How to Cite | Publication History | PlumX Article Matrix
Rahamat Unissa, Merugula Sowmya, Yadla Sagarika, Veeravarapu Divya, Mundrathi Anusha, Ashwini Kumar Chauhan, Sowmya konakanchi and Sindhu Chowdary
Department of Pharmaceutical Biotechnology, Faculty of Pharmacy, Malla Reddy College of Pharmacy, Maisammaguda, Dhulapally, Secunderabad, Osmania University, Telangana, India.
Corresponding Author E-mail: srunissa@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2436
ABSTRACT: The aims of the present study were to extract, purify, characterize and evaluate the in vitro anti -proliferative activity of l-arginase against the cancer cells. L-Arginase is a therapeutic l-arginine depleter, was found to possess excellent antiproliferative activity against various arginine auxotrophic cancer cells. It depletes the levels of l-arginine by converting it into l-ornithine and urea. The marine isolate, Idiomarina sediminium H1695 used in the present study was isolated from coastal areas of Andhra Pradesh, India. The culture was grown and maintained fresh on the nutrient agar slants and preserved under refrigeration. The production of the enzyme was carried out under optimal conditions using submerged fermentation technique. The enzyme was purified to near homogeneity by ammonium sulphate precipitation technique followed by standard chromatographical methods. It was then characterized and evaluated for the anti-proliferative activity against A375-C6 and HCT-116 cells. The results revealed that the enzyme was purified to 29.87 folds after gel filtration chromatography. The purity of the enzyme was confirmed by SDS-PAGE which showed single band of protein with molecular mass 37 k Da. The purified enzyme of Idiomarina sp. exhibited maximum activity at temperature 37°C and pH 7.5. I.C.50 values for A375-C6 and HCT-116 cell lines were found to be 3.47 and 5.765 U/ml respectively. L-Arginase produced by Idiomarina sp. could be a suitable target for arginine auxotrophic cancers. Further animal studies must be carried out to develop the enzyme in the form of chemotherapeutic drug.
KEYWORDS: A375-C6 and HCT-116 cell linesargininosuccinate synthetase; H1695; Idiomarina sediminium
Download this article as:| Copy the following to cite this article: Unissa R, Sowmya M, Sagarika Y, Divya V, Anusha M, Chauhan A. K, konakanchi S, Chowdary S. Purification, Kinetic Characterization and Anti-Proliferative Activity of A Therapeutic Protein from Marine Sources. Biosci Biotech Res Asia 2017;14(1). |
| Copy the following to cite this URL: Unissa R, Sowmya M, Sagarika Y, Divya V, Anusha M, Chauhan A. K, konakanchi S, Chowdary S. Purification, Kinetic Characterization and Anti-Proliferative Activity of A Therapeutic Protein from Marine Sources. Biosci Biotech Res Asia 2017;14(1). Available from: https://www.biotech-asia.org/?p=21651 |
Introduction
Cancer is a dreadful disease characterized by abnormal proliferation of cells.1 Hence they need continuous supply of huge amount of nutrients for their growth and survival. Most of these cancer cells are dependent upon l-arginine for their growth and development, depletion of which leads to their death.2 Degradation of tumor cells using l-arginine depleters was found to be one of the promising technique for the treatment of l-arginine auxotrophic (ASS–) cancer cells.
L-Arginase is a metalloenzyme that is involved in the irreversible hydrolysis of l-arginine to l-ornithine and urea.3 Its presence has been found in all kingdoms of life ranging from unicellular prokaryotes to multicellular eukaryotes. Apart from anti-proliferative activity, it was also found useful in treatment of various physiological disorders such as rheumatoid arthritis, allergic asthma, acute neurological disorders, liver injury etc.4-7 It can also be used in the form of biosensor for estimating the levels of l-arginine in clinical samples.8
L-Arginase can convert l-arginine to l-ornithine and urea but its use for degrading tumour cells is also limited due to its low substrate affinity and stability problems at physiological p H. The properties of an enzyme usually depends upon the source from which it has been isolated. Hence considering the fact that marine water is saline in nature and chemically closer to blood plasma, it could provide enzyme which is compatible with human blood. Various marine samples were collected and several strains having the ability to produce the enzyme, have been isolated, out of which strain giving the highest yield was selected for the further studies (identified as Idiomarina sediminium).9 Production of the enzyme was carried out under submerged fermentation and various parameters were optimized for maximal production of the enzyme [10]. Moreover, literature reports suggests that the enzyme isolated from different sources differs in terms of physiological, biochemical, catalytic and immunological properties. Hence the enzyme thus produced was purified, characterized and evaluated for the presence of anti-proliferative activity against two cancer cells.
