Manuscript accepted on : 20 December 2016
Published online on: --
Plagiarism Check: Yes
Mohammad Sadegh Shirsalimian1, Abbas Akhavan Sepahy1*, Mohammad Ali Amoozegar2, Seyed Mehdi Kalantar3 and Reza Dabbagh4
1Department of Microbiology, Faculty of Biological Sciences, Islamic Azad University North Tehran Branch, Tehran, IR Iran.
2Extremophiles Laboratory, Department of Microbiology, Faculty of Biology and Center of Excellence in Phylogeny of Living Organisms, College of Science, University of Tehran, Tehran, IR Iran.
3Genetics Department, Shahid Sadoughi University of Medical Sciences, Yazd, IR Iran.
4Nuclear Sciences and Technology Research Institute, NFCS, Tehran, IR Iran.
Corresponding Author E-mail: bioshk@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2404
ABSTRACT: Despite the extreme environmental conditions, hot arid deserts have a number of microhabitats that allow the evolution of unique extremophiles so as to being adapted to desiccation and ionizing radiation. There have been several attempts to demonstrate a link between radiation resistance and desiccation tolerance phenotypes. The diversity of ionizing radiation resistant bacteria was investigated in soil and surface sand samples collected from arid Gandom Beryan area located in the Lut desert in Iran, by exposing to different periods of dehydration in a desiccator. The surviving bacteria were recovered after plating on R2A, TSA and TGY agar media. After discarding the spore-forming isolates; Twelve orange, pink, yellow and white pigmented colonies forming Gram-positive cocci-shaped were obtained. The isolated strains on R2A agar were more diverse (9 isolates) compared with those on TSA (3 isolates) and TGY (without any isolates). A gamma radiation and desiccation resistant, coccoid Gram-positive, lemon-yellow to pale-orange pigmented actinobacterium, designated strain A10, was isolated from a mixture of sand samples. Morphological and Biochemical characteristics and phylogenetic analysis based on 16S rRNA gene sequences revealed that strain A10 belonged to the genus Kocuria and was closely related to Kocuriapolaris CMS 76orT (99.4 % similarity). Strain A10 was shown to be resistant to gamma radiation up to 4 kGy and remained viable after desiccation for 28 days.
KEYWORDS: Mesophilic; Halotolerant; Kocuriapolaris; Lut Desert; Iran
Download this article as:| Copy the following to cite this article: Shirsalimian M. S, Sepahy A. A, Amoozegar M. A, Kalantar S. M, Dabbagh R. Isolation of a Mesophilic and Halotolerant Strain of Kocuriapolaris From Gandom Beryan Area in the Lut Desert of Iran, Moderately Resistant to Gamma Radiation and Desiccation. Biosci Biotech Res Asia 2016;13(4). |
| Copy the following to cite this URL: Shirsalimian M. S, Sepahy A. A, Amoozegar M. A, Kalantar S. M, Dabbagh R. Isolation of a Mesophilic and Halotolerant Strain of Kocuriapolaris From Gandom Beryan Area in the Lut Desert of Iran, Moderately Resistant to Gamma Radiation and Desiccation. Biosci Biotech Res Asia 2016;13(4). Available from: https://www.biotech-asia.org/?p=17431 |
Introduction
Although all of the challenges are found in the extreme environments, a broad diversity of microorganisms was isolated from these habitats that are called “extremophiles”. Hot arid deserts with harsh environmental conditions such as low water availability, large temperature difference between day and night, high levels of solar irradiance and lower amounts of nutrients are classified among extreme environments1. Microorganisms, with high resistance to extreme desiccation and ionizing radiation which are two main factors for oxidative stress conditions, make up a group of extremophiles. Generating free radicals known as reactive oxygen species (ROS), these two environmental stresses are the major exogenous sources causing oxidative stress in a living cell. Oxidative stress is characterized by induced diverse damages to DNA as well as proteins and lipids. A number of cellular defense mechanismsto combat oxidative stress in desiccation tolerant and radiation resistant microorganisms include efficient DNA repair systems, pigments such as carotenoids, ROS scavenging enzymes (e.g. catalases, superoxide dismutases and peroxidases) and antioxidant complexes containing divalent manganese were been described2.
