How to Cite | Publication History | PlumX Article Matrix
T. V Mehtiyev
Central District Hospital, Ministry of Health of Azerbaijan, Azerbaijan. Sheki, M. Rasulzade str., 164, AZ 5503.
ABSTRACT: Hyperglycemia, dyslipidemia, oxidative stress, as well as such related medical conditions as hypertension, androgen-deficient states, unhealthy lifestyle (absence of physical activity, obesity and smoking) are risk factors for the development of both endothelial dysfunction (EnD) and erectile dysfunction (ED) in patients with type 2 diabetes mellitus. Therefore, the aim of our study is to reveal the role of decompensation of the disease and the duration of diabetes mellitus and age-related androgen deficiency (ARAD) in the development of this pathology. The study involved 261 patients with diabetes mellitus living in Azerbaijan at the age from 30 to 60 years and having a history of diabetes mellitus for the period from 6 months to 30 years. International Index of Erectile Function scale was applied to evaluate erectile function in patients. For the diagnosis of endothelial dysfunction we used different routine studies (anamnesis, general medical examination, clinical, biochemical and hormonal tests) as well as special studies - pharmaco-doppler sonography, assessment of peak systolic velocity, end-diastolic velocity, and modified method of examination by Mazo E.B. et al. The study revealed correlation between the index of erectile function and the level of glycated hemoglobin, and increasing duration of the disease. Also we have found that prolonged hyperglycemia in conjunction with hypoglycemia episodes leads to depression and sexual disadaptation. We offer to evaluate these facts as risk factors on a par with age factors. Results of the study suggest that long-term decompensation of diabetes mellitus (HbAIc > 6.5%), and an increase in the duration of diabetes mellitus increase the risk of endothelial dysfunction by 51.9%. Arteriogenic erectile dysfunction is observed against the background of the endothelial dysfunction. All signs of ARAD were revealed in 43.7% of men with type 2 diabetes mellitus enrolled in the study. The presence of ≥3 risk factors for cardiovascular disease in patients with type 2 diabetes mellitus increases the risk of erectile dysfunction by 83.9%.
KEYWORDS: diabetes mellitus; age-related androgen deficiency; endothelial dysfunction; erectile dysfunction
Download this article as:| Copy the following to cite this article: Mehtiyev T. V. The Role of the Risk Factors on the Development of Erectile Dysfunction in Patients with Type II Diabetes Mellitus. Biosci Biotech Res Asia 2015;12(2) |
In patients with type 2 diabetes mellitus (T2DM) decompensation of diabetes (HbAIc >6.5) leads to the development of endothelial dysfunction (EnD), while the presence of vascular disease leads to diabetic micro- and macrovasculopathies [1]. In these patients hyperglycemia, dyslipidemia, hypertension, obesity, sedentary lifestyle, and smoking play a significant role in the development of EnD. These are also risk factors for the development of cardiovascular disease (CVD) [2, 3]. Developing of oxidative stress that is associated with decompensated diabetes mellitus, decreased activity of endothelial NO-synthase deficiency of androgens and depression play an important role in the development of EnD and erectile dysfunction (ED) [4, 5]. EnD results in the broken vasoconstrictor/vasodilator balance, reduced production of NO, which is the main mediator providing relaxation of smooth muscle cells of the arteries and trabecules with subsequent increase in the inflow of arterial blood and the development of penile erection [6, 7]. Clinical studies have demonstrated that there is a causal relationship between T2DM and low testosterone levels [8]. On the other hand, an addition of androgen-deficient state to T2DM has a negative predictive value [9]. Consequently, the presence of androgen-deficient state, as well as the history and the course of diabetes mellitus are major risk factors in the development of ED.
Aim of the study
To reveal the role and limitations of diabetes mellitus decompensation, and androgen-deficient state on the development of ED in T2DM patients.