Chemicals
Chemicals used in the present study for the preparation of the media were obtained from Hi-Media laboratories. Remaining chemicals were purchased from Sigma Aldrich, Bengaluru, India.
Bacterial strain
The bacterial strain used in the present study for the production of l-arginase is Idiomarina sediminium H1695 .It was isolated from the marine samples collected from the coastal areas of Andhra pradesh. It was tested for the enzyme production and identified by sequencing method.
Inoculum
Fresh cultures were used for the preparation of the inoculum. Adequate amount of the sterile water was added into the NA slants of the microorganism, mixed well and poured into sterile nutrient broth medium. It was then incubated for one day at 370C in an incubator. 10% of suspension was used as inoculum.
Fermentation medium and fermentation conditions for l-arginase production [10]
Production of the enzyme was carried out under optimal conditions in an optimal nutritional media by submerged fermentation technique. Conditions which were found suitable for optimal production of the l-arginase were – p H 9, temperature 37°C, agitation rate of 200rpm and incubation time of 120hrs respectively (in our previous studies).
Table 1: Composition of optimal production medium
| Ingredients |
Quantities (gms/100ml.)
|
| Casein | 2 |
| L-Arginine | 2 |
| NaCl | 2.5 |
| KH2PO4 | 0.1 |
| K2HPO4 | 0.1 |
| MgSO4.7H2O | 0.05 |
| CaCl2 | 0.01 |
| NaNO3 | 0.01 |
| Tri sodium citrate | 0.01 |
| Maltose | 1.5 |
| Sea water | 100ml |
Purification
Mass of the bacterial cells were separated from the medium by centrifugation. The concentration of the protein as well as the enzyme activity present in the crude extract were estimated. The protein present in the crude extract was precipitated by ammonium sulphate fractionation method.
Ammonium Sulfate precipitation
Ammonium sulphate fractionation method was carried out in an ice bath at 40C under mild stirring conditions. Certain quantities of solid ammonium sulphate were added to the enzyme solution to get 10% and then successively raised up to 20-60% saturation. Upon precipitation the sample was centrifuged for 45 minutes at 6000rpm.The pellet was then dissolved in phosphate buffer saline. The protein content, enzyme activity and specific activity of pellet as well as supernatant were quantified after each step of ammonium sulphate precipitation. The fractions thus obtained were pooled and concentrated by dialysis.
Ion exchange Chromatography
The dialysate from the previous step was passed through DEAE-Sepharose column (1.2 X8.2 cm), previously equilibrated with Tris HCl Buffer pH 7.2 (0.01M) containing 10 mM NaCl and then eluted with 0.01M Tris HCL buffer pH 7.0 containing NaCl (0.1– 0.5 M) in gradient. The volume of fraction was 1ml at flow rate 1ml / min. Each fraction thus collected was analysed for enzyme and protein concentration. Active fractions were pooled and concentrated by lyophilization.
Gel filtration Chromatography
The concentrated enzyme from previous step was applied to the Sephadex –G-100 column (1.2 x 8.2cm) that was pre equilibrated with 0.01 M Tris HCL buffer at p H 7.2.The enzyme was eluted with same buffer conditions, then 1ml fractions were collected in each tube with flow rate of 1ml/min and quantified for enzyme and protein content. Active fractions were concentrated by dialysis and lyophilized.
The purity of the enzyme was further confirmed by SDS-PAGE (Sodium dodecyl sulphate polyacrylamide gel electrophoresis) as described by Laemmli.10
Analytical Techniques
Urea Concentrations
Enzyme activity was measured in terms of the rate of hydrolysis of l-arginine to l-ornithine and urea. The amount of the urea formed was quantified by the method followed by Archibald.11 The reaction mixture was prepared by adding 0.2ml of glycine buffer (pH =9.5), 0.5 ml of appropriately diluted enzyme and 0.1ml of manganese chloride. The mixture was incubated at 370C for 10 min.0.1ml of l-arginine was added to the mixture and incubated for further 30 min, after which the reaction was stopped by adding 1ml of perchloric acid solution. The volume was made up to 2ml with the distilled water .To this 1ml of 3:1 mixture of phosphoric acid and sulphuric acid was added and mixed well. Then 0.1ml of 4% α-isonitropropiophenone was added and sealed. The tubes were then placed in boiling water bath. After one hour, the solution was removed and cooled at room temperature. The absorbance was taken at 540nm.
