How to Cite | Publication History | PlumX Article Matrix
Shapoor Heidari Chaleshtori and Mehrdad Ataie Kachoie
Department of Herbal Medicine, College of Food and Drug, Shahrekord Branch, Islamic Azad University, Shareakord, Iran.
Corresponding Author E-mail: Mehrdad.ataie@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2331
ABSTRACT: Abstract Application of various types of fertilizers can effect on biological activities of Calendula officinalis. The present investigation was aimed to study the chemical components and antimicrobial effects of C. officinalisgrown under chemical and biological conditions on methicillin-resistant Staphylococcus aureus.Four hundred samples of hospital infections were collected and cultured. MRSA strains were subjected to the disk diffusion and GC-Mass. One-hundred out of 400samples of hospital infections were positive for MRSA (25%). All isolates were also positive for mecA gene. Forty different chemical components were detected in the C. officinalis. The most variable components were found in the control group (1,8-cineole (30.456%), γ-terpinene (25.547%), terpinolene (4.584%), α-terpineol (4.490%) and trans-β-ocinene (4.153%)). Application of biologic and chemical fertilizers caused significant increase in the levels of some chemical components (P<0.05).MRSA strains harbored the highest levels of resistance against tetracycline (95%), ampicillin (92%), penicillin (90%), gentamycin (88%) and ciprofloxacin (77%). Control group had the highest antimicrobial effects but essential oil of the C. officinalis enriched with both fertilizers were effective on resistant MRSA. Use of C. officinalis growthunder bothchemical and biologic fertilizers has been recommendedas a primary approach for synthesis of effective antibiotic.
KEYWORDS: Calendula officinalis; Chemical components; Antimicrobial effects; Methicillin resistant Staphylococcus aureus; Biologic fertilizer; Chemical fertilizer
Download this article as:| Copy the following to cite this article: Chaleshtori S. H, Kachoie M. A. Chemical composition and antimicrobial effects of Calendula officinalis grown under chemical and biological conditions on the Methicillin-resistant Staphylococcusaureus isolated from hospital infections. Biotech Res Asia 2016;13(3). |
| Copy the following to cite this URL: Saeedpour B. Assessment of the ecological potential of Shaskoh protected area for determining suitable ecotourism sites. Biotech Res Asia 2016;13(3). Available from: https://www.biotech-asia.org/?p=16165 |
Introduction
Staphylococcus aureus (S. aureus) is a gram-positive coccal bacterium and a one of the most important causative agents of various types of hospital infections including systemic infections (such as urinary, respiratory and blood infections, pneumonia, sinusitis and food poisoning),as well as skin and soft-tissue infections (such as wounds,abscess, burns,furunclosisand impetigo), [1-3]. Both community-associated and hospital-acquired infections with Staphylococcus aureus (S. aureus) have increased in the past 20 years, and the rise in incidence has been accompanied by a rise in antibiotic-resistant strains—in particular, methicillin-resistant S aureus (MRSA) [4, 5]. MRSA strains have global significance regarding the high prevalence of infections in the cases hospitalized in hospitals [4, 5]. The gene for methicillin resistance, mecA, is carried on a 21- to 67-kb element which has an active presence in all infective strains of bacterium[6]. MRSAstrains of clinical infections harbored the high levels of resistance against the extensive ranges of antibiotics including beta-lactam antibiotics, which include the penicillins (methicillin, dicloxacillin, nafcillin, oxacillin, etc.) and the cephalosporinsand other types of antimicrobial agents including erythromycin, cotrimoxazole, tetracycline, penicillin, gentamicin, cefexim and clindamycin [7-10]. According to the high prevalence of MRSA resistance against commonly used antibiotics in hospitals, the need for application and prescription of novelantimicrobial agents is essential.
In recent years, much attentions have been done to the prescription of medicinal plants for treatment of various types of infectious diseases[11]. Medicinal plants are a suitable sources of antimicrobial agents.Calendula officinalis(C. officinalis) is one of the most commonly used medicinal plants among Iranian people which is native to the Mediterranean regions[12]. C. officinalis, commonly known as pot marigold, is an annual herb and belongs to Asteraceae family. Flowers are monoecious (individual flowers are either male or female, but both sexes can be found on the same plant) and are pollinated by Bees. It is noted for attracting wildlife.C. officinalis can be broadly applied as an antiseptic, anti-inflammatory and cicatrizing as well as a light antibacterial and antiviral agent[11, 13-15].The plant contains esquiterpenes glycosides, saponins, xanthophylls, triol triterpenes, flavonoids, volatiles, δ- cadinene, α-cadinol, 1,3,5-cadinatriene and α-muurolol which show anti-oxidative and antimicrobial effects [11, 13-16].
