How to Cite | Publication History | PlumX Article Matrix
Natalya Fyodorovna Beresten1, Elena Sergeevna Pavochkina1, Irina Arcadievna Ozerskaya1, Mikhail Vasulievich Medvedev2 and Vyacheslav Mikhaylovich Kitaev2
1Federal state budgetary educational institution of additional professional education Russian medical Academy of postgraduate education Barrikadnaya str., 2/1, bld. 1, Moscow, 125993, Russia.
2Federal state budgetary educational institution of additional professional education “The Institute of advanced training of Federal medical-biological Agency” Volokolamskoe sh. 91, Moscow, 125371, Russia.
DOI : http://dx.doi.org/10.13005/bbra/2357
ABSTRACT: to study the characteristics of the wall motion of the superior mesenteric and common femoral arteries in patients with arterial hypertension using the pulsed-wave tissue Doppler imaging. the study involved 140 patients (64 men and 76 women aged from 35 to 68 years) who were divided into 2 groups. The first group (n=72) consisted of patients with arterial hypertension of the 2 and 3 degrees. In the second (control) group (n=68) were included almost healthy volunteers. By means of the pulsed-wave tissue Doppler among the surveyed patients was evaluated the phase structure of the motion of the front and rear walls of the superior mesenteric artery and common femoral artery. were obtained the differences of phase structure of motion of the front and rear walls of the superior mesenteric and common femoral arteries in healthy persons and patients with arterial hypertension. These results allow us to conclude that during the remodeling of arteries in hypertension appear some peculiarities of the motion of their walls.
KEYWORDS: superior mesenteric artery; common femoral artery; pulse-wave tissue Doppler; arterial hypertension; remodeling
Download this article as:| Copy the following to cite this article: Beresten N. F, Pavochkina E. S, Ozerskaya I. A, Medvedev M. V, Kitaev V. M. Remodeling of Arteries in Arterial Hypertension. New Diagnostic Approaches to Assess the Remodeling of Elastic Arteries. Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Beresten N. F, Pavochkina E. S, Ozerskaya I. A, Medvedev M. V, Kitaev V. M. Remodeling of Arteries in Arterial Hypertension. New Diagnostic Approaches to Assess the Remodeling of Elastic Arteries. Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=16742 |
Introduction
Currently the study of remodeling of the arteries is very important. It allows detecting early symptoms of cardiovascular damage.
Remodeling is a complex process of pathophysiological changes in the structure and geometry of the arteries, occurs at the disease, aging and injury (Kovaleva, Demidenko, 2009; Polivoda, Cherepok, Sychev, 2004; Jacobsen, 2011). Remodeling appear at first as an adaptive process, and then gives way to the disruption of the structure of blood vessels in response to a direct damage by toxic substances and metabolites because of the influence of atherogenic factors or changes in hemodynamic load. Then a structural damage of blood vessels manifest by the violations of their functions, which consistently leads to circulatory disorders of the organs and violation of their functional properties (Dzau, Gibbons, 1993). The process of remodeling of arteries in arterial hypertension (AH) is the most studied and described.