There have been several studies regardingthe possible correlation between microbial desiccation tolerance and radioresistance phenotypes3,4. Due to the low environmental background gamma radiation doses on the earth, probably desiccation has been as a contributing factor to evolve extreme resistance against ionizing radiation during evolutionary process. The reason for this lies in the fact that the drylands were more common on the earth’s surface, compared to environments with high ionizing radiation flux3. An illustration of this is the isolation of various radioresistant bacterial species such as Deinococcus, Hymenobacter, Kocuria, Geodermatophilus, Kineococcus5 and cyanobacterium Chroococcidiopsis from some arid deserts around the world6.
Based on the aridity index (AI), named as the ratio of precipitation to potential evapotranspiration, areas having AI less than 1 are considered as deserts which constitute over one-fifth of total land on the earth7. In the view of climate scientists, regions with average annual rains (AAR) of less than 400 mm per year are classified as dry lands or deserts8.
The Lut desert with an area of 199000 km2, between 28° and 32° N latitude, is located in eastern part of Iran and is the 25th largest desert in the world. According to NASA’s satellite temperature records, some parts of the Lut desert have experienced surface temperature more than 70°C between 2003 and 2010, which makes it a candidate for the title of “the hottest place on the earth”.Gandom Beryan (translated as Toasted Wheat) hill extends an area of about 200 km2 and is located in 80 km north of Shahdad in Kerman province, Iran (Figure 1). Surface and hillside of this area is covered in black volcanic lava with which a considerable amount of sun’senergy is absorbed, leading to extraordinary increased temperature. Also, a saline river, locally called Shoor River, as the only permanent river, flows at the heart of the Lut desert9. The Shoor river water has a total salt concentration of approximately 13.4% which is about 4 times that of normal seawater.
 |
Figure 1: The location of the Gandom Beryan hill and sampling site used in this study. |
In order to understand microbial diversity of arid environments, many previous studies have been performed on the Atacama desert in northern Chile that were mainly conducted by NASA’ astrobiology program as a Martian surface model on earth8. Little is known about the abundance and diversity of desiccation tolerant and radio resistant microorganisms in the Lut desert and to date, the only report is the isolation of two ionizing radiation strains of Deinococcus spp. from a soil sample collected from this unique habitat10.
Members of the genus Kocuria are non-endospore forming, non-motile, Gram-positive cocci which are classified within the family Micrococcaceae in the Actinomycetals order11. At the time of the writing, the genus Kocuria comprises of 20 species (http://www.bacterio.cict.fr/k/kocuria.html) which have been isolated from a wide variety of environmental habitats such as air12, rhizoplane13, desert soil5 and salt-fermented seafood14. Resistance to various oxidative stress-promoting agents including ionizing radiation, desiccation and UV-C radiation has been reported for the genus Kocuria15,16. We isolated a new strain of Kocuria sp. from surface sand samples of Gandom Beryan hill in the Lut desert of Iran. This strain was shown resistance against desiccation and gamma radiation.
Materials and Methods
Chemicals
All chemicals and culture media were obtained from Merck and Quelab Laboratories Inc. Genomic DNA extraction kit and PCR master mix were purchased from CinnaGen and Ampliqon, respectively.
Sampling and isolation methods
In this study, desiccation tolerance was used as the selective factor to isolate ionizing radiation resistant strains. Surface sand and soil were collected from Gandom Beryan hill in the Lut desert (31° 01′ 00” N, 57° 40′ 20” E) (Figure 1) and kept at the ambient temperature until processed. Five grams of each sample in 4 replicates were placed in a sterile glass petri dish and incubated for 1, 2, 4 and 8weeks in a desiccator containing silica gel at 30°C17. A petri dish was removed from desiccator at above mentioned intervals of time, appropriate dilutions prepared in sterile 0.85% NaCl solution (w/v) and plated on R2A, TSA and TGY agar media. After incubation at 30°C for 14 days, single colonies were purified on the same medium by successive cultivation.