According to the high beneficial aspects of planting of C. officinalis, farmers have moved to use from various types of agricultural fertilizers to increasing the quality and quantity, controlling the pests and accelerate the harvesting time of plant. Application of these agricultural fertilizers and especially biologic and chemical (urea) manures can effects on the chemical composition and also therapeutic activities of essential oil extracted from C. officinalis. In some cases, significant changes have been occurred in the composition and performance of medicinal plants [17, 18].
According to the high prevalence of MRSA strains in Iranian cases of hospital infections, economic,cosmetic, and pharmaceutical valuesof C. officinalis andlack of published data on the influence of chemical and biologic fertilizers on components and antimicrobial activities of C. officinalis, the present study was carried out to evaluate thechemical components and antimicrobial effects of various treatments of C. officinalis on the MRSA strains isolated from various types of hospital infections in Iran.
Materials and methods
Ethical issues
The present study was accepted by the ethical committees of the Baqiyatallah University of Medical Sciences and Hajar Hospital, Iran. Written informed consent was obtained from all of the study patients or their parents. Written consent was also signed by the Research Adjutancy of the Islamic Azad University of Shahrekord (IAUSHK 110224) and ethical committees of the educational Hospitals, Tehran, Iran.
Samples collection and MRSA identification
From March to October 2015, overall 500clinical samplesfrom various types of infections were collected from hospitalized patients of Iranian hospitals and were immediately transferred to the laboratory in cooler with ice-packs.
Twenty-fivemicroliters of each samples were inoculated on Mueller–Hinton broth (MHB, Merck, Germany) supplemented with 6.5% NaCl and homogenized. The suspension was incubated for 16–20 h at 37 °C. One milliliter of the enriched MHB media was added to 9 ml of phenol red mannitol broth containing ceftizoxime (5 μg/ml) and aztreonam (75 μg/ml) (PHMB) and incubated for 16–20 h at 37 °C. The surface of the selective isolation medium MRSA ID was inoculated with a sterile loop. The plates were incubated for 24 h at 37 °C (when the colonies were difficult to identify the incubation was protracted for another 24 h). Typical green colonies were primary known as MRSA. Five selected typical colonies per plate were subcultured on Tryptone Soya Agar (TSA, Merck, Germany). Typical colonies were tested with the Staphytect Plus test (Oxoid), a latex agglutination test for the detection of clumping factor, Protein A and certain polysaccharides found in MRSA.
PCR confirmation of MRSA
All of the MRSA strains were cultured on Tripticase Soy Broth (TSB) and were incubated at 37 °C for 18-24h. Genomic DNA was extracted from bacterial colonies using the DNA extraction and purification kit (Cinagen, Iran) according to the manufacture instruction. Presence of MRSA strains were confirmed using the PCR-based amplification of mecA gene [19]. Reaction was performed in a final volume of 50 µL containing 5 µL 10 × buffer + MgCl2, 2 mM dNTP, 2 unit Taq DNA polymerase, 100 ng genomic DNA as a template, and 25 picomole of each primer (5′- AAATCAGATGGTAAAGGTTGGC-3’ and 5′-AGTTCTGCAGTACCGGATTTGC-3′) (533 bp). PCR was performed using a thermal cycler (Eppendorf Mastercycler 5330, Eppendorf-Nethel-Hinz GmbH, Hamburg, Germany) under the following conditions: an initial denaturation for 1 minutes at 94°C and 40 cycles including 95°C for 30 s, 55 °C for 30 s and 72 °C 1 min, and a final extension at 72°C for 5 minutes. S. aureus ATCC 6538 was used as a positive control and distilled water was used as a negative control. Fifteen microliters of PCR products in all reactions were resolved on a 1.5% agarose gel containing 0.5 mg/ml of SYBR Green in Tris–borate–EDTA buffer at 90 V for 1 h, also using suitable molecular weight markers. The products were examined under ultraviolet illumination.
Treatments of Calendula officinalis
Various treatments of C. officinaliswere produced on the farm.After planting plants in the same conditions, the rebellion began to different treatments.The first treatment of C. officinalis was treated with the chemical fertilizer which contain urea. The second treatment was treatedusing biologic fertilizer. The third treatment was treatedwithboth ureaand biologic fertilizers. The control group was growth in a routine condition without any fertilizer. All other conditions including process of irrigation,lighting, soil type, temperature and humidity were similar between treatments. After growing, the C. officinalisflowers were collected and immediately transferred to the Medicinal and Aromatic Research Center of the Islamic Azad University of Shahrekord.
Extraction of essential oil
Five-hundred grams of fresh flowers of each treatment were hydro distilled separately for 3 h in an all-glass Clevenger apparatus in accordance with the British pharmacopoeia method [20].