Features of remodeling are largely determined by the structure of the arterial wall. It is known that the vascular wall consists of smooth muscle cells (SMC) surrounded by a highly structured extracellular matrix, consisting mainly of collagen of the I and III types, elastin and proteoglycans. Basic mechanical properties associated with the blood vessel’s function are: elongation, ductility, stiffness and elasticity. The ratio of elastin and collagen is the main determinant of arterial stiffness. Elastin stabilizes the structure of the wall, in combination with collagen prevents the irreversible deformation of the vessel by the pulsating blood flow; proteoglycans contribute to the compressibility of the vessel wall (Basu, Sen, Tyagi, 2010). By reducing the formation of desmosine the crosslinking in the structure of elastin are produced in insufficient quantities or are not produced at all. As a result, the elastic tissues become thinner, become flabby and loose (Рatel, Fine, Sandig, Mequanint, 2006). Clinically these disorders can lead to the development of aneurysm and rupture of the aorta, to the defects of the heart valves. Collagen provides rigidity of the wall, its resistance to tearing (Nissen, Cardinale, Udenfriend, 1978). Synthesis and maturation of collagen is a complex multistep process that starts in the cell, and culminating in the intercellular matrix. A special role in the regulation of its synthesis play hormones and vitamin C. Glucocorticoids inhibit collagen synthesis by inhibiting the activity of enzymes of prolylysilhydroxylase. Insufficient hydroxylation of residues of Proline and Lysine increases the sensitivity of collagen to the action of collagenase and non-specific proteases. The proportion of collagen in the blood vessels increases with age and its synthesis can be accelerated in pathological conditions, for example, in hypertension. Metalloproteinase MMP-9 is actively involved in the process of remodeling the aortic vascular bed through the regulation of cell proliferation, cell migration and contractile properties of the collagen of the vascular wall (Galis, 2002). Collagenases MMP-1, MMP-13 are carrying out the enzymatic digestion of fibrillar collagen of the I and III types (Jacobsen, 2011; Diez, 1996).
The nature of the lesion and functional consequences of arterial remodeling depend on the caliber of the arteries.
Remodeling of large vessels in patients with arterial hypertension, especially are characterized by the increase in the thickness of the walls of the arteries, their expansion and by the increase in rigidity. So the increase of aortic stiffness is a major cause of the increase of the pulse pressure and systolic blood pressure in the elderly (Milnor, 1989). The maximum change in the aortic wall is observed in the subendothelial layers of the media in the form of increasing the content of collagen and elastin fragmentation with possible calcification. The disorganization of the fibrous structures of elastin in the aortic wall with the presence of portions of mucoid degeneration or medionecrosis can cause aortic dissection and even rupture (Virmani, Avolio, Mergner, 1991). For elastic-type vessels are characteristic the hypertrophic remodeling and the parallel development of hypertension and atherosclerosis. These two processes lead to the violation of the transport function of blood vessels, which is associated with a decrease in the diameter of the nutrient artery and the subsequent development of ischemia of organs and tissues, seal blood vessels. Dysfunction of the arteries increases a pulse pressure and postload on the heart (Virmani, Avolio, Mergner, 1991; Boutouyrie, Laurent, Benetos, 1992).
An important role in the progression of arterial hypertension (AH) play the small arteries and arterioles that are related to the resistive vessels (Park, 2001). In remodeling of resistance vessels there is an increase in total peripheral resistance, which leads to the lower perfusion in the organs. The development of eutrophic inward remodelling is characteristic for the resistive vessel in AH (Mulvany, Aalkjaer, 1990).
Were described and highlighted several types of vascular remodeling in hypertension (Mulvany, Aalkjaer, 1990). Depending on changes in the diameter of vessel are distinguished: concentric “inside” remodeling, which decreases the lumen of the vessel, and eccentric “outwards”, in which the lumen of the vessel increases. Depending on the decrease/increase of cellular components of the vascular wall, or in the absence of its changes are identified several types of remodeling: eutrophic, hypertrophic and hypotrophic.
1) Eutrophyc inward remodeling is characterized by reduction of the lumen of the vessel during the change of thickness of the medial layer and is accompanied by the increasing ratio of wall/lumen of the vessel.
2) Eutrophyc outward remodeling is characterized by an increase of the lumen of the vessel without changing its cross sectional area.
3) Hypertrophic inward remodeling is characterized by the increasing of ratio of wall/lumen of the vessel caused by thickening of the medial layer
4) Hypotrophic outward remodeling is characterized by an increase of the lumen of the vessel with a decrease in the area of its cross section.
Concentric (inward) vascular remodeling usually occurs at the increased intravascular pressure or reduced blood flow, while the eccentric (outer) remodeling develops at the increasing blood flow (Walsh, Smith, Kim, 2000). Eutrophyc inward remodeling was previously described in the resistance arteries at a mild course of hypertension.