Total Protein
The concentration of the protein present in the sample was determined by Lowry’s method by taking absorbance values at 660nm and the values were expressed in mg.13
Kinetic Properties of l-Arginase
In the catalytic characterization of purified l–arginase the parameters such as p H, temperature, metal ions, substrate specificity and determination of Km and V max values were studied.
Evaluation of Anti-Proliferative Activity in Vitro
The purified enzyme thus obtained was evaluated for in vitro anti-proliferative activity against A375-C6 and HCT-116 cell lines by MTT assay.14
Cell Culture
Cancer cell lines were obtained from American Type Culture Collection. Cells were maintained in RPMI – 1640 medium in CO2 incubator at 370 C with 98 % humidity and 5% CO2 gas environment. Details of the cancer cells are given under table: 2.
Table 2: Details of Cancer cell lines
| Cell line | Morphology | Origin | Species | Supplier |
| A375-C6 | Epithelial like | Skin from human | Human | American Type Culture Collection |
| HCT-116 | Epithelial | Colon | Human | American Type Culture Collection |
The enzyme l-arginase obtained from Idiomarina sediminium H1695 was tested for anti-proliferative activity against A375-C6 and HCT-116 cell lines by using MTT assay as described by Mossman, 1983.14 Stock samples were diluted with RPMI medium to required concentrations of l-arginase ranging from 0.01 to 100 IU/ml. 100µL of cells were added in 96 well plate at the density 5 × 105 cell/ml and incubated at 37oC in 5% CO2, 95 % air for 24 hrs. Then the cells were treated with various concentrations of samples in total volume (200 μl/well) for 24 hr. At 21 hrs, cells were centrifuged at 2000 rpm for 10 minutes and resuspended with 180 μL RPMI medium to rinse treated samples. A volume of 20 μl MTT solution (5 mg/ml) was added to each well and incubated at 37oC for another 3 hrs. Then the medium was aspirated to about 180 μL from each well. The formazan crystals formed were dissolved with 180 μL of dimethyl sulfoxide (DMSO). An optical density (OD) of formazan was detected by a dual wavelength UV spectrometer at 570 -650 nm reference wavelength. The percentage of cytotoxicity was compared with the untreated cell as control and determined with the equation as below.
Percentage cytotoxicity (%) = (OD of test sample / OD of control) × 100 %
The plot of per cent cytotoxicity versus sample concentration was used to calculate the concentration lethal to 50 per cent of the cells (IC50).
Data are reported as the mean + standard deviation (S.D) for at least three replicates. The percentages of cell viability were presented graphically using Microsoft excel computer program.
Results
Production of l-arginase using Idiomarina sp. under optimal conditions gave an overall yield of 137.79 mg of protein with 215.36 U of enzyme activity in the crude extract. It showed a good specific activity of 1.56 U/mg. Results of the purification of the protein are given in the table 1.Further purification of the protein by ammonium sulphate fractionation method decreased the total enzyme activity to 182.02 U with protein content of 14.68 mg respectively. But the specific activity of the enzyme was increased to 12.39 U/mg. Sample containing higher amount of the enzyme obtained in the previous step was further purified by passing through DEAE sepharose column. 1ml of the fractions were collected per minute and each fraction was quantified. Total three protein peaks were observed in the chromatogram with enzyme activity in only one peak. Fractions no. 14 yielded 6.132 mg of protein with 118.2 U of enzymatic activity per ml. It showed specific activity of 19.275 U/mg. The fraction showing highest enzymatic activity and protein content i.e. 14th fraction was loaded in Sephadex G-100 column of 1.2 X 8.2 cm dimension. Column was eluted with Tris-HCl buffer pH 7.2 (0.01M).1ml of fraction per minute were collected and quantified.9th fraction yielded 2.051 mg of protein with 95.71 U of enzymatic activity.