GC-mass analysis
In order to study the chemical compositions of 4 different treatments of C. officinalis flowers, the GC-mass analysis method was used using an Agilent 6890 Series II gas chromatograph (Palo Alto, USA) coupled to an Agilent 5973 quadrupole mass spectrometer with electron ionization mode (EI) generated at 70 eV (ion source at 230 °C and transfer line at 280 °C). The GC was performed using a J&W DB-5 (5% diphenyl- 95% dimethyl silicone) capillary column (30 m x 0.25 mm i.d. x 0.25 µm film), and helium was used as a carrier gas (1 mL min-1). The initial temperature was programmed from 35 °C to 60 °C (at 1 °C min-1), to 170 °C (3 °C min-1), to 200 °C (8 °C min-1), and to 280 °C (15 °C min-1), and maintained at 280 °C for 5 min. The injector port (splitless mode, 0.5 min) was at 250 °C. Retention indexes were calculated with reference to nalkanes. All compounds were identified by comparison of both the mass spectra (Wiley 275 library) and the retention index data found in the literature [21].
Antimicrobial effects of various treatments of the Calendula officinalis
Agar disc diffusion method was used for screening of antibacterial activity of C. officinalisflowers [14]. MRSA isolates of human hospital infections were spread on to Nutrient Agar (NA, Merck, Germany) medium. Paper discs were separately impregnated with 25µl of the 0.5 mg/mL plant essential oil and placed on the inoculated agar plates. All the plates were allowed to stay at room temperature for 30 min to allow diffusion of the essential oilthen incubated at 37 ºC for 24 hrs. Susceptibility of MRSA isolates against the essential oilof C. officinalisflowers were also compared with the commercially antimicrobial disks. Susceptibility of MRSA isolates were tested against ampicillin (10 u/disk), gentamycin (10 µg/disk), penicillin (10 u/disk), cotrimoxazole (30 µg/disk), lincomycin (2 µg/disk), ciprofloxacin (5 µg/disk), clindamycin (2 µg/disk), imipenem (30 u/disk), tetracycline (30 µg/disk), cefexime (5 µg/disk) and azithromycin (15 µg/disk) antibiotic agents (Oxoid, UK). The diameter of the zone of inhibition produced by essential oiland also each antibiotic disc was measured and interpreted based on theprotocol of the Clinical and Laboratory Standards Institute (CLSI) [22]. S. aureus ATCC 25923 was used as quality control organism in antimicrobial susceptibility determination.
Statistical analysis
Antimicrobial effects of the C. officinalisflowers and each antimicrobial agent were tested 3 times.Results were transferred to a Microsoft Excel spreadsheet (Microsoft Corp., Redmond, WA) for analysis. Statistical analysis was performed using SPSS/19.0 software (SPSS Inc., Chicago, IL) for significant relationship between the antibiotic susceptibility of MRSA strains of hospital infections. The chi-square test and Fisher’s exact 2-tailed test analysis were performed in this study. Statistical significance was regarded at a P value < 0.05.
Results and discussion
Of 400 samples of hospital infections collected from various types of hospital infection samples of Iranian health centers and hospitals, 100 samples (25%) were positive for presence of MRSA. Presence of the mecA gene was identified in all of the MRSA isolates of the hospital infections using the PCR method. Figure 1 shows the results of the gel electrophoresis for amplification of the mecA gene.
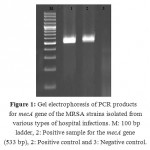 |
Figure 1: Gel electrophoresis of PCR products for mecA gene of the MRSA strains isolated from various types of hospital infections. M: 100 bp ladder, 2: Positive sample for the mecA gene (533 bp), 2: Positive control and 3: Negative control. |
Frequency of chemical components in various treatments of C. officinalis essential oil is shown in table 1. Totally, 40 different chemical components were detected in the essential oil of the C. officinalis flowers. Control group of C. officinalisharbored the most variable chemical components.Totally, 1,8-cineole (30.456%), γ-Terpinene (25.547%), Terpinolene (4.584%), α-Terpineol (4.490%) and Trans-β-ocinene (4.153%) were the most commonly detected components in the control group. Increase in the levels of1,8-cineole, trans-β-ocinene, α-copaene, α-bourbonene, α-Terpinyl acetate, β-Bisabolene, spathulenol, α-cadinol, γ-Cadinene, α-cadinene and α-Bisabolol in the C. officinalis group treated with urea manure, 1,8-cineole, trans-β-ocinene, α-copaene, α-Terpinyl acetate, α-muurolene, spathulenol, γ-Cadinene, α-cadinene and T-muurolol in the group treated with biologic manure and finally 1,8-cineole, Trans-β-ocinene, α-copaene, α-Terpinyl acetate, β-Bisabolene, spathulenol, β-Eudesmol, α-Cadinol, γ-cadinene, α-cadinene and α-Bisabolol in the group treated with both urea and biologic manures were significant (P< 0.05).