16S rRNA gene sequencing
To extract DNA, strain A10 was grown on R2A agar at 30°C for 3 days, and then bacterial colonies transferred to one microtube. Genomic DNA was extracted using a DNPTM Kit (CinnaGen) according to the manufacture’s protocol. The almost entire 16S rRNA gene was amplified with bacterial universal primers 8F (5ˊ-AGAGTTTGATCCTGGCTCAG-3ˊ) as forward and 1492R (5ˊ-GGTTACCTTGTTACGACTT-3ˊ) as reverse. PCR reaction was performed in a Reddy 2X PCR MasterMix (Ampliqon) using by a Thermocycler (Veriti 96-well Thermal Cycler, Applied Biosystems). The quality of PCR products were confirmed by electrophoresis on 1% agarose gel and then purified and sequenced by Macrogen services (Seoul, South Korea).
Phylogenetic analysis
Almost complete 16S rRNA gene sequence of the strain A10 (˃1400 bp) was compared to other published known sequences which are available in the GenBank database using BLASTn program (http://www.ncbi.nlm.nih.gov) and through EzTaxon server (http://www.extaxon.org), and then aligned with the highest 16S rRNA gene sequences obtained from EzTaxon using ClustalX software18. A phylogenetic tree was constructed by the neighbor-joining method19 with bootstrap values based on 1000 replicates20 contained in the MEGA software package, version 6.
Morphological and Biochemical characterization
Cell morphology was observed by using light microscopy with cultures grown on agar medium at 30°C. Gram staining was carried out by using the standard Gram reaction and was confirmed by using the KOH lysis test method21. Wet-mount method was used to examine motility. Colony morphology was observed on R2A agar and Antarctic bacterial medium (ABM; 0.5% peptone, 0.2% yeast extract and 1.5% agar; w/v) plates after incubation at 30°C for 48 h. All the Biochemical tests were performed at 30°C. Catalase, oxidase, urease and gelatinase activities, production of indole, starch hydrolysis, methyl red and Voges-Proskauer tests were assessed as described by Holding and Collee22. Growth at different temperatures (4, 10, 20, 25, 30, 37°C), the pH range (pH 4-10, at intervals of 1.0 pH unit) and various NaCl concentrations (0, 1, 3, 5, 7, 10, 15 %; w/v) were performed on ABM agar plates. The pH was adjusted by using 5M NaOH or HCl. Single carbon source tests were carried out using minimal medium : 1.05% K2HPO4, 0.45% KH2PO4, 0.1% (NH4)2SO4, 0.5% carbon source23.
Desiccation tolerance assay
Strain A10 was grown in ABM broth medium at 30°C for 24 h. Cells were collected by centrifugation, then washed and suspended in 0.85% NaCl solution to achieve an approximate concentration of 107-108 cfu ml-1. 25 µl aliquots were added to each well of standard 96-well microplate and dried inside a desiccator containing silica gel as a desiccating agent at 30°C. After 1, 2 and 4 weeks, a microplate was removed and 250 µl of sterile 0.85% NaCl solution was added to each well and pipetted to rehydrate the desiccated cells24. The number of survivors for each desiccation time were enumerated by serial dilution and plating on ABM agar. Plates were incubated at 30°C for 5 days. The percentages of survival were evaluated using viable cells present in the original inocula. Tolerance to desiccation in strain A10 was compared to Deinococcus radiodurans R1 (ATCC 13939) and E.coli (PTCC 1329) as desiccation resistant and sensitive strains, respectively. D.radiodurans R1 was grown in TGY broth (1% tryptone, 0.1% glucose, 0.5% yeast extract) at 30°C and E.coli grown in Luria-Bertani medium at 37°C.
Gamma radiation resistance assay
A washed cell suspension was prepared as described above. The final cell suspension, in triplicate, was divided into 1 ml aliquots in sterile 1.5 ml Eppendorf tubes and irradiated at room temperature from a gamma cell 60Co source (Gamma Beam-150 Type B)at different doses(up to 6 kGy in steps of 1 kGy) of gamma radiation. An unexposed tube served as a non-irradiated control. After irradiation, the treated samples were serially diluted in sterile 0.85% NaCl, plated on solid ABM medium and incubated at 30 °C for 5 days to determine the number of colony-forming units (CFUs). D. radiodurans R1 and E.coli were used as positive and negative controls, respectively. The survival ratios were calculated by dividing the surviving cells after irradiation to viable cells in the unexposed control.