Sdspage
The crude extract and the samples obtained from three steps of purification process was passed through SDS PAGE to check for the purity of the sample. Crude extract i.e. lane no. 2 showed many bands showing presence of different proteins. Ammonium sulphate precipitation extract also showed the presence of several proteins. Samples form IEC showed the presence of three band indicating presence of three proteins. Finally GFC extract showed single band of purified protein. From the SDSPAGE, the molecular weight of the enzyme was found to be 37k Da (fig.3)
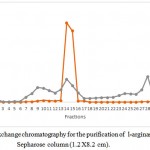 |
Figure 1: Ion exchange chromatography for the purification of l-arginase using DEAE-Sepharose column (1.2 X8.2 cm).
|
Table 3: Details of purification steps
|
Step |
Total Enzyme Activity (IU) |
Total Protein (mg) |
Specific Activity (IU/mg) |
% Recovery |
Purification Fold |
| Crude
extract |
215.36 | 137.79
|
1.56 | 100 | 1 |
| Ammonium sulphate fractionation | 182.02 | 14.68 | 12.39 | 84.51 | 7.942 |
| IEC | 118.18 | 6.132 | 19.27 | 54.87 | 12.35 |
| GFC | 95.71 | 2.051 | 46.61 | 44.44 | 29.87 |
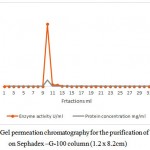 |
Figure 2: Gel permeation chromatography for the purification of l-arginase on Sephadex –G-100 column (1.2 x 8.2cm)
|
 |
Figure 3: Electrophoretic analysis of l-arginase from Idiomarina sp. at various stages of purification.Lane1 molecular weight markers, Lane 2 – crude extract, Lane 3- ammonium sulphate fractionation, Lane 4- GFC, Lane 5- IEC. Kinetic properties of l-arginase
|
The l-arginase isolated for Idiomarina sp. showed activity at wide range of p H. Maximal activity was found at p H 7.5 as shown in the fig. 4.
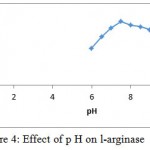 |
Figure 4: Effect of p H on l-arginase
|
L-Arginase obtained in our study showed good activity at broad range of temperature i.e. between 30-50°C being optimal activity at 37°C (fig. 5).
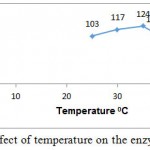 |
Figure 5: Effect of temperature on the enzyme activity
|
Manganese was the most efficient metal ion for enzyme activity. Other ions were found to repress enzyme activity (fig. 6).
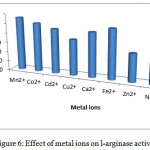 |
Figure 6: Effect of metal ions on l-arginase activity.
|
The substrate specificity of the enzyme was evaluated against various substrates such as l-arginine, l-asparginine etc. The enzyme show good affinity towards l-arginine with 112U/ml. Very less specificity towards d-arginine and least towards others (fig. 7). The enzyme was inhibited in the presence of 2mercaptoetanol, dithiothrietol and EDTA (fig. 8).A Line weaver Burk analysis gave Km and Vmax value of 1.26± 0.41 mM and 138.88 ± 0.24 U/ml/min, respectively as shown in the fig. 9.
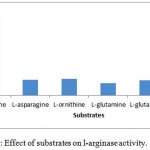 |
Figure 7: Effect of substrates on l-arginase activity.
|
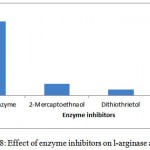 |
Figure 8: Effect of enzyme inhibitors on l-arginase activity.
|
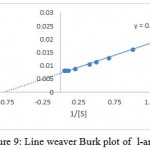 |
Figure 9: Line weaver Burk plot of l-arginase
|
In vitro anticancer activity of l-arginase the results of anti-proliferative activity are given under the table 4.