Table 1: Frequency of chemical composition of various treatments of Calendula officinalis essences.
| Number | Chemical components | Distribution in various treatments (%) | |||
| Control* | Treatment 1 | Treatment 2 | Treatment 3 | ||
| 1 | α-Thujene | 0.459 | – | – | – |
| 2 | α-Pinene | 3.032 | – | 0.553 | |
| 3 | Camphene | 0.256 | – | – | – |
| 4 | Sabinene | 0.293 | – | – | – |
| 5 | P-Pinene | 0.490 | 0.784 | – | – |
| 6 | 1-Octen-3-ol | 0.291 | – | – | – |
| 7 | 1,8-cineole | 30.456 | 53.755 | 43.512 | 50.431 |
| 8 | p-cymene | 2.495 | – | – | – |
| 9 | α-Terpinene | 0.283 | – | – | – |
| 10 | β-caryophyllene | 1.289 | – | – | – |
| 11 | Trans-β-ocinene | 4.153 | 7.61 | 11.562 | 15.834 |
| 12 | Benzene acetaldehyde | 1.354 | – | – | – |
| 13 | γ-Terpinene | 25.547 | – | – | – |
| 14 | cis-Sabinene hydrate | 2.928 | 3.319 | 2.319 | 1.863 |
| 15 | Terpinolene | 4.584 | 1.044 | 0.954 | 0.753 |
| 16 | α-phellandrene | 0.390 | – | – | – |
| 17 | α-terpineol | 0.676 | 0.403 | 1.345 | 0.945 |
| 18 | Carvacrol | 3.146 | 1.343 | 1.060 | 0.877 |
| 19 | Terpinene-4-ol | 0.369 | – | – | – |
| 20 | α-Terpineol | 4.490 | 1.493 | 1.263 | 0.967 |
| 21 | n-Dodecane | 0.279 | – | – | – |
| 22 | Carvacrol methy ether | 0.301 | – | 0.412 | 0.287 |
| 23 | α-copaene | 0.318 | 3.425 | 5.759 | 6.996 |
| 24 | α-bourbonene | 0.510 | 1.598 | 1.645 | 2.035 |
| 25 | α-Terpinyl acetate | 0.520 | 1.899 | 2.321 | 3.413 |
| 26 | Eugenol | 0.676 | 0.392 | 0.216 | 0.02 |
| 27 | n-Tetradecane | 3.146 | 1.343 | 1.060 | 0.895 |
| 28 | )E-(Caryophyllene | 0.369 | – | – | – |
| 29 | α-muurolene | 2.805 | 2.327 | 4.359 | 3.125 |
| 30 | β-Bisabolene | 0.622 | 2.880 | 2.985 | 5.612 |
| 31 | )E-(γ-Bisabolene | 0.301 | – | 0.412 | 1.534 |
| 32 | Spathulenol | 0.318 | 1.529 | 5.579 | 4.525 |
| 33 | β-Eudesmol | 0.510 | 1.598 | 1.765 | 3.456 |
| 34 | α-Cadinol | 0.520 | 1.899 | 2.078 | 2.684 |
| 35 | γ-Cadinene | 3.319 | 5.652 | 12.345 | 15.675 |
| 36 | Cadina1,4-diene | 0.950 | 1.044 | 1.887 | 1.345 |
| 37 | α-cadinene | 1.434 | 3.714 | 7.342 | 9.823 |
| 38 | α-Bisabolol | 0.024 | 0.452 | 0.793 | 1.372 |
| 39 | α-cadinol | 0.616 | 0.845 | 2.564 | 1.231 |
| 40 | T-muurolol | 0.324 | 1.178 | 2.422 | 4.102 |
Table 2 represents the antibiotic susceptibility pattern of MRSA strains isolated from hospital infections against various treatments of the C. officinalisessential oil.We found that the control group had the highest antimicrobial effects on the MRSA strains of human clinical samples, followed by treatments No 3 and 2. Treatment No 1 had the lowest antimicrobial effects. In the other hand, the prevalence of susceptibility of MRSA against the control group, treatments No 3, 2 and 1 were45%, 30%, 24% and 18%, respectively (P<0.05). MRSA strains harbored the highest levels of resistance against treatment No 1 (41%), followed by treatments No 2 (40%) and 1 (36%). Statistically significant difference was seen between the types of C. officinalis and prevalence of resistance (P<0.05).