Materials and methods of the study
The study involved 261 male T2DM patients living in Sheki-Zagatalsky, and Balaken-Gabalinsky districts of Azerbaijan. The age of the patients was from 30 to 60 years (51.7 ± 8.5). The duration of diabetes mellitus was from 6 months to 30 years (17.4 ± 7.5). The study used individual medical records proposed by the World Health Organization (WHO). International Index of Erectile Function (IIEF) scale was used to evaluate erectile function in patients. According to the IIEF scale, 22-25 points were accepted as the normal range, 17-21 points – mild, 12-16 points – moderately mild, 8-11 – moderate, and 5-7 points –severe degree of the disorder [10, 11]. Hospital scale was used in order to assess the level of anxiety and depression [12]. Androgen-deficient condition or age-related androgen deficiency (ARAD) were diagnosed in the presence of the following signs listed below:
- clinical symptoms of hypogonadism,
- 37 points on the AMS questionnaire,
- total serum testosterone <12 nmol/L and/or free testosterone level <0.225 nmol/L)
The study of androgen status includes the assessment of clinical androgen deficiency using the standard international AMS questionnaire (Aging Males’ Symptoms). This questionnaire consists of 17 questions with the answers by 5-point scale, which allow to reveal sexual, psychological and somatic disorders in patients [13, 14].
Routine tests were applied for the diagnosis of ED (anamnesis, general medical examination, clinical and biochemical studies) as well as special studies: pharmaco-doppler sonography, assessment of peak systolic velocity (PSV), end-diastolic velocity (EDV) of the blood flow. Determination of serum total testosterone as well as sex hormone-binding globulin (SHBG) was performed by radioimmunoassay method with strategy electronic sr 300 (Germany) analyzer using reagents of the company Beckman Coulter (USA). Blood sample withdrawn in 8-10 a.m. was used for the analysis.
The level of free testosterone was determined using an electronic calculator-converter, developed by T. Fiers and J.M. Kaufman (available online at http://issam.ch/freetesto.htm). Consultation of urologist, ophthalmologist, psychiatrist were applied if appropriate. The level of glycated hemoglobin was measured in patients with diabetes mellitus in order to identify the state of decompensation. Vibration, temperature and tactile sensitivity were assessed in order to detect diabetic neuropathy. Modified method of examination by Mazo E.B. et al. was used in all the patients at the beginning and at the end of the study to assess an endothelial function of cavernous arteries [15]. The method consisted of the following: the diameter of the cavernous arteries is assessed by ultrasonography (US); then the pressure in a. brachialis is determined; then the cuff is placed at the base of the genitals and the air is forced in the cuff with the pressure bigger than the systolic pressure of the patient by 10 mmHg. The compression is preserved for 5 minutes. The lack of blood flow in the distal part of the cavernous artery is assessed by ultrasonography. After 5 minutes the cuff is removed. The diameter of distal parts of the cavernous arteries in the cuff area is determined by ultrasonography. The percentage of the increase in cavernous artery diameter (PICAD) is used to assess endothelial function of cavernous arteries:
PICAD = 100% x (D2-D1)/D1,
where D1 is an average assessment of the decompression diameter of both cavernous arteries; D2 is an average assessment of postcompression diameter of both cavernous arteries.
If PICAD is less than 50%, this indicates dysfunction of the cavernous arteries.
To determine endothelial dysfunction, blood pressure in a. brachialis is determined first of all. Then the air is inflated in the cuff with the pressure higher by 50 mmHg. The compression is preserved for 5 minutes. Pre- and postcompression change in the diameter of the artery is determined in %. If the increase is less than 15%, it is regarded as endothelial dysfunction.
Doppler sonography of cavernous arteries and a. Brachialis was carried out on the “Medison X-8” apparatus with LA 523 10-5 line probe.
Evaluation of the received digital data was carried out by the determining of Student’s t- test and Pearson’s χ2 test.