Hypertrophic inward remodeling was identified at the experimental deoxycorticosterone acetate-salt arterial hypertension (DOCA-salt hypertension), renovascular hypertension on the model of “one kidney – one clip” and in patients with symptomatic hypertension (Intengan, Schiffrin, 2000). In patients with essential hypertension during the antihypertensive therapy was marked the Eutrophyc outward remodeling. In contrast, at the anti-hypertensive therapy in SHR-rats, it is noted hypotrophic outward remodeling. Differences in the effect of antihypertensive drugs on the remodeling of the medial layer of blood vessels in experimental animals and patients with essential hypertension were due to the multifactorial nature of the disease in humans (Mulvany, 1992).
Histological characteristics of eccentric remodeling are: thinning of the vessel wall, reducing smooth muscle component of the media, its degeneration, fobroelastic intimal thickening, fragmentation of the elastic membrane with secondary fibrosis, reduction of extracellular matrix and reduction of the ratio of the thickness of the wall of the vessel to the inner diameter (Intengan, Schiffrin, 2000). In hypertrophic remodelling is an increase in the mass of the blood vessel by thickening its muscle layer (tunica media) and/or subendothelial layers of the intima due to hyperplasia of SMC in blood vessels of large and medium caliber or due to the hypertrophy of SMC in resistive vessels, and combinations of these processes (Dzau, Gibbons, 1993). The increase in the thickness of the complex intima-media is associated with activation, proliferation and migration of SMC, and also with the restruction of cellular elements and extracellular matrix of the vascular wall. The result is an increase in the thickness of the vascular wall, while the elastic properties of the arteries are reduced. Appear the stiffness and rigidity of arteries (Mulvany, Aalkjaer, 1990).
It is difficult to estimate a process of remodeling of arteries in hypertension. The main method for assessing the degree of remodeling (changes in the vascular elasticity) is sphygmometry – determination of pulse wave velocity (2). In clinical practice there is a great need in new instrumental, cost-effective research opportunities in the remodeling of large arteries. Study of the process of the remodeling of large arteries optimizes diagnosis of the impaired blood flow and the functions of internal organs in arterial hypertension, which in turn helps to identify adequate therapy and prevent the occurrence of complications.
Methods
In the study were involved 140 patients (64 men and 76 women) aged from 35 to 68 years (average age was 52,16 ± 8.9 years). All the examinees were divided into 2 groups. These groups were formed depending on the degree of elevation of clinical AP and in terms of the data of 24-hours arterial blood pressure monitoring (ABPM) according to the recommendations of the ESH/ESC, RMSAH/ARSSC (Russian medical society for arterial hypertension and All-Russian scientific society of cardiology). The first group (n=72) consisted of patients with arterial hypertension of the 2 degree (SBP 160-179 mm Hg and/or DBP 100-109 mm Hg; or according to ABPM the average daily BP – 140–147 /88-93 mm Hg) and the 3 degree (SBP 180 and higher mm Hg and DBP 110 and higher mm Hg; or according to ABPM the average daily BP – 148/94 mm Hg). In the second (control) group (n=68) were included almost healthy volunteers (with clinical BP less than 139/89 mm Hg, with a daily BP less than 125/80 mm Hg). History of the surveyed had no data of the regular antihypertensive medication. Blood pressure increase was observed within an average of 6.49 ± 3.88 years. From the survey were excluded patients with secondary forms of hypertension, patients with diabetes, acquired and congenital heart defects, disorders of contractile function of the left ventricle (ejection fraction less than 50%), with a significant atherosclerotic lesions of peripheral arteries, aneurysm of the abdominal aorta, occlusive lesions of the abdominal aorta, inflammatory diseases.