Table 2: Antibiotic susceptibility pattern of MRSA strains isolated from hospital infections against various treatments of the Calendula officinalis methanol extracts.
| Study groups | Susceptibility pattern of 100 isolated strains of MRSA (%) | ||
| Susceptible | Intermediate | Resistant | |
| Control | 45 (45) | 30 (30) | 25 (25) |
| Treatment 1 | 18 (18) | 41 (41) | 41 (41) |
| Treatment 2 | 24 (24) | 36 (36) | 40 (40) |
| Treatment 3 | 30 (30) | 34 (34) | 36 (36) |
Table 3 shows the antibiotic susceptibility pattern of MRSA strains isolated from hospital infections against commonly used antibiotics. We found that the MRSA strains harbored the highest levels of resistance against tetracycline (95%), ampicillin (92%), penicillin (90%), gentamycin (88%) and ciprofloxacin (77%). MRSA strains harbored the highest levels of susceptibility against imipenem (95%), azithromycin (76%), cotrimoxazole (66%), clindamycin (65%) and lincomycin (59%). Significant difference was also seen between the types of antibiotic disk and pattern of antibiotic resistance (P<0.05). Antibiotic susceptibility of the treatment No 3 of the C. officinalis was entirely higher than tetracycline, ampicillin, penicillin, gentamycin, cefexime and ciprofloxacin (P<0.05).
Table 3: Antibiotic susceptibility pattern of MRSA strains isolated from hospital infections against commonly used antibiotics.
| Antibiotic agents | Susceptibility pattern of 100 isolated strains of MRSA (%) | ||
| Susceptible | Intermediate | Resistant | |
| Ampicillin | 3 (3) | 5 (5) | 92 (92) |
| Gentamycin | 3 (3) | 9 (9) | 88 (88) |
| Penicillin | 4 (4) | 6 (6) | 90 (90) |
| Cotrimoxazole | 66 (66) | 22 (22) | 12 (12) |
| Lincomycin | 59 (59) | 26 (26) | 15 (15) |
| Ciprofloxacin | 10 (10) | 13 (13) | 77 (77) |
| Clindamycin | 65 (65) | 23 (23) | 12 (12) |
| Imipenem | 95 (95) | 4 (4) | 1 (1) |
| Tetracycline | 1 (1) | 4 (4) | 95 (95) |
| Cefexime | 12 (12) | 16 (16) | 62 (62) |
| Azithromycin | 76 (76) | 14 (14) | 10 (10) |
As far as we know, the present investigation is the first prevalence report of chenmical composition and antimicrobial effects of various treatments of the C. officinalis on the MRSA strains isolates from various type of hospital infections. As said, 25% of samples were positive for MRSA which was substantially high. Extraordinary, lopsided and indiscriminate prescription of methicillin and also other types of antimicrobial agentsarethe main reasons for the high prevalence of MRSA strains in clinical infection samples. Contamination of the hospital environment and lack of personal hygiene especially in patients who were carrier of MRSA strains is another reason for the high prevalence of MRSA. Deficiency of appropriate sanitizer maybe the other reason.
MRSA strains of our research harbored the highest levels of resistance against tetracycline, ampicillin, penicillin, gentamycin, cefexime and ciprofloxacin antibiotics which was similar to the results of various previously published articles [23-29]. All of these researches have suggested the synthesis, formulation and application of novel antimicrobial agents to overcome occurrence of high antibiotic resistance in the MRSA strain of human and even animal clinical samples.
We found that enrichment of the agricultural soils with chemical and biologic fertilizers can change chemical components of essential oil extracted from them. However, the variety of chemical components and even percent of some of them in the control group which was growth in the normal condition were higher than those of chemical, biological and both groups but the levels of some specific components were increased. Totally, 1,8-cineole, β-bisabolene, trans-β-ocinene, α-copaene, spathulenol, α-bourbonene, α-terpinyl acetate, T-muurolol,α-muurolene, α-cadinol, γ-cadinene, α-cadinene,β-eudesmol,and α-bisabololchemical components had been increased in the essential oils of treatment groups compared with control. However, the antimicrobial effects of the control group were higher than other treatments but we found that antibiotic resistant strains of MRSA had the higher susceptibility to treatment No 3 than control group (P<0.05). In keeping with this, the antimicrobial effects of control group were higher than treatments No 2 and 1 (P<0.05). It seems that application of both biologic and chemical fertilizers can improve the antimicrobial effects of C. officinalis against MRSA.
In a study which was conducted by Davary Panah and Farahvash (2014) [30]the effects of biological and chemical fertilizers were significant (P<0.01 and P<0.05) on all of parameters of C. officinalis(plant height, stem diameter, essential oil, total fresh weight and total dry weight).The y showed the highest amount of essential oil was achieved in thegroup enriched with both nitroxine fertilizer andurea fertilizers which was similar to our findings. Rafiee et al. (2013) (31), Rahmani et al. (2009) (32), Bieski et al. (2013) (33), Jevdovic et al. (2013) (34) and Arab et al. (2015) (35) reported similar results. Unfortunately, there were no published data on the effects of fertilizers on the chemical components of C. officinalis essential oil.