Results of the study
195 (74.7%) patients out of 261 involved in the study revealed decompensation of diabetes mellitus (HbAıc >6.5%). The state of decompensation was not revealed in 66 (25.3%) patients. 3* or more risk factors for CVD were identified in 219 examined (83.9%) patients, including 203 patients (77.8%) with arterial hypertension and 212 (81.2% – də) overweight or obese patients, 84 (32.1%) patients with depression, and 208 (79.8%) smokers. Psychogenic ED was revealed in 68 (26.1%) patients, arteriogenic ED was revealed in 148 (56.7%) patients, vein occlusive ED was revealed in 25 (9.6%) patients, and mixed vasculogenic ED – in 20 patients. As a result of the studies of endothelial dysfunction and a.Brachialis, and cavernous arteries, we found that patients with arteriogenic ED occur much more frequently than patients with ED of other genesis. In 48 patients suffering from arteriogenic ED and percentage assessment of postcompressive dilation of a.Brachialis below 15% the PICAD index was 28.1±1.3. In other words, it was less than 50% (Table 1). Signs of ejaculation and diabetic polyneuropathy were revealed in patients with mixed vasculogenic ED on the background of autonomous diabetic neuropathy.
Table 1: Percentage of increase in cavernous artery diameter (PICAD)
| Groups | PICAD score |
| Control (n = 50) | 83,7 ± 4.4 |
| All patients (n = 261) | 56.7 ± 6.1 ** |
| psychogenic ED (n = 68) | 80.9 ± 5.4 |
| arteriogenic ED (n = 148) | 28.1 ± 1.3 *** |
| vein occlusive ED (n = 25) | 81.6 ± 5.6 |
| mixed vasculogenic ED (n = 20) | 80.9 ± 4.6 |
Note: statistically significance in comparison with the control group:
** – P <0.01; *** – P <0.001
Monitoring of EnD and ED prevalence in the range of 3 years of age was performed, given that cardiovascular disease is a risk factor. For this purpose, patients were divided into three age groups: 30-39, 40-49, 50-59 years (Table 2). The prevalence of EnD and the ED at the age of 0-39 is 2-3 times less as compared with other age groups.
Table 2: Detection of endothelial and erectile dysfunction in patients with type 2 diabetes mellitus according to the age
| Age | Patients with EnD
(N = 148) |
Patients with ED
(N = 219) |
| 30-39 | 17
11.5 ± 2.6% |
39
17.8 ± 2.6% |
| 40-49 | 62
41.9 ± 4.1% |
79
36.1 ± 3.2% |
| 50-59 | 69
46.6 ± 4.1% |
101
46.1 ± 3.4% |
In 219 (83.9%) of 261 T2DM patients the value of IIEF was below normal. These patients were identified with erectile dysfunction of varying degrees. From 219 patients with erectile dysfunction, 64 (29.2%) were diagnosed with severe form, 35 (15.9%) with mild form, and 120 (54.8%) were diagnosed with moderate form of ED. In 55 patients with moderate ED form (25.1%) it was classified as moderately mild ED, and in 65 (29.7%) patients – as the moderate degree of ED. With the increase in the levels of glycated hemoglobin, we found a correction with increasing cases of severe ED. The results are shown in Table 3. Despite the fact that cases of ED are present both in compensated and decompensated diabetes mellitus, the incidence of the severe forms increases in case of severe decompensation of diabetes. These are vasculogenic and especially arteriogenic ED form to be the most common in these patients.
Table 3: Correlation between levels of glycated hemoglobin and IIEF
| The level of glycated hemoglobin (HbAIc) | The severity of ED according to IIEF | χ2; p | |||
| Severe | Moderate | Moderately mild | Mild | ||
| Decompensated diabetes (HbA Ic> 6.5) (n = 163) | 57
35.0 ± 3.7% |
49
30.1 ± 3.6% |
46
28.2 ± 3.5% |
11
6.7 ± 2.0% |
χ2 = 47.9;
p <0.001 |
| Compensated diabetes (HbA Ic <6.5)
(N = 56) |
7
12.5 ± 4.4% |
6
10.7 ± 4.1% |
19
33.9 ± 6.3% |
24
42.9 ± 6.6% |
|
ED of varying degree was revealed in 94 (77.7%) of 121 T2DM patients with the duration of the disease of <5 years, in 67 (83.6%) of 73 patients with the duration of the disease of 5-10 years, and in 61 (95.5%) of 64 patients with the duration of the disease of more than 10 years. The number of patients with a short duration of the disease is greater, while the prevalence of ED cases in this group is less than that in the other groups (Figure 1).