Table 4: Results of MTT assay
| Cancer cell line | IC50 |
| A375-C6 | 3.94±0.11 |
| HCT-116 | 4.82±0.21 |
Discussions
Purification of the enzyme l-arginase was achieved by 60 % of ammonium sulphate, ion exchange and gel filtration chromatography respectively. The specific activity increased from 1.56 to 46.61 U/mg for the crude extract and the final preparation respectively. The final sample was examined using SDSPAGE which revealed that it contained only one band of protein with molecular weight 37k Da. Other bacterial species produced l-arginase with variable molecular weights. The molecular weight of the l-arginase isolated from Pichia pastoris, P. chrysogenum, was found to be 37.8 and 37 k Da respectively.15,16 However, L-arginase from B. licheniformis, Iris hollandica and soybean has 33, 36.5, 35 and 60 k Da, respectively.17,18,19 Maximum l-arginase activity was observed when the enzyme was incubated at p H 7.5.A similar p H value was obtained for rat and bovine arginase.20,21 Mammalian l-arginase appears to show more alkaline optima between 9-10.5, while McGee et al reported an acidic pH optimal value of 6.1 for Helicobacter pylori.22,23 Optimum temperature of 370C was obtained for Idiomarina sp. Arginase from different sources have shown relatively high optimal temperature values. Helix pomata and Helix aspersa showed optimal temperature between 60 and 65 0C.24 L-Arginase obtained from various other sources showed optimal temperatures at 25, 30, 35 and 37 0C.23,25,26,27 Based upon Line weaver burke analysis Km and Vmax values were found to be 1.26± 0.41 mM and 138.88 ± 0.24 U/ml/min, respectively. This indicates the high substrate specificity. L-Arginases obtained from different microorganisms has different substrate affinities and probably plays different physiological roles in the enzyme activity. Higher Km values (4.8, 6 and 25 m M) for l-arginase from Penicillium chrysogenum, F. gigantica and marine mollusk Chitan latus respectively have been reported.16,28,29 On other hand, a lower km value was obtained for l-arginase from P. americana was 0.33m M.L-Arginase is a metalloenzyme that showed preference for manganese which correlates with the results obtained in the studies of Jenkinson et al ,1996,Dabir et al.,2005and Mohamed et al.,2005.In our studies l-arginase was strongly inhibited by dithiothrietol and Mercaptoethanol which comply with the results obtained in McGee et al., 2004 studies. Chelating agents such as EDTA also inhibited the enzyme activity. The I.C.50 value of A375-C6 in our studies was found to be 3.94±0.11 whereas for l-arginase isolated from Penicillium chrysogenum it was 0.18 ± 0.09. I.C50 values of Idiobacterium sp. l-arginase against HCT-116 was found to 4.82±0.21.
Conclusions
L-Arginase isolated from Idiomarina sp. has several beneficial properties needed for an ideal therapeutic enzyme. It showed a good activity and stability over wide range of p H and temperature’s .Substrate specificity of the enzyme towards l-arginine is also very high. Hence the desired effect can be achieved with small amounts of the enzyme. It also showed a very good activity against A375-C6 and HCT-116 cells. Based upon the results obtained in our present study we conclude that the l-arginase isolated from Idiomarina sediminium is physiologically active and stable enzyme with good activity against cancer cells line. Hence it could be a good target against the melanoma and colon cancers.
Acknowledgments
Authors are thankful to the authorities of Malla Reddy College of Pharmacy for providing the facilities to carry out this work.
References
- unissa R., Sudhakar M andreddy a. S. k. Condition Optimization and Production of Extracellular l-Arginine Deiminase from Vibrio alginolyticus 1374. Current Biotechnology. 2015;(4):254-260.
- unissa R., Sudhakar M and reddy a. S. k. Evaluation of in vitro Anti-proliferative Activity of l-arginine deiminase from Novel Marine Bacterial Isolate. British Microbiology Research Journal. 2016;13(5):1-10.
CrossRef - Borkovich K. A and Weiss R. L. Purification and characterization of arginase from Neurospora crassa. Journal of Biological Chemistry . 1987;262(15):7081–7086.
- Corraliza I and Moncada S. Increased expression of arginase II in patients with different forms of arthritis. Implications of the regulation of nitric oxide. The Journal of Rheumatology. 2002;29(11):2261-2265.
- Esch F., Lin K. I., Hills A., Zaman K., Baraban J. M., Chatterjee S., Rubin L., Ash D. E and Ratan R. R. Purification of a multipotent antideath activity from bovine liver and its identification as arginase: nitric oxide-independent inhibition of neuronal apoptosis. J Neurosci. 1998;18(11):4083-4095.
- Munder M. Role of arginase in asthma: potential clinical applications. Expert Review of Clinical Pharmacology. 2010;3(1):17-23.
CrossRef - Morris C. R., Poljakovic M., Lavrisha L., Machado L., Kuypers F. A and Morris S. M. Jr. Decreased Arginine Bioavailability and Increased Serum Arginase Activity in Asthma. American Journal of Respiratory and Critical Care Medicine. 2004;170:148-153.