Results
Identification of the microorganism
The Lut desert of Iran contains a collection of unique geological phenomena and records of the world. Gandom Beryan, a black basaltic hill, is one of these. Also, NASA’ aqua/MODIS satellite recorded one of the highest surface temperature on earth in this large desert9. During a study ofthe culturable diversity of desiccation tolerant and radiation resistant bacteria in the soil and sand dunes of Gandom Beryan area, a large number of colonies were obtained after different dehydration periods in a desiccator. Isolates which were found by microscopic examination to be spore-forming species were discarded. A total of 12 pigmented colonies were recovered on R2A, TSA and TGY agar plates. Most of these isolates were obtained on R2A agar (9 isolates). R2A medium with a lower level of nutrients if is combined with longer incubation times can to be an excellent choice for recovering and stimulating the growth of oligotrophic bacteria found in desert soils.
A mucoid lemon-yellow pigmented colony on R2A agar medium, designated as A10, was isolated after 8 weeks incubation of a mixture sand sample in a desiccator. Colonies on ABM agar are smooth, round with an entire margin, translucent and pale-orange in color. Cells are non-motile, aerobic, non-spore forming, Gram-positive cocci, occurring in diads and tetrads. Strain A10 Grows between 4 and 37°C and Optimal growth occurs at 25-30°C on ABM agar. Also, it was able to grow in the presence of 1-10% NaCl (w/v) with an optimal growth at 5% NaCl (w/v). ABM agar adjusted to pH 6–9 supports growth. The strain was positive for catalase, oxidase and starch hydrolysis but negative for indole, methyl red, Voges-Proskauer, urease and gelatinase. Lactose, D-maltose and L-arginine can be utilized as sole carbon sources for growth whereas L-arabinose and D-mannitol are not.
The almost complete 16S rRNA gene was sequenced. A search on the EzTaxon database showed that strain A10 belonged to the genus Kocuria and exhibited the most similarity with the 16S rRNA gene sequence from Kocuriapolaris CMS 76orT (99.4%) and Kocuriarosea DSM 20447T (99.2%). A phylogenetic dendogram which was generated by the neighbor-joining method confirmed the taxonomic position of the strain A10. (Figure 2).
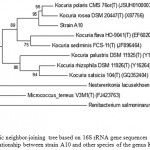 |
Figure 2: Phylogenetic neighbor-joining tree based on 16S rRNA gene sequences showing the phylogenetic relationship between strain A10 and other species of the genus Kocuria.
|
Bootstrap values (expressed as percentages of 1000 replications) greater than 50% are given at nodes. Bar, 0.5% sequence variation.
Resistance to desiccation and gamma radiation
There is a close relation between desiccation tolerance and ionizing radiation resistance mechanisms3. Desiccation tolerance for the strain A10 after exposure to different periods of dehydration was investigated, as well as D. radiodurans R1 and E. coli strains were used for comparison. After 4 weeks in a desiccator containing silica gel, strain A10 remained viable and exhibiting approximately 0.7% survival, whereas the viability of D. radiodurans R1 decreased to about 54% over the same time period which was in agreement with previous studies16,24. The survival percentages for strain A10 after 1 and 2 weeks were 18% and 6.6%, respectively. Also, the viability of the E. coli reduced dramatically upon a week of desiccation, so that there were about 200 viable organisms per 100 µl of original culture. The E. coli showed very little or no survival after 2 weeks of desiccation. Based on Figure 3, the Kocuria sp. A10 showed more resistant to desiccation than E.coli but has a lower tolerance to desiccation in comparison with D. radiodurans R1. It should be noted that as desiccator was opened at each time, the relative humidity within desiccator increased and after resealing, it decreased again. Therefore, the survival rates of bacteria may be affected by cycles of desiccation and partial dehydration3.