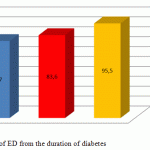 |
Figure 1: Dependency of ED from the duration of diabetes |
Vasculogenic and mixed severe forms of erectile dysfunction are more common in patients with diabetes with the duration of more than 10 years.
Analysis of the hormonal status in men with T2DM showed that the average level of total testosterone was 12.6 ± 0.4 nmol/L, the level of sex hormone-binding globulin was 45.2 ± 2.9 nmol/L, and the level of free testosterone was 0.22 ± 0.01 nmol/L. Total score according to the AMS questionnaire was determined and amounted to 35.4 ± 1.3. It was assessed in order to estimate the androgenic status in the group of men with type 2 diabetes mellitus.
According to the results, hypogonadism was diagnosed in 114 (43.7%) men with T2DM by all the three criteria and the level of total testosterone. Besides, according to the AMS questionnaire the prevalence of hypogonadism was greater by 10% and amounted to 54% (n = 141) (Table 4).
Table 4: Indicators of androgen status in T2DM patients with hypogonadism and without it
| Indicators | The group of T2DM patients with hypogonadism
n = 114 |
The group of T2DM patients without hypogonadism
n = 147 |
| Total testosterone, nmol/l | 10.3 ± 0.3 | 14.7 ± 0.5 **** |
| Free testosterone, nmol/l | 0.19 ± 0.01 | 0.24 ± 0.02 * |
| SHBG, nmol/L | 37.9 ± 3.4 | 51.4 ± 4.4 * |
| The total score according to the AMS questionnaire | 40.8 ± 1.4 | 30.8 ± 1.6 *** |
* p <0.05, *** p <0.001, **** p <0.0001 significance of the differences between groups
The prevalence of hypogonadism was analyzed in three age ranges in men with T2DM. For this purpose patients were divided into three age groups: 30-39, 40-49 and 50-59 (Table 5). Obviously, the prevalence of hypogonadism increased in T2DM patients, as well in the general population of men with age. Thus, if only 17% men in the age of 30-39 years had hypogonadism, its prevalence was 3-fold more common in men in the age of 40-49 years. This has a reliable significance compared to the age group of 30-39 years. This trend continues in the older age.
Table 5: The frequency of androgen deficiency in T2DM men in three age ranges
| Age ranges | Patients with hypogonadism |
| 30-39
n = 70 |
12
17% |
| 40-49
n = 85 |
44
52% ** |
| 50-59
n = 106 |
57
54% ** |
** <0.01, significance of differences compared to the age group of 30-39 years
In general, ED of varying degree was detected in 84% (n = 219) of T2DM men. Erectile function was normal only in 16% (n = 42) of men. The mean total score according to IIEF in the group were in the range of 16.8±0.6. The mean total score according to IIEF in patients with hypogonadism and T2DM was equal to 14.2±0.5 points, while in the group without hypogonadism it was equal to 19.0 ± 0.9 points. The difference between the two groups is statistically significant (Figure 2).
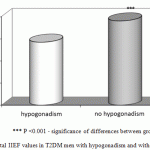 |
Figure 2: Total IIEF values in T2DM men with hypogonadism and without it |
*** P <0.001 – significance of differences between groups
Mild-moderate (62.6%) and moderate (20.2%) ED were mainly detected in the group of T2DM men with hypogonadism, while the frequency of mild and severe forms was less than 15% (Table 6). Clinical picture is somewhat different in the group of patients without hypogonadism. Patients generally have a mild form of ED (47.3%). All other violations are detected much less than in 25% of cases.