CrossRef - Kumar K., Phathak T and Walia S. L-arginase Based Biosensor for Detection of L-arginine in Juice Samples. Nat. Prod. Plant Resour. 2012;2(4):494-499.
- unissa R., Sudhakar M and reddy a. S. k. Selective isolation and molecular identification of l-arginase producing bacteria from marine sediments. World journal of pharmacy and pharmaceutical sciences. 2015;4(6):998-1006.
- unissa R., Sudhakar M and reddy A. S. k. Optimized production of l-arginase a tumour inhibitor Isolated from marine bacteria . Int j pharm bio sci. 2015;6(2):506–517.
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685.
CrossRef - R.M. Colorimetric determination of urea. J. Biol Chem. 1945;157:507-518.
- Lowry O. H., Rosebrough N. N., Farr A. L., Randall R. Y. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265-275.
- Mosmann T. J Immunol Methods. 1983;65:55-63.
CrossRef - Zhang X., Liu J., Yu X., Wang F., Yi L., Li Z., Liu Y and Ma L. High-level expression of human arginase I in Pichia pastoris and its immobilization on chitosan to produce L-ornithine. Zhang et al. BMC Biotechnology. 2015;15:66.
CrossRef - Ashraf S. El-Sayed .,Ahmed A. S., Ayman A. D., Amgad M. R. Purification and immobilization of L-arginase from thermotolerant Penicillium chrysogenum KJ 185377.1 with unique kinetic properties as thermostable anticancer enzyme. Archives of Pharmacal Research. 2014. DOI 10.1007/s12272-014-0498-y.
CrossRef - Simon J. P and Stalon V. Purification and structure of arginase of Bacillus licheniformis. Biochimie. 1976;58(11–12): 1419–1421.
- Boutin J. P. Purification properties and subunit structure of arginase from Iris bulbs. European Journal of Biochemistry. 1982;127(2):237–243.
CrossRef - Kang J. H and Cho Y. D. Purification and properties of Arginase from Soybean, Glycine max. Axes. Plant Physiol. 1990;93(3):1230–1234.
CrossRef - Gasiorowsk I., Porembska Z., Jachimowicz J and Mochnacka I. Isoenzymes of arginase in rat tissues. Acta Biochim Pol. 1970;17(1):19-30.
- Wheatley D. N., Philip R and Campbell E. Arginine deprivation and tumor cell death: Arginase and its inhibition. Molecular and Cellular Biochemistry. 2003;244:177-185.
CrossRef - Jenkinson C. Groody. W. Cederbaum. Comparative properties of arginases. Comp. Biochem .Physiol. 11 4B(1):107-132.
- McGee D. J., Jabaleta J.,Viator R. J.,Testeman O. L., Ochoa A. C., Mendz G. L. Purification and characterization of Helicobacter pylori arginase RocF unique features among l-arginase superfamily . J. Biochem. 2001;271:1952-1962.
- Bret R., Girard C.,Riou J. Sur cetaines properties des arginase du tissue hepatopancreatique d’helix pomatia lin etd Helix aspera mull. Biochimie. 1972;54:421-430.
CrossRef - Grazi E and Magri E. Molecular characteristics of chicken liver arginase. Biochem J. 1972;126:667-674.
CrossRef - Ikemoto M., Tabata M., Murachi T and Totani M. Purification and properties of human erythrocyte arginase. Ann Clin Biochem. 1989;26(6):547-53.
CrossRef - Dabir S., Dabir P and Somvanshi B. Purification, properties and alternate substrate specificities of arginase from two different sources: Vigna catjang cotyledon and buffalo liver. Int J. Biol Sci. 2005;1(3):114-122.
CrossRef - Mohammed S. A., Fahmy T. M., Mohamed T. M., Hamdy S. M. Urea cycle of Fasiola gigantica .Purification and characterization .Comp. Biochem. B.Mol. Biol. 2005;142(3):308-16.
CrossRef - Carvajalal N., Kessi E., Bidart J., Rojas I. Properties of l-arginase from foot muscle of Chiton latus .A Comp. Physiol. (Part B). 1988;90(2):385-388.

This work is licensed under a Creative Commons Attribution 4.0 International License.





