos T, Silva JP, Lobo JS, Costa P, Amaral MH, Pintado MM, Gomes AM, Malcata FX, Freitas AC. Influence of L-cysteine, oxygen and relative humidity upon survival throughout storage of probiotic bacteria in whey protein-based microcapsules. International Dairy Journal. 2011 Nov 30;21(11):869-76.

This work is licensed under a Creative Commons Attribution 4.0 International License.





