Manuscript accepted on : 07 August 2017
Published online on: --
Plagiarism Check: Yes
Gudaniya Kasthuri, A. Neethi Reddy, P. Manasa Roopa and Deveeka. k. Zamare
Sreenidhi Institute of Science and Technology Department of Biotechnology.
DOI : http://dx.doi.org/10.13005/bbra/2552
ABSTRACT: The development of drug resistance in pathogens due to over exploitation of the drugs is urging the need of finding alternative method for controlling the growth of these pathogens. The dental biofilms development leads to the dental caries which when neglected can be serious. The oral biofilm is a complex colonization of different pathogens, whereas Streptococcus mutans paves the path for the formation of these stubborn biofilms by makeing use of dietary sugars and accumulates on tooth surface through exopolysaccharides (EPS). The current life style and eating habits are favoring the plaque development leading in to dental caries. Here we amide to eliminate the dread of development of drug resistance in to the pathogens by mechanical damage and combination of treatment as an alternate option for destruction of Streptococcus mutans. This was accomplished by the application of Iron Nanoparticles (FeNP). It was observed that the antimicrobial activity of natural and synthetic drugs can be enhanced by the synergy of FeNP. The popular natural products like Syzygium aromaticum (Clove) buds, Azadirachta indica (Neem) leaves and Camellia sinensis (Green tea) leaves were tested for their activity against the Streptococcus mutans. The enhanced antimicrobial activity were tested by treating Streptococcus mutans with different combination of treatments like only plant extract, Plant extract with FeNP, plant extract with FeNP and Amoxicillin to obtain a comparative analysis of its effects. The study showed that the activity of antimicrobial agent can be enhanced when added with FeNP. The FeNP were green synthesized from the extracts of Syzygium aromaticum (Clove) buds, Azadirachta indica (Neem) leaves and Camellia sinensis (Green tea) leaves and was characterized by UV,FTIR and SEM studiesand zeta potential studies.
KEYWORDS: Application of iron nanoparticles;antimicrobial activity; Dental biofilm
Download this article as:| Copy the following to cite this article: Kasthuri G, Reddy A. N, Roopa P. M, Zamare D. K. Application of Green Synthesized Iron Nanoparticles for Enhanced Antimicrobial Activity of Selected Traditional and Commonly Exploited Drug Amoxicillin Against Streptococcus mutans. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Kasthuri G, Reddy A. N, Roopa P. M, Zamare D. K. Application of Green Synthesized Iron Nanoparticles for Enhanced Antimicrobial Activity of Selected Traditional and Commonly Exploited Drug Amoxicillin Against Streptococcus mutans. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27093 |
Introduction
Organized Clusters of bacterial cells firmly adherent to surfaces and enmeshed in a 3D matrix of extracellular polymeric substances, such as exopolysaccharides (EPS) leads to the formation of biofilms leading to many infectious diseases [L. Hall-Stoodleyet.al 2004, D. Lebeaux et.al 2014]. One of the most prevalent and costly oral disease dependent on the biofilms formation is the dental caries. This Caries causing biofilms also known as plaque [ H. Koo,et.al 2013] which are developed when virulent species, such as Streptococcus mutans and other bacteria, utilize dietary sugars to accumulate on tooth surface through EPS production, and acidify the local environment [H. Koo,et.al 2013, N. Takahashi et.al 2011]. Streptococcus mutans, a Gram-positive facultative anaerobic coccus that is part of the normal flora of the mouth, has been consistently associated with human dental caries [Loesche, 1986]. This condition can lead to severe and chronic periodontal problems such as gingivitis and chronic periodontitis.
Inhibition of the biofilm has been always the topic of concern among the researchers round the world. Application of nonmaterial for the inhibition of this biolilm due to their incredibly high surface to volume ratio which provides tremendous face in diffusion, therefore this study was amid to find out different potential combination of antimicrobial agents for the inhibition of Streptococcus mutans which lays out the foundation for the other fungi and bacteria pathogens.The antibacterial properties of some medicinal plants are been in use for quite a long time. Among them the Syzygium aromaticum (Clove), Azadirachta indica (Neem) and Camellia sinensis (Green tea) are benign used traditionally for the dental health and are known for their antimicrobial and antioxidant properties. But with the current changing life style and changing food habits relying merely on this product do not solve the problem related to oral health issues. [Sharma.et.al 2016] all though many commercial products in the field of dental care claims to have incorporation of these natural ingredients in their products but the selection of the best still remains an unsolved puzzle for the layman.