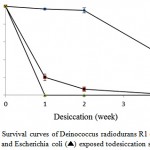 |
Figure 3: Survival curves of Deinococcus radiodurans R1 (♦), strain A10 (■) and Escherichia coli (▲) exposed todesiccation stress. |
Strain Kocuria sp. A10, D. radiodurans R1 and E. coli were tested for their resistance to different doses of gamma radiation. As shown in Figure 4, strain A10could survive up to 4 kGy and showed no loss of viability at 1 kGy dose of gamma radiation. Strain A10 cells were moderately resistant to gamma radiation with survival rates of 49%, 29% and 20% at 2, 3 and 4 kGy doses of gamma radiation, respectively. D. radiodurans R1 culture showed highly radioresistance up to 6kGy gamma rays dose without loss of survival. The cell numbers in E. coli culture was reduced dramatically after receiving a 1 kGy dose of gamma radiation.
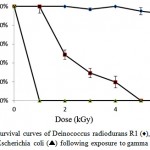 |
Figure 4: Survival curves of Deinococcus radiodurans R1 (♦), strain A10 (■) and Escherichia coli (▲) following exposure to gamma radiation. |
Discussion
According to the “desiccation adaptation hypothesis”, dehydration was a key selection pressure for the evolution of the extraordinary radio resistance in Deinococcus radiodurans, as one of the most radiation resistant organisms known. Indeed, ionizing radiation and prolonged desiccation have the similar effects on cellular macromolecules2. Therefore, arid desert habitats with a low water activity is proposed as a logical candidate for the evolution of resistance against ionizing radiation.
Kocuria polaris was isolated by Reddy et al from a cyanobacterial mat sample in McMurdo Dry Vally, Antarctica. Kocuria spp. as a moderately radio resistant and desiccation tolerant bacteria has been reported previously16. Rainey et al isolated a Kocuria sp. from arid soil of Sonoran desert exposed to 5 to 9 kGy of gamma irradiation. In this study, a radio resistant strain of Kocuria sp., named A10, was isolated from sand dunes of arid Gandom Beryan area in the Lut desert by using desiccation conditions for the isolation of radioresistant bacteria. These results provided another substantial evidence of an evolutionary relation between ionizing radiation resistance and desiccation tolerance.
The Lut desert, the natural habitat of Kocuria sp. A10, is constantly exposed to some extreme environmental stresses including high levels of solar irradiance, starvation and dehydration. Strain A10 was1800 times more resistant to desiccation than E. coli. However, D. radiodurans R1 showed higher resistance to 28 days of desiccation (65 times) when compared to strain A10.
Kocuria polaris A10 tolerates up to 10% NaCl and optimal growth occurs with 5% (w/v) NaCl. Based on these results, strain A10 has been adapted to a wide range of salt concentrations like other halo tolerant species of the genus Kocuria25. We hypothesized that the presence of a saline river in the vicinity of Gandom Beryan hill (shown in Figure 1) can justify its high tolerance to salinity. Salt deposits is resulted from evaporation of the Shoor River’s brine which can to be moved by strong winds in the Lut desert. Moreover, in contrast to its nearest phylogenetic neighbor, psychrophilic K. polaris CMS 76orT, strain A10 grows between 4 and 37 °C with an optimal growth at 25-30°C. Life in a hot desert such as the Lut desert with a high temperature difference between day and night requires such a temperature behavior.
Researches isolated a psychrotrophic Kocuria sp. ASB 107 from Ab-e-Siah radioactive spring in Ramsar, Mazandaran province, Iran. Radioactivity of Ab-e-Siah spring is approximately 146.5 Bq. l-1. The equivalent dose around the spring is about 13.48 µSv.h-126. The D10 value of ASB 107 strain (the radiation dose necessary to provide a 90% reduction in viability) for gamma rays was 2 kGy. Interestingly, radio resistance level of Kocuria sp. A10 at 2 kGy dose of gamma rays was 5 times higher than that of Kocuria sp. ASB 107.D10value for Kocuria sp. A10 was about 4.5–5 kGy. These results can confirm the “desiccation adaptation hypothesis’’. In other words, the Lut desert compared to Ab-e-Siah radioactive spring has been more suitable habitat for the evolution of high resistance against ionizing radiation.
It is well known that most radio resistant bacteria use two main strategy to achieve high radio resistance, including protection-based mechanisms by ROS-scavenging capacity such as Mn2+ complexes, carotenoids and antioxidant enzymes; and repair based mechanisms such as efficient well-developed DNA repair systems2. Finding any relevant relation among the factors mentioned to radio resistance in Kocuria sp. A10 needs further study. In addition, challenging strain A10 by other sources of oxidative stress enhances better understanding regarding the mechanisms that confer resistance against oxidative stress.