In a study which was conducted by Efstratiou et al. (2012) [14], results showed that the C. officinalis extracts represented exceptional antibacterial activity against Pseudomonas aeruginosa, Bacillus cereus, Escherichia coli, Staphylococcus aureus, Klebsiellaaerogenes, Enterococcusfaecalis and Klebsiellapneumonia. Chakraborthy (2008) [36] reported that the lowest Minimum Inhibitory Concentration (MIC) values of C. officinalis were observed for ethanol extract, chloroform extract, water extract and petroleumether extract against the bacteria. They showed that the extracts of C.officinalis leaves were significantly effective against bothGram-positive and Gram-negative organisms. Similar studies about the high antimicrobial effects of the C. officinalis on pathogenic bacteria have been reported previously by Faria et al. (2011) [37] (Brazil), Bissa and Bohra (2011) [38] (India) and Rigane et al. (2013) [39] (Tunisia). High antimicrobial effects of the C. officinalis is due to its antimicrobial chemical components. Recent study revealed that triterpenoid like calendulaglycoside, triterpenoid saponin like faradiol,asorhamnetin3-O-neohesperidoside, quercetin and isorhamnetin are the main chemical components of the C. officinalis which are responsible for antioxidative, anti-cancer, antimicrobial, anti-inflammatory and wound healing effects [40]. In fact, application of biologic and chemical fertilizers caused to increase in the levels of these chemical components of C. officinalis. Other researchers showed that the mode of antimicrobial action of the C. officinalis may be related to its ability to inactivate microbial enzymes, inhibition of DNA gyrase, inhibit cytoplasmic membrane function[41, 42].
Results of the documented reports revealed that the main compounds within Calendula are the triterpenoids[43, 44] which are claimed to be the most important anti-inflammatory and antimicrobial components within the plant. Other constituents identified in Calendula such as the saponins, micronutrients, flavonoids, and polysaccharides, may also be responsible for the antimicrobial, anti-inflammatory, antioxidant, and wound healing effect of the plant[43-45]. The antimicrobial activity of essential oil of C. officinalis is attributed to its main chemical components including citral (aldehyde), geraniol (primary alcohol), eugenol (phenol), menthol (secondary alcohol) and cinnamic aldehyde (aldehyde) [46]. Compounds such as linalool, citral, geraniol, or thymol are more antiseptic agents in the essential oil of the C. officinalis[47].
In conclusion, the results of our survey revealed that enrichment of agricultural soil with biologic and chemicalfertilizerscan improve the medicinal and especially antimicrobial effects of C. officinalis. As it showed, MRSA had the high prevalence and also antibiotic resistance in Iran. In addition, enrichment of C. officinaliswith both biologic and chemical fertilizers had the highest effects on the antimicrobial activities of extracted essential oil on the MRSA.Simultaneous application of both types of fertilizers improved the percent of some important chemical components. MRSA strains whichhad a high levels ofresistance against variety of tested antibiotics (tetracycline, ampicillin, penicillin, gentamycin, ciprofloxacin imipenem, azithromycin, cotrimoxazole, clindamycin and lincomycin), were susceptible against the essential oil extracted from the C. officinalisenriched with both types of fertilizers. We recommended the use of C. officinalis grown in soil enriched with bothchemical and biologic fertilizers as a primary approach for synthesis and formulation of novel antibiotic drug for treatment of resistant strains of MRSA in hospital. In keeping with this, thoughtful prescription of antibiotics is another required option to prevent from extension of MRSA strains.
Acknowledgements
The authors would like to thankDr. F. Safarpoor Dehkordi at the Department of Food Hygiene and Quality Control, University of Tehran, Iran and all of the staffs of the Medicinal and Aromatic Plants Research Center of the Islamic Azad University of Shahrekord, Iran for their important technical and clinical support. The present study was supported by the Islamic Azad University of Shahrekord, Iran (IAUSHK 2001394).
References
- Noskin GA,Rubin RJ,Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E.The burden of Staphylococcus aureus infections on hospitals in the United States: an analysis of the 2000 and 2001 Nationwide Inpatient Sample Database. Arch Intern Med 2005; 165: 1756-61.
CrossRef - Solberg CO. Spread of Staphylococcus aureus in hospitals: causes and prevention. Scand J Infect Dis2000; 32: 587-95.
CrossRef - Lee BY,Bailey RR,Smith KJ, Muder RR, Strotmeyer ES, Lewis GJ, Ufberg PJ, Song Y, Harrison LH. Universal methicillin-resistant Staphylococcus aureus (MRSA) surveillance for adults at hospital admission: an economic model and analysis. Infect Control Hosp Epidemiol 2010; 31: 598-606.
CrossRef - David MZ,Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev2010; 23: 616-87.
CrossRef - Tverdek FP,Crank CW,Segreti J. Antibiotic therapy of methicillin-resistant Staphylococcus aureus in critical care. Crit Care Clin 2008; 24: 249-60.
