How to Cite | Publication History | PlumX Article Matrix
Studies on Storage and Viability of Jamun Seeds (Syzygium cuminii Skeels)
Ch. Allaylay Devi1, G. S. K. Swamy2 and Nagesh Naik2
1Department of Fruit Science, Indira Gandhi Krishi Vishwavidhyalaya, Raipur - 492012.
2Department of Fruit Science,K.R.C. College of Horticulture, Arabhavi - 591 218, Belgaum Karnataka, India.
Corresponding Author E-mail: chongthamallaylaydevi@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2408
ABSTRACT: An experiment was conducted during 2015-2016 to study the storage and viability of jamun seeds (Syzygium cuminii Skeels). Studies revealed that the seeds possessed short viability due to recalcitrant. This could be overcome by finding a suitable storage temperature and container for storage of jamunseeds.This study showed significant differences among the various storage and treatments. The seeds stored at refrigerated (5⁰C) gave higher germination per cent at zero, 15, 30, 45, 60, 75 and 90 days after extraction (DAE)(78.56%, 73.52%, 43.33%, 60.41%, 60.09%, 53.76% and 54.36% respectively) compared to seed of other storage conditions. Seeds treated with Poly bag + Trichoderma harzianum (86.68%, 66.29%, 22.64%, 35.13%, 33.21%, 28.78% and 30.75% respectively)at zero, 15, 30, 45, 60, 75 and 90 days after extraction (DAE) gave higher germination followed by seeds kept in poly bag and seeds treated with Poly bag + Charcoal powder. Seeds stored at room temperature are not recommended for jamun seed storage because seed deterioration rate under such condition is higher within 15 days of extraction. The use of bio-control agents and moisture holding media are therefore recommended as an approach for extending the viability and potential to increase the germination of jamun seeds.
KEYWORDS: Jamun; Poly bag; Fungicides; Bio control agents; Paper bag
Download this article as:| Copy the following to cite this article: Devi C. A, Swamy G. S. K, Naik N. Studies on Storage and Viability of Jamun Seeds (Syzygium cuminii Skeels). Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Devi C. A, Swamy G. S. K, Naik N. Studies on Storage and Viability of Jamun Seeds (Syzygium cuminii Skeels). Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=17333 |
Introduction
Jamun (Syzygium cuminii Skeels) is an important, underexploited, indigenous fruit crop of our country. It belongs to Myrtaceae family. It has gained tremendous importance and recognition in recent past not only because of its hardy nature but also for its uncomparable medicinal and nutritional properties. The fruits are good source of iron, minerals, sugars and proteins. The fruits are used in the preparation of delicious beverages, jellies, jam, squash, wine, vinegar, etc. The vinegar prepared out of juice extracted from slightly unripe fruits is stomachic, carminative and diuretic, apart from having cooling and digestive properties (Thaper, 1958). The fruit syrup is a remedy for diarrhoea. The seed powder has antidiabetic properties and is a lotion for the cure of ringworm (Dastur, 1952). The importance of fruit can be judged by the premium price it fetches at metros. It has been reported that it is sold in Mumbai at the rate of rupees 150 to 300 per kilogram of fresh ripe fruits. Maximum number of jamun trees is found scattered throughout the tropical and subtropical regions of the country. Seed contain an alkaloid jambosin and a glycoside, jambolin or antimallin, which reduces or stop diastatic conversion of starch into sugars. The volatile oil from the jamun seeds can be extracted & used as an effective medicine against diabetes, heart & liver trouble (Vijayanandet al., 12 and Singh, 10).Seeds are recalcitrant and are relatively high in moisture content and possess a characteristic feature of losing their viability during desiccation.Desiccation below critical level leads to loss of viability. Prolonging the viability of seeds for more days would facilitate the availability of seeds for multiplication and also use by local farmers throughout the year.
To increase the productivity, there should be availability of good planting material along with proper management practices. The crop can be propagated sexually, through seeds and asexually by the use of grafting. Most of the planting material is produced from seeds and there are reports that do not present satisfactory germination. Since the seeds exhibit slow and less germination, pre-germination treatments may enhance the germination potential of jamun seeds. Therefore the objectives of this study are to determine the suitable storage temperature and containers for maintaining the high germination and viability rate of jamun seed after three months storage.
Materials and Methods
The present investigation was carried out during the year 2015-2016 at K.R.C. College of Horticulture, Arabhavi. The experiment was laid in Factorial completely randomized design (FCRD) with two factors viz. seeds stored in refrigerated at 5⁰C and seeds kept at room temperature. The treatments comprised of seeds kept in T1– Poly bag (300 gauge thick), T2– Poly bag + Charcoal powder (20g per kg of seed), T3– Poly bag + Bavistin (2g per kg of seed), T4– Poly bag + Saw dust (half kg per kg of seed), T5– Poly bag + Trichoderma harzianum (10 g per kg of seed) and T6– Control (Paper bag) with three replications. Observations were recorded in respect of days for initiation of germination, for fifty per cent germination and for complete germination at zero, 15, 30, 45, 60, 75 and 90 days interval. Germination percentage was recorded. The germination percentage was worked out after complete germination, i.e., after stoppage of germination. It was calculated by dividing total number of seeds sown with the number of seeds germinated and was multiplied by 100.