Table 6: Identification of erectile dysfunction among those with and without hypogonadism using the level of total testosterone
|
Parameters |
ED according to IIEF | |||
| severe form | moderate form | moderately mild form | mild form | |
| Hypogonadal | 4.6% | 20.6% ** | 62.6% *** | 12.2% |
| No hypogonadism | 3.5% | 7.7% | 13% | 47.3% ** |
** P <0.01, *** P <0.001 – significance of differences in the frequency of various degrees of erectile dysfunction in T2DM patients with hypogonadism and without it.
Discussion
The results obtained in the study are consistent with the literature data. McVary K.T. showed that there is correlation between the increase in the duration of diabetes, as well as the increased levels of glycated hemoglobin and erectile dysfunction [16]. Kurbatov D.T. et al. note that violation of erectile function in T2DM patients occurs 3 times more often than in the general population and the risk of affection of the small vessels of the corpora cavernosa and nerve terminal departments increases [5]. Romeo İ.K. et al. showed that in case of diabetes mellitus decompensation, cases of depression were much more common in comparison with the general population of T2DM patients. The author also noted their role in the development of erectile dysfunction [17]. All signs of ARAD were revealed in 43.7% of men with type 2 diabetes mellitus enrolled in the current study. These findings are consistent with the other studies. According to professor Vertkin A.L., ARAD is diagnosed in every second male T2DM patient [18]. The group of British scientists also demonstrated that low testosterone levels were detected in 42% of T2DM patients having risk factors [16].
We also studied the ED among patients with hypogonadism, since the presence of two violations adversely affects not only the quality of life, but also the life expectancy of men being in the reproductive age. On the other hand, endocrine disorder (including T2DM) and androgen deficiency as well as the age can cause ED [19]. According to the recommendations of the International Society for Sexual Disorders, the European Society of Urology and the American Association for Sexual Medicine, it is necessary to determine the level of testosterone in the blood in patients with erectile dysfunction and/or loss of libido (class 2a recommendation, level A of evidence) [13]. Our results show that ED is detected in all patients with hypogonadism, whereas ED is detected in 78% of cases in those with normal levels of testosterone. On the other hand, the degree of erectile dysfunction increases in case of hypogonadism. It is obvious that the presence of decompensation of T2DM increases the likelihood of ED [20]. On the other hand, the presence of androgen-deficient state also contributes to the development of ED. Given the complexity and multifactorial development of erectile dysfunction in T2DM patients, some authors propose to allocate it as a separate pathogenic form [21].
Thus, the results of this study confirmed the regularity of the inverse correlation between age and testosterone levels. Age and the presence T2DM are among the most powerful factors contributing to the increased incidence of androgen deficiency states and the development of ED. However, the presence of several risk factors, including duration of decompensation and the duration of T2DM can accelerate this process. The study noted correlation between the index of erectile function and the level of glycated hemoglobin, as well as increased duration of the disease. The presence of ≥3 CVD risk factors increases the risk of erectile dysfunction.
Conclusions
- Long-term T2DM decompensation (HbAIc >6.5%), an increase in the duration of T2DM increase the risk of endothelial dysfunction by 51.9%. Arteriogenic erectile dysfunction is observed against the background of endothelial dysfunction.
- All signs of ARAD were revealed in 43.7% of men with type 2 diabetes mellitus enrolled in the study.
- The presence of ≥3 CVD risk factors in T2DM patients increases the risk of erectile dysfunction by 83.9%. Severe erectile dysfunction forms are present more often in patients being in the state of prolonged decompensation of diabetes mellitus.
References
- Ametov, A.S. Type 2 diabetes mellitus.Problems and solutions. M.: GEOTAR-Media, 2012, pp. 183-208.
- Mazo, E.B., Hamidov, S.I., & Iremashvili, V.V. (). Erectile dysfunction. 2nd ed. with revision and supplements. M.: “Medical News Agency” LLC, 2008, p. 240.