CrossRef - Wielders CL,Fluit AC,Brisse S, Verhoef J, Schmitz FJ. mecA gene is widely disseminated in Staphylococcus aureus population. J Clin Microbiol 2002; 40: 3970-5.
CrossRef - Chambers HF,Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol2009; 7: 629-41.
CrossRef - Xia J,Gao J,Kokudo N, Hasegawa K, Tang W. Methicillin-resistant Staphylococcus aureus antibiotic resistance and virulence. Biosci Trends 2013; 7: 113-21.
- Chambers HF. Methicillin-resistant Staphylococcus aureus. Mechanisms of resistance and implications for treatment. Postgrad Med2001; 109: 43-50.
- Ansari S,Nepal HP,Gautam R, Rayamajhi N, Shrestha S, Upadhyay G, Acharya A, Chapagain ML. Threat of drug resistant Staphylococcus aureus to health in Nepal. BMC Infect Dis 2014; 14:157.
CrossRef - Pan SY,Zhou SF,Gao SH, Yu ZL, Zhang SF, Tang MK, Sun JN, Ma DL, Han YF, Fong WF, Ko KM. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid Based Complement Alternat Med 2013; 2013: 627375.
CrossRef - Arora D,Rani A,Sharma A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn Rev 2013; 7: 179-87.
CrossRef - Ukiya M,Akihisa T,Yasukawa K, Tokuda H, Suzuki T, Kimura Y. Anti-inflammatory, anti-tumor-promoting, and cytotoxic activities of constituents of marigold (Calendula officinalis) flowers. J Nat Prod 2006; 69: 1692-6.
CrossRef - Efstratiou E,Hussain AI,Nigam PS, Moore JE, Ayub MA, Rao JR. Antimicrobial activity of Calendula officinalis petal extracts against fungi, as well as Gram-negative and Gram-positive clinical pathogens. Complement Ther Clin Pract 2012; 18: 173-6.
CrossRef - Butnariu M,Coradini CZ. Evaluation of Biologically Active Compounds from Calendula officinalis Flowers using Spectrophotometry. Chem Cent J2012; 6: 35.
CrossRef - Martins FS,da Conceição EC,Bandeira ES, Silva JO Junior, Costa RM. The effects of extraction method on recovery rutin from Calendula officinalis L. (Asteraceae). Pharmacogn Mag 2014; 10: S569-73.
- 17. Pan C1, Zhan R, Ding P, Xu H. Effects of various organic fertilizers on growth and mineral nutrient absorption of Morindar officinalis. Zhong Yao Cai 2002; 25:699-701..
- Mohammad Reza Haj Seyed Hadi*, Mohsen Abarghooei Fallah, Mohammad Taghi Darzi. Influence of Nitrogen Fertilizer and Vermicompost Application on Flower Yield and Essential Oil of Chamomile (Matricaria Chamomile L.). J ChemHealth Risks 2015; 5: 235–244
- Murakami K,Minamide W,Wada K, Nakamura E, Teraoka H, Watanabe S. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J Clin Microbiol 1991; 29: 2240-4.
- British Pharmacopoeia. H. M. S. Office. 2, London, 1980: 109-110.
- Adams RP. Identification of essential oil components by gas chromatography / mass spectroscopy. Illinois:Allured Publishing Corporation, 1995: 469.
- Clinical and Laboratory Standards Institute (CLSI), Performance standards for antimicrobial susceptibility testing. Twenty-second informational supplement M100-S21. Wayne Pa: 2012.
- Tokajian S, Haddad D, Andraos R, Hashwa F, Araj G.Toxins and AntibioticResistance in StaphylococcusaureusIsolated from a MajorHospital in Lebanon. ISRN Microbiol 2011; 2011: 812049.
CrossRef - Virdis S, Scarano C, Cossu F, Spanu V, Spanu C, De Santis EP. Antibiotic Resistance in Staphylococcus aureus and CoagulaseNegativeStaphylococciIsolated from Goats with SubclinicalMastitis. Vet Med Int 2010; 2010: 517060.
CrossRef - Udo EE,Al-Sweih N,Dhar R, Dimitrov TS, Mokaddas EM, Johny M, Al-Obaid IA, Gomaa HH, Mobasher LA, Rotimi VO,Al-Asar A. Surveillance of antibacterial resistance in Staphylococcus aureus isolated in Kuwaiti hospitals. Med Princ Pract 2008; 17: 71-75.
CrossRef - Alghaithy AA, Bilal NE, Gedebou M, Weily AH. Nasalcarriage and antibioticresistance of Staphylococcusaureusisolates from hospital and non-hospitalpersonnel in Abha, SaudiArabia. Trans R Soc Trop Med Hyg 2000; 94: 504-507.
CrossRef - Młynarczyk A, Młynarczyk G, Łuczak M, Grzesik A, Lewandowska M, Jeljaszewicz J. Antibioticresistance of Staphylococcusaureusstrainsisolated in twodifferentWarsawhospitals. Med Dosw Mikrobiol 2001; 53: 217-225.