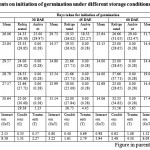 |
Table 1: Effect of different treatments on initiation of germination under different storage conditions |
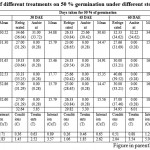 |
Table 2: Effect of different treatments on 50 %germination under different storage conditions |
Results and Discussion
The spread in acreage of Syzygium cuminii Skeels is mainly through seeds, as sexual propagation is traditionally followed for large-scale multiplication. Grafting is one of the most effective asexual methods for large scale selected clonal multiplication. The essential component for this endeavour is the availability of large-scale healthy seedling rootstocks. In the context of conflicting reviews from literature on seed germination behaviour was executed. The results highlighted that the seeds of jamun has short viability and slow germination rate.
Among the different germination inducing treatments, the jamun seeds treated with poly bag + Trichoderma harzianumin general responded well with high seed germination.Seedling emergence is primarily a function of the moisture and temperature (Savage etal., 1994). It was observed that seeds treated with poly bag + Trichoderma harzianumrecorded minimum number of days for initiation of germination(26.78, 27.57, 13.99, 12.68, 14.47, 15.00 and 15.13 respectively), minimum number of days took for 50 per cent germination (29.73, 32.86, 15.79, 14.47, 16.74, 15.22 and 15.79, respectively) at zero, 15, 30, 45, 60, 75 and 90 Days after extraction (DAE) which was on par with poly bag + Charcoal powder and poly bag + Bavistin (Table 1 to 7). Maximum number of days for initiation was recorded when seeds treated with control (14.06-30.64) followed by saw dust Table 1 to 7. And similar results were also obtained for seeds of control took maximum number of days for completion of germination (37.26-18.37) Table 1 to 7.This behaviour was irrespective of the month of sowing. These observations are in line with findings of (Jubeset al., 1975).Availability of moisture in the soil also plays an important role to initiate germination of seeds sown in any of the months. Early seed germination begins with the imbibition of water by the seed and increase in fresh weight due to increasing water uptake. The imbibition phase was followed by a period where there is little water uptake called the lag phase. The fresh weight of the seed begins to increase again as water uptake drives the emergence of the radicle. The time needed for seed to germinate is related to the difference in the seeds threshold water potential and the seeds water content. The difference is the basis for the hydro time model (Gummerson, 1986 and Bradford, 1990).Maximum number of days took for 50 per cent germination with seeds of control (Table 1 to 7). This might be due to as recalcitrant seeds must not dry below 30 to 50 per cent or else they will lose their ability to germinate (Chin and Roberts, 1980). Seed stored at ambient condition become very much prone to fungal attack which leads to loss of seed viability and vigor (Mittal, 1998).
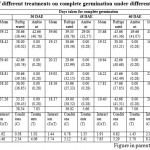 |
Table 3: Effect of different treatments on complete germination under different storage conditions |
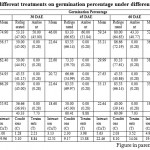 |
Table 4: Effect of different treatments on germination percentage under different storage conditions |
In case of storage conditions, the highest germination percentage was obtained when the seeds were stored in refrigerated conditions compared (43.33-78.56) to seed stored at ambient conditions (0.28-83.76) Table 1 to 7. This might be due to decline in the endogenous levels of gibberellins in litchi seeds and suggested that this decline was the limiting factor for the maintenance of viability and/or germination of these seeds, Dhillon and Sharma (1986). Temperature affects both seed germination percentage and germination rate (Kotowski, 1926). Germination rate is invariably low at low temperature but increases gradually as the temperature rises, similar to a chemical rate reaction curve (Koller, 1972). Water stress can reduce the germination percentage (Doneen and Macgillivray, 1943 and Hanks and Thorp, 1956).
Among the different moisture retention media viz., fungicide and bio-agents treated to jamun, poly bag and fresh culture of Trichoderma harzianum (10g/kg) treated seeds kept in poly bag recorded the highest per cent germination in 0, 15, 30 45, 60, 75 and 90 DAE respectively (Table 1 to 7). This may be due to Polythene bags with a wall thickness from 0.1 to 0.25 mm is thick enough to prevent excessive moisture loss and thin enough to allow some gas exchange for short-term storage of recalcitrant seeds (Anandalakshmiet al., 2005). T. harzianum might have released growth promoting substances which result in rapid germination of seeds and also caused suppression of soil borne pathogens (Priyarani et al., 1999 and Mukhopadhyay, 1987). While least per cent germination was recorded in the seeds treated with saw dust (0.5 kg per kg seed) kept in poly bag (Table 1 to 7). Seed stored in paper bags did not germinate. These containers did not prevent seeds from desiccation. The moisture contents of the seeds stored in paper bags allowed free movement of air and moisture leading to poor hygienic condition inside the container. This could be the reason for poor germination of seeds stored in these containers (Anandalakshmiet al., 2005).