All patients underwent a comprehensive examination in the morning, including the measurement of clinical BP – 3 times with 1 minute intervals, daily blood pressure monitoring and ultrasound examination of the arteries with high peripheral resistance: superior mesenteric artery (SMA) and right common femoral artery (RCFA). Analysis of the speed of displacement of the anterior and posterior wall (AW and PW), SMA and RCFA were conducted in the mode of pulsed–wave tissue Doppler imaging (PTD) on the ultrasound scanners MyLab 70” (Esaotе, Italy), “VIVID7 (GE, USA), “HDI 5000” (Philips) using a phased array transducer, 1-4 MHz and a multifrequency linear transducer of 5.0-10.0 MHz with a simultaneous ECG recording in case of delay of breathing of the patient for 5-10 cardiac cycles. The control volume was located in the region of the longitudinal image of the AW and PW of SMA at the site of 0.5 cm distal to the origin of the SMA from the aorta and in the area of AW and PW of RCFA on the area of 2-3 cm proximal to bifurcation of RCFA. The starting point of time phases was considered the R-wave of the ECG.
Statistical analysis of data was performed using software package Statistica 8. The level of statistical significance was adopted – 0.05. The significance of differences of relative frequencies of occurrence of the set indicators was assessed by χ2-Pearson.
Results
All patients did not differ according to age, the body surface area, body mass index. Were not revealed any intergroup differences according to the heart rate.
Qualitative analysis of the motion of the front and rear walls of SMA using pulsed-wave tissue Doppler is presented in figure 1. On each schedule of the Doppler frequency offset that reflects the speed of motion of the front and rear walls of the SMA, were obtained images of the antegrade wave (S1), which appeared in the result of the systolic expansion of the SMA by the pulse wave at the measurement site. Antegrade wave (S2) has arisen as a result of the reflection pulse wave from the peripheral arteries. Retrograde wave (D) was formed during early diastole as the result of the elastic traction of the arterial wall. In the phase of late diastole, as there is a decreasing in the diameter of SMA appeared a retrograde wave (E).
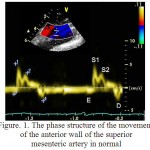 |
Figure 1: The phase structure of the movement of the anterior wall of the superior mesenteric artery in normal
|
It is important to note that in the control group the relative frequency of occurrence of a four-phase structure of the motion of the front and rear walls of the SMA was higher (0,944 and 0,875) than in the 1st group (0.069 and 0, respectively, at p<0.05). In the group of patients with hypertension a relative frequency of unilateral motions of the anterior and posterior walls of SMA amounted to 0.750, that is lower than in the control group – 0,764 (p<0.05), and a multidirectional motion of the walls of SMA amounted to 0,250, which is higher than in the control group – 0,236 (p<0.05). A relative frequency of occurrence of systolic positive peak S1 and diastolic negative peak of AW and PW in healthy and in patients with hypertension amounted to 1.00 (p<0.05). A relative frequency of occurrence of diastolic negative peak E of AW of SMA in patients with hypertension accounted 0,176, which is lower than in the group of healthy individuals – 1.00 (p<0.05). A relative frequency of diastolic peak E of PW of SMA in patients with hypertension accounted 0,147, which is lower than in the group of healthy individuals – 0,972 (p<0.05). A relative frequency of occurrence of the third antegrade systolic peak S2 of AW of SMA was lower in patients with hypertension (0,074) than in the control group (0,944, p<0.05). A relative frequency of occurrence of peak S2 of PW of SMA was absent in patients with AH and accounted 0,880 (p<0.05) in the control group.
For RCFA is characteristic the multidirectional motion of AW to the sensor and the AW from the sensor (figure 2). The peculiarities of structure of the wall motion of RCFA on the curve of PTD is in the succession of the high speed positive peak S in the phase of systole and a negative peak D in the phase of diastole for the front wall and the negative peak S and positive peak D for the posterior wall (figure 3). Peak S was resulted because of the systolic expansion of RCFA by the pulse wave, and the peak D was formed during a diastole as the result of the elastic traction of the arterial wall.