Radiation resistant and desiccation tolerant bacteria have turned into an exciting topic in microbiology. Engineering radiation-resistant bacteria is anideal candidate for bioremediation of radioactive waste sites27. Many studies on biodiversity of extremophiles in the Atacama Desert, as a model of extraterrestrial life on Mars, have been performed by NASA8. Also, understanding radio protective defense mechanisms in radiation resistant bacteria has opened not only a new promising approach for delaying aging, but also an alternative treatment for cancers2. Gandom Beryan hill in the Lut desert of Iran has not been characterized from a microbiological standpoint until today. The study of the microbial biodiversity of this area through both culture-dependent and -independent methods can help achieve the above aims in the future. In this study, Gandom Beryan hill in the Lut desert was selected for the isolation of radiation resistant bacteria. A mesophilic and halo tolerant strain of Kocuria polaris, named A10, was isolated from a mixed sand sample collected from this arid area. Strain A10 was resistant at doses of 1 to 4 kGy of gamma radiation and maintained its viability over the period of 4 weeks of desiccation. A bioremediation strategy by using radio resistant microorganisms, such as Kocuria spp, can be developed to decontaminate the radioactive waste. Genetic engineering can be used to confer multiple resistance mechanisms against extreme environmental conditions in Kocuria polaris A10. Therefore, arid Gandom Beryan hill has been targeted by our research team for the isolation of radio resistant bacteria in long term.
Acknowledgement
The authors would like to thank all the staff members of the Biotechnology Research Center of Islamic Azad University of Shahrekord Branch in Iran for their sincere support.
References
- Chanal, A., Chapon, V., Benzerara, K., Barakat, M., Christen, R. The desert of Tataouine: an extreme environment that hosts a wide diversity of microorganisms and radiotolerant bacteria. Environ Microbiol., 2006; 8(3): 514-525.
CrossRef - Slade, D., Radman, M. Oxidative stress resistance in Deinococcusradiodurans. Microbiol. Mol. Biol. Rev., 2011; 75(1): 133-191.
CrossRef - Mattimore, V., Battista, J.R. Radioresistance of Deinococcus radiodurans: functions necessary to survive ionizing radiation are also necessary to survive prolonged desiccation. J. Bacteriol., 1996; 178(3): 633-637.
CrossRef - Tanaka, M., Earl, A.M., Howell, H.A., Park, M.J., Eisen, J.A. Analysis of Deinococcusradiodurans‘s transcriptional response to ionizing radiation and desiccation reveals novel proteins that contribute to extreme radioresistance. Genetics., 2004; 168(1): 21-33.
CrossRef - Rainey, F.A., Ray, K., Ferreira, M., Gatz, B.Z., Nobre, M.F. Extensive diversity of ionizing-radiation-resistant bacteria recovered from Sonoran Desert soil and description of nine new species of the genus Deinococcus obtained from a single soil sample. Appl. Environ. Microbiol., 2005; 71(9): 5225-5235.
CrossRef - Billi, D., Friedmann, E.I., Hofer, K.G., Caiola, M.G., Ocampo-Friedmann, R. Ionizing-radiation resistance in the desiccation-tolerant cyanobacterium Chroococcidiopsis. Appl. Environ. Microbiol., 2000; 66(4): 1489-1492.
CrossRef - Makhalanyane, T.P., Valverde, A., Gunnigle, E., Frossard, A., Ramond, J.B. Microbial ecology of hot desert edaphic systems. FEMS. Microbiol. Rev., 2015; 39(2): 203-221.
CrossRef - Azua-Bustos, A., Urrejola, C., Vicuña, R. Life at the dry edge: microorganisms of the Atacama Desert. FEBS. Lett., 2012; 586(18): 2939-2945.
CrossRef - Yazdi, A., Emami, M.H., Shafiee, S.M. Dasht-e lut in iran, the most complete collection of beautiful geomorphological phenomena of desert. Open Journal of Geology., 2014; 4(6): 249-261.