The investigation revealed that freshly extracted jamun seeds gave higher per cent germination. It was gradually reducing when days increased after extraction. Seeds stored at refrigerated condition can extend the seed viability. The seeds kept in polybag and seeds treated with poly bag + Trichoderma harzianum (10 g/kg) recorded higher seed germination compared to other treatments.
Reference
- Anandalakshmi, R., Sivakumar, V., Warrier, R. R., Parimalam, R., Vijayachandran, S. N. and Singh, N.B., Seed storage studies in Syzigium cuminii, J. Tropical For. Sci., 2005.17(4): 566-573.
- Bradford, K.F., A water relation analysis of seed germination rates. Plant Physiology, 1990.94:840-849.
- Chin, H.F. and Roberts, E.H., Recalcitrant Crop Seeds.Tropical Press, 1980. Kuala Lumpur.
- Dastur, J.P., Medicinal Plants of India and Pakistan, 1952. 2nd Edition, D.B. Taraperevala Sons, Bombay.
- Dhillion, B.S. and Sharma, S.B., Endogenous level of gibberellins in relation to fruit cracking in litchi (Litchi chinensisSonn.) J. Res., Punjab Agril. Uni., 1986. 23(3): 432-434.
- Doneen, L.D. and Macgillivray, J.H., Germination (emergence) of vegetable seed as affected by different soil conditions. P. Physio., 1943. 18: 524-529.
CrossRef - Gummerson, R.J., Citusmychorrhizae potential benefits and interactions with pathogens.Hort. Sci., 1986. 21: 1302-1306.
- Hanks, R.S. and Thorp, F.C., Seedling emergence of wheat related to soil moisture content, bulk density, oxygen diffusion rate and crust strength. Proceeding American Society of Soil Sci., 1956.20: 307-310.
- Hartman, G. E. and Nelson, E.B., Mechanism of protection of seed and seedlings by biological seed treatments; Implications for practical disease control. In Seed Treatment: Prog. Pros., 1994.BCPC Monograph No. 57, pp.283-292.
- Jeffs, K. A. and Tuppen, R. J., Requirements for efficient treatment of seeds.In Seed Treatment.Ed. Jeffs, K.A., BCPC Publications, Surrey U.K., 1986.pp. 17-50.
- Jubes J. T., Martinez H., Padilla E. and Oste C. A., Effects of mechanical scarification, substrate, seed position and giberellic acid on germination in cherimoya. Review Agron. Noroeste Argentina, 1975.12: 161-172
- Koller, D., Environmental control of seed germination.In Seed Biology.Vol.2, 1972. Ed. Kozlowski, T.T., Academic Press, New York.
- Kotowski, F., Temperature relations to germination of vegetable seeds Proceedings of American Society for Hort. Sci., 1926. 23:176-184.
- Melett, L.M.M., Regional Situation of the culture of passion South-east State of Sao Paulo.In; Technical Meeting On Research Passion Fruit.,1999. p:15-19.
- Mittal, R. K., Hansen, H. J. & Thomsen, K., .Effect of seed treatments and storage temperatureon storability of Syzigium cuminii seeds.IUFRO Seed Symposium Recalcitrant Seeds.Forest Research Institute Malaysia, 1998.Kepong.
- Mukhopadhyay, A. N., Biological control of soil borne diseases of vegetables and pulses by Trichoderma species. Indian Phytopath.,1987. 40 (2): 276-277.
- Priyarani, Aggarwal, A. and Mehrotra, R.S., Growth responses in Acacia nilotica inoculated with VAM fungi (G. mossae), Rhizobium sp. And Trichoderma harzianum, Indian Phytopath.,1999.52 (2): 151-153.
- Savage F., Phelps, W.E.K. and Steckel, J.R.A, Seedling emergence modelling and the timing of irrigation durimg vegetable crop establishment.Fourth National Symposiumon Stand Establishment of Horticultural Crops, 1994. Davis, CA, pp: 103-108.
- Thaper, AR., Farm Bulletin, 1958. No. 42, ICAR, New Delhi
- Vasconcellos, M.A.S. and Cereda, E., The cultivation of sweet passion fruit, In : San Jose, A.R., Passion Fruit Production and Marketing, 1994.P:71-83.
- Yamashiro, T., and Langraf, J.H., Fusarium resistant graft of passion fruit (P. edulis f. flavicarpaDeg.). In: Brazilian Congress of Fruit, 1979. 5: 918-921.

This work is licensed under a Creative Commons Attribution 4.0 International License.