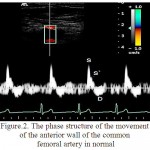 |
Figure 2: The phase structure of the movement of the anterior wall of the common femoral artery in normal
|
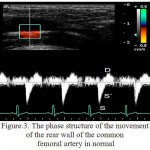 |
Figure 3: The phase structure of the movement of the rear wall of the common femoral artery in normal
|
On the range of speed of the walls of RCFA on the descending part was recorded positive peak VS` because of the reflection of the pulse wave from the peripheral arteries.
In the control group the relative frequency of occurrence of multi-directional motion of the anterior and posterior walls of RCFA were 0.960, which is higher than in patients of the 1st group (0,910; p<0.05) and the relative frequency of occurrence of unilateral motion of the anterior and posterior walls of RCFA were 0,042, which is lower than in patients of the 1st group (0,059, p<0.05).
In the control group the relative frequency of occurrence of the three-phase structure of the motion of the anterior and posterior walls were 0,972, which is higher than in the 1st group (on the front wall of RCFA a relative frequency of occurrence of waves S, S`, D amounted to 0.074, p<0.05, on the posterior wall of RCFA – 0,044, p<0.05). In the 1st group the relative frequency of occurrence of additional peaks S” for PW and AW of RCFA made – 0,926 and 0,955 (p<0.05), respectively.
Discussion
Thus, for the SMA it is characteristic the unidirectional motion of the anterior and posterior walls. In normal the schedule of Doppler frequency offset, that reflects the speed of motion of the anterior and posterior walls of the BWA has four-phase curve, consisting of successive waves E, S1, S2, D (figure 1). The structure of the wall motion of the SMA, according to the PTD, is similar to the structure of the wall motion of abdominal aorta (Huang, 2008; Laviades, Varo, Diez, 2000), probably due to the similarity of the structure of the layers of the walls.
As there is the lowering of elasticity of SMA due to degenerative changes and its remodeling in hypertension on a patients` schedule of Doppler frequency peak was disappeared peak E. Also was marked a blunting and fusion of peaks S1, S2, a reducing of their amplitude. Violation of the phase of the motion of structure of the wall was associated with the violation of the elastic properties of the SMA, which led to the early pulse wave reflection and wave S2 was superimposed on the velocity profile of the wall of SMA in the phase of early systole. The absence of E wave was probably due to the fact that in the increased stiffness of SMA in the phase of late diastole a diameter of the SMA remained virtually unchanged and the E wave was not forming (figure 4).
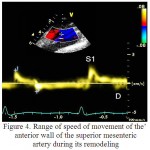 |
Figure 4: Range of speed of movement of the anterior wall of the superior mesenteric artery during its remodeling
|
For the RCFA it was characteristic the multidirectional motion of the anterior and posterior walls, with the registration of a three-phase structure, consisting of successive systolic waves S,S` and a diastolic wave D. In the remodeling of the artery it was noted the emergence of several blunt and with low amplitude systolic peaks S“, which is due to the fact that in the vascular system with a reduced elasticity, the passage of the pulse wave and its reflection is faster. In this way the reflected wave was superimposed on the temporal profile in the late systolic phase (figure 5).
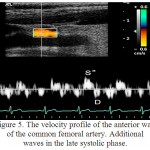 |
Figure 5: The velocity profile of the anterior wall of the common femoral artery. Additional waves in the late systolic phase.
|
Insights
In a clinical practice it is possible to assess the remodeling of large arteries in arterial hypertension using pulsed-wave tissue Doppler imaging.
Qualitative analysis of the motion of the front and rear walls of the superior mesenteric artery and the common femoral artery revealed differences in the phase structure of the wall motion in healthy individuals and in patients with arterial hypertension.
In arterial hypertension the pulsed wave tissue Doppler spectrum of the wall motion of the superior mesenteric artery has a biphasic curve of the motion (fusion, blunting and a decrease in the amplitude of systolic peaks S1 and S2, presence of an early diastolic peak D, absence of the late diastolic peak E). In arterial hypertension the pulsed wave tissue Doppler spectrum of the wall motion of the common femoral artery is characterized by the appearance of multiple blunt, reduced in the amplitude the additional peaks S”.