- Rijal KR,Pahari N,Shrestha BK, Nepal AK, Paudel B, Mahato P, Skalko-Basnet N. Prevalence of methicillinresistantStaphylococcusaureus in schoolchildren of Pokhara. Nepal Med Coll J 2008; 10: 192-195.
- Deng JJ, Zhu JN, Yang CL, Shu M, Xiao GG, Su M, Zhou W. Clinical distribution and drug resistance of Staphylococcus aureusisolated from hospitalized children. Sichuan Da Xue Xue Bao Yi Xue Ban 2013; 44: 159-161.
- Davary Panah D, Farahvash F. The effect of biological and chemical fertilizers on yield of Calendula officinalis in greenhouse conditions. J Nov Appl Sci 2014; 3: 1435-1438
- Rafiee H, Mehrafarin A, Qaderi A, Kalate Jari S, Naghdi Badi H. Phytochemical, Agronomical and Morphological Responses of Pot Marigold (Calendula officinalis L.) to Foliar Application of Bio-stimulators (Bioactive Amino Acid Compounds). J MedPlants 2013; 12: 48-61.
- Rahmani N, Daneshian J, Aliabadi Farahani H. Effects of nitrogen fertilizer and irrigation regimes on seed yield of calendula (Calendula officinalis L.). J Agric Biotechnol Sustainable Dev 2009; 1: 024-028.
- Bielski S, Szwejkowska B. Effect of fertilization on the development and yields of pot marigold (Calendula officinalis L.). Herba Polonica 2013; 59: 5-12.
CrossRef - Jevdovic R, Todorovic G, Kostic M, Protic R, Lekic S, Zivanovic T, Secanski M. The effects of location and the application of different mineral fertilizers on seed yield and quality of pot marigold (Calendula officinalis l.). Turk J FieldCrops 2013; 18: 1-7
- Arab A, Zamani GR, Sayyari MH, Asili J. Effects of Chemical and Biological Fertilizers on Morpho-Physiological Traits of Marigold (Calendula officinalis L.). Eur J Med Plants 2015; 8: 60-68.
CrossRef - Chakraborthy GS. Antimicrobial activity of the leaf extracts of Calendula officinalis (linn.). J Herb Med Toxicol 2008; 2: 65-66.
- Faria RL,Cardoso LM,Akisue G, Pereira CA, Junqueira JC, Jorge AO, Santos Júnior PV. Antimicrobial activity of Calendula officinalis, Camellia sinensis and chlorhexidine against the adherence of microorganisms to sutures after extraction of unerupted third molars. J Appl Oral Sci 2011; 19: 476-82.
CrossRef - Bissa S, Bohra A. Antibacterial potential of pot marigold. J Microbiol Antimicrobial 2011; 3: 51-54.
- Rigane G, Ben Younes S, Ghazghazi H, Ben Salem R. Investigation into the biological activities and chemical composition of Calendula officinalis L. growing in Tunisia. Int Food Res J 2013; 20: 3001-3007.
- Muley BP, Khdabadi SS, Banarase NB. Phytochemical Constituents and Pharmacological Activities of Calendula officinalis Linn (Asteraceae): A Review. Trop J Pharm Res 2009; 8: 455-465.
CrossRef - Cushnie TP, Lamb AJ. Antimicrobial activity of flavonoids. Int J Antimicrob Agents 2005; 26: 343 56.
CrossRef - Shah Pratibha J, Williamson Manita T. Antibacterial and Synergistic activity of Calendula officinalis Methanolic Petal Extract on Klebsiella pneumoniae Co-producing ESBL and AmpC Beta Lactamase. IntJCurrMicrobiolApp Sci 2015; 44: 107-117.
- Arora D, Rani A, Sharma A. A review on phytochemistry and ethnopharmacological aspects of genus Calendula. Pharmacogn Rev2013; 7:179-87.
CrossRef - Butnariu M, Coradini CZ. Evaluation of Biologically Active Compounds from Calendula officinalis Flowers using Spectrophotometry.Chem Cent J 2012; 6: 35.
CrossRef - Faria RL, Cardoso LM, Akisue G, Pereira CA, Junqueira JC, Jorge AO, Santos Júnior PV. Antimicrobial activity of Calendula officinalis, Camellia sinensis and chlorhexidine against the adherence of microorganisms to sutures after extraction of unerupted third molars. J Appl Oral Sci 2011; 19:476-82.
CrossRef - Hartman D, Coetzee JC. Two US practitioners’ experience of using essential oils for wound care. J Wound Care 2002; 11: 317-20.
CrossRef - Bruneton J. Farmacognosia. Zaragoza, 2001: 477.

This work is licensed under a Creative Commons Attribution 4.0 International License.