- Mamedov, N. (). Menshealthproblemsincardiology//Moscow, 2013, p.29-44.
- Integrated approach in the clinical practice (). / Translated from English. / Under supervision ofAlan Gregoire, John P. Proyor. M .: Medicine, 2000, p. 240.
- Kurbatov, D.G., Rozhivanov, R.V., & Priymak, D.V. (). Erectile dysfunction in patients with diabetes mellitus (literature review).Russian Medical Journal. 2009; 17 (25): 1672-1676.
- Damulin, I.V., & Esilevsky, Y.M. (2014). Erectile dysfunction. Current State of Urology, 3, 95-101.
- Mamedgasanov, R.M., & Mehtiyev, T.V. (2013). Andropause and erectile dysfunction in men of reproductive age with type 2 diabetes mellitus. Problems of Endocrinology, 1, 3-8.
- Laaksonen, D.E., Niskanen, R., Purnonen, K., Nuyssonen, , Tuomainen, T.P., & Valkonen, V.P. (2004). Testosterone and sex-hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabetes Care, 27, 1036-1041.
- Hak, A.E., Witteman, J.C.M., De Jong, F.H., Geerlings, M.I., Hofmann, A., & Pols, H.A.P. (2002). Low levels of endogenous androgens increase the risk of atherosclerosis in elderly men: The Rotterdam Study. J ClinEndocrinolmetab, 87, 3632-3639.
- Rosen, R.C., Riley, A., Wagner, G., Osterloh, I.H., Kirkpatrick, J., & Mishra, A. (1997). The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology, 49, 822-830.
- Cappelleri, J., Rosen, R., Smith, M. et al. (1999). Diagnostic evaluation of the erectile function domain of the International Index of Erectile function. Urology, 54, 346-351.
- Zigmond, A., & Snaith, R. (1990). The Hospital Anxiety and Depression Scale. ActaPsychiatricaScandinavica, 67, 361-370.
- Morales, A., & Lunenfeld, B. (2002). International Society for the Study of the Aging Male. Investigation, treatment and monitoring of late onset hypogonadism of males. Official recommendations of IS-SAM. International Society for the Study of the Aging Male. Aging Male; 5(2), 74-86.
- Wang, C., Nieschlag, E., & Swerdloff, R. (2008). Investigation, treatment and monitoring of late-onset hypogonadism in males. ISA, ISSAM, EAU, EAA and ASA recommendations. European Journal of Endocrinology, 159, 507-514.
- Mazo, E.B., Hamidov, S.I., Ovchinnikov, R.I. et al. (2005). Postcompression test in the diagnosis of vasculogenicerectiledysfunction. Urology, 4, 64-69.
- McVary, K.T. (2007). Erectile dysfunction. New Engl. J. Med., 357, 2472-2481.
- Romeo, J.H., Seftel, A.D., Madhun, Z.M., & Aron, D.C. (2000). Sexual function in men with diabetes type 2: association with glycemic control. J Urol, 163, 788-791.
- Vertkin, A.L., Arinina, E.N., & Adonina, E.V. (2008). Men’s health: evolution issues and revolutionary decisions. Pharmateka, 9, 39-43
- Geoff, H. (2009). The role of androgens in men’s health. A guide for healthcare professionals (6-68). National Services for Health Improvement. UK.
- Hak, M., Kitagawa, Y., Nakamura, N., Kadano, M., Mogami, S., Hirata, C., Ichio, N., & Wada, K. (2004). Association between serum testosterone and carotid atherosclerosis in men with type 2 diabetes mellitus. Diabetes Care, 26, 1869-1873.
- Diabetes Control and Complication Trial Research Group:The effect of intensive treatment of diabetes mellitus on the development and progression of long-term complications in insulin dependent diabetes mellitus (1993). NEnglMed., 329, 977-980.

This work is licensed under a Creative Commons Attribution 4.0 International License.