Reference
- Basu, P., Sen, U., Tyagi, N. (2010) Blood flow interplays with elastin: collagen and MMP: TIMP ratios to maintain healthy vascular structure and function(vol. 6, pp. 215–228.). Vasc Health Risk Manag.
- Boutouyrie, P., Laurent, S., Benetos, A. et al. (1992). Opposing effect of ageing on distal and proximal large arteries in hypertensives (vol. 10(6), pp. 87-91.). J. Hypertension.
CrossRef - Diez, J., Hernandez, M. (1996). Is the extracellular degradation of collagen type I fibers depressed in spontaneously hypertensive rats with myocardial fibrosis? (vol. 94, p. 2998). Circulation.
- Dzau, V.J., Gibbons, G.Н. (1993). Vascular remodeling mechanisms and implications (vol. 21(1), pp. 1–5.). J. CardiovascPharmacol.
- Galis, Z. S. (2002). Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling (vol. 91, p. 852.). Circulation Research.
CrossRef - Huang, Y. (2008). Abdominal aortic wall motion of healthy and hypertensive subjects: evaluation of tissue Doppler velocity imaging (vol. 36(4), p. 218–225). J. Clin. Ultrasound.
CrossRef - Intengan, H.D., Schiffrin, E.L. (2000). Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants (vol. 36, pp. 312-318). Hypertension.
CrossRef - Jacobsen, J. C. B. (2011). Significance of microvascular remodeling for the vascular flow reserve in hypertension (vol. 1(2), pp. 117–131). Interface Focus.
- Laviades, C, Varo, N, Diez, J. (2000). Transforming growth factor-b in hypertensives with cardiorenal damage (vol. 36, pp. 517–22). Hypertension.
- Milnor, W.R. (1989). Haemodynamics. 2nd ed. Baltimore: Williams & Wilkins Co.
- Mulvany, M. (1992). Vascular growth in hypertension (vol. 22, pp. 7–11). J. Cardiovasc. Pharmacol.
- Mulvany, M.M., Aalkjaer, C. (1990). Structure and function of small arteries (vol. 70, pp. 921-961). Physiology Rev.
CrossRef - Nissen, R., Cardinale, G.J., Udenfriend, S. (1978). Increased turnover of arterial collagen in hypertensive rats (vol. 75, pp. 451–453). Proc. Natl. Acad. Sci. USA.
CrossRef - Park, J.B. (2001). Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension (vol. 19, pp. 921-930). J Hypertens.
CrossRef - Virmani, R., Avolio, A.P., Mergner, W.J. et al. (1991). Effect of aging on aortic morphology in populations with high and low prevalence of hypertension and atherosclerosis (vol. 139, pp. 1119-1129). Amer. J. Pathology.
- Walsh, K., Smith, R.C., Kim, H.S. (2000). Vascular cell apoptоsis in remodeling, restenosis, and plaque rupture (vol. 87(3), pp. 184-188). Circ Res. 2000.
- Kovaleva, O. N., Demidenko, V. A. (2009). Diagnostic value of determining the intima-media complex to evaluate the features of remodeling and atherosclerotic vascular lesions (vol. 1(20), pp. 45-48). Practical angiology.
- Polivoda S. N., Cherepok, A. A., Sychev, R. A. (2004). Remodeling of elastic-type arteries in patients with hypertensive disease: diagnostic importance of pulse pressure (vol. 82(9), pp. 35-39). Klinich. medicine.
- Рatel, A, Fine, B, Sandig, M, Mequanint, K. (2006) Elastin biosynthesis: The missing link in tissue-engineered blood vessels (vol. 71, pp. 40–49). Cardiovasc Res.

This work is licensed under a Creative Commons Attribution 4.0 International License.