CrossRef - Mohseni, M., Abbaszadeh, J., Omran, A.N. Radiation resistant of native Deinococcus spp. isolated from the Lout desert of Iran “the hottest place on Earth”. Int. J. Environ. Sci. Technol., 2014; 11(7): 1939-1946.
CrossRef - Stackebrandt, E., Koch, C., Gvozdiak, O., Schumann, P. Taxonomic Dissection of the Genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int. J. Syst. Evol. Microbiol., 1995; 45(4): 682-692.
- Zhou, G., Luo, X., Tang, Y., Zhang, L., Yang, Q. Kocuriaflava sp. nov. and Kocuriaturfanensis sp. nov., airborne actinobacteria isolated from Xinjiang, China. Int. J. Syst. Evol. Microbiol., 2008; 58(6): 1304-1307.
CrossRef - Kovács, G., Burghardt, J., Pradella, S., Schumann, P., Stackebrandt, E. Kocuriapalustris sp. nov. and Kocuriarhizophila sp. nov., isolated from the rhizoplane of the narrow-leaved cattail (Typha angustifolia). Int. J. Syst. Evol. Microbiol., 1999; 49(1): 167-173.
- Park, E.J., Roh, S.W., Kim, M.S., Jung, M.J., Shin, K.S. Kocuriakoreensis sp. nov., isolated from fermented seafood. Int. J. Syst. Evol. Microbiol., 2010; 60(1): 140-143.
CrossRef - Asgarani, E., Soudi, M.R., Borzooee, F., Dabbagh, R. Radio-resistance in psychrotrophic Kocuria sp. ASB 107 isolated from Ab-e-Siah radioactive spring. J. Environ. Radioact., 2012; 113: 171-176.
CrossRef - Gholami, M., Etemadifar, Z., Bouzari, M. Isolation a new strain of Kocuriarosea capable of tolerating extreme conditions. J. Environ. Radioact., 2015; 144: 113-119.
CrossRef - Chen, M., Alexander, M. Survival of soil bacteria during prolonged desiccation. Soil. Biol. Biochem., 1973; 5(2): 213-221.
CrossRef - Thompson, J.D., Gibson, T.J., Plewniak, F., Jeanmougin, F., Higgins, D.G. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic. Acids. Res., 1997; 25(24): 4876-4882.
CrossRef - Saitou, N., Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol., 1987; 4(4): 406-425.
- Felsenstein, J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution., 1985; 39(4): 783-791.
CrossRef - Cerny, G. Studies on the aminopeptidase test for the distinction of gram-negative from gram-positive bacteria. Eur. J. Appl. Microbiol. Biotechnol., 1978; 5(2): 113-122.
CrossRef - Holding, A., Collee, J. Chapter I Routine Biochemical Tests. Methods. Microbiol., 1971; 6(1): 1-32.
- Reddy, G.S., Prakash, J.S., Prabahar, V., Matsumoto, G.I., Stackebrandt, E. Kocuriapolaris sp. nov., an orange-pigmented psychrophilic bacterium isolated from an Antarctic cyanobacterial mat sample. Int. J. Syst. Evol. Microbiol., 2003; 53(1): 183-187.
CrossRef - Fredrickson, J.K., Shu-mei, W.L., Gaidamakova, E.K., Matrosova, V.Y., Zhai, M. Protein oxidation: key to bacterial desiccation resistance. The. ISME. J., 2008; 2(4): 393-403.
CrossRef - Tang, S.K., Wang, Y., Lou, K., Mao, P.H., Xu, L.H. Kocuriahalotolerans sp. nov., an actinobacterium isolated from a saline soil in China. Int. J. Syst. Evol. Microbiol., 2009; 59: 1316-1320.
CrossRef - Dabbagh, R., Ghafourian, H., Baghvand, A., Nabi, G., Riahi, H. Bioaccumulation and biosorption of stable strontium and 90Sr by Oscillatoriahomogenea cyanobacterium. J. Radioanal. Nucl. Chem., 2007; 272: 53-59.
CrossRef - Daly, M.J. Engineering radiation-resistant bacteria for environmental biotechnology. Curr. Opin. Biotechnol., 2000; 11(3): 280-285.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