However constant advance research in the field of oral health is demanding all the time due to the increasing awareness among consumers. In this study we have proposed a cost-effective and safe strategy to overcome the dental platonic problem by application of green synthesized Iron nanoparticles (FeNPs) for enhancing the activity of natural products like Clove, Neem, Green tea and also commercially available synthetic antibiotic like amoxicillin which are used widely for the treatment of dental caries.
FeNps were green synthesized with the help of Clove buds, Neem leaves, Green tea leaves and characterized by UV, FTIR, SEM and zeta potential studies. Iron nanoparticles are safe and nontoxic hence they become a secure option for its biomedical applications. This study confirmed the enhanced antimicrobial activity of the natural and synthetic product with synergism with FeNp.
Materials and Methodology
Reagents
Ferric Chloride was obtained from Sigma Aldrich and concentration of 0.001 M was acquired by using deionized (Di) water. The Syzygium aromaticum (Clove) buds, Azadirachta indica (Neem) leaves and Camellia sinensis (Green tea) leaves were procured from authorized medicinal plant dealers in Hyderabad. The material was thoroughly washed with deionized water three times and grinded in to a paste and dried in to powdered form with help of hot air oven. 100 gram of powered from the sample obtained were boiled with 100ml of deionzed water. The extract was then filtered through Whatman No. 1 filter paper and further centrifuged 2500 rpm for 15 minutes and supernatant was collected and stored at 40c till further used.
Green Synthesis of Iron Nanoparticles and Characterization of Nanoparticles
For green synthesis of Iron Nanoparticles both the precursors (Aqueous extract of medicinal plant source and 0.1m Fecl3) were added in 1:1 ration at 500c with constantan stirring. The palate was then separated by centrifugation, double washed with Di water and stored for further characterization and anti pleacktonic studies. After the synthesis of nanoparticles, they were run under UV-Vis spectroscopy, FTIR (Fourier Transform Infrared Spectroscopy, SEM (Scanning Electron Microscopy) and zeta potential studies for characterization.
Microbial Sample Collection
The samples were collected by scraping the tooth surface with cotton swab. The swab was then dipped into sterilized nutrient broth and then incubated overnight at 37°C. The culture from the overnight grown culture was used further for studies by streaking on selective medium i.e tryptone-yeast-cysteine-sucrose-bacitracin (TYCSB) obtained from Sigma Aldrich and prepped as instructed by the manufacturer.
Minimum Inhibitory Concentration Studies
Determination of minimum inhibitory concentration values of FeNPs , amoxicillin, Syzygium aromaticum, Azadirachta indica, Camellia sinensis extracts, and synergistic effect of this three extract, FeNp and amoxicillin together in 1:1:1 ratio were studied against Streptococcus mutans using the broth microdilution method. The microbial suspensions were prepared as described CLSI (Clinical and Laboratory Standards Institute). The MIC was determined over a range from 120 – 0.015 μg/mL by the serial dilution method, as described CLSI-2013.
Minimum Bactericidal Concentration
Minimum bactericidal concentration (MBC) was determined from MIC range using Spread plate method. Mueller Hinton agar in Petri dishes were sub-cultured from tubes without growth and incubated at 37°C for 24 h. The petri dishes were observed macroscopically. The highest dilution that yielded no bacterial colony on a solid medium was taken as MBC.
Zone of Inhibition Studies
Petri plates containing 20ml (TYCSB) were seeded with 24hr culture of bacterial strains. Wells were prepared in to the plates. 20 µl of the Syzygium aromaticum, Azadirachta indica, and Camellia sinensis extracts, suspended Iron nanoparticles (FeNP) and amoxicillin suspension were added into the wells individually. The other set of plates were loaded with the combination of FeNP and plant extract i.e. FeNP + Syzygium aromaticum (FeNPSa), FeNP + Azadirachta indica (FeNPAi), FeNP + Camellia sinensis (FeNPCs). The enhanced activity of amoxicillin was studied by suspending the FeNp +Plant extract +Amoxicillin, (FeNPSa+Am, FeNPAi+Am and FeNPCs+Am)
The plates were then incubated at 37°C for 24 hours. The antibacterial activity was assayed by measuring the diameter of the inhibition zone formed around the well (NCCLS, 1993) amoxicillin disc was used as a positive control.
Statistical Analysis
Experiments were performed in triplicate. Data are represented as a mean with standard deviation. For statistical analysis ANOVA was performed followed by a Tukey’s HSD post hoc test, and the value 0.05 was considered to be significant.
Results and Discussion
Green Synthesis of Iron Nanoparticles
The iron nanoparticles were green synthesized with the help of aqueous extract of the selected plant samples. The synthesis is due to the reduction activity of the photochemical. The UV band observation, FTIR (Fourier Transform Infrared Spectroscopy, SEM (Scanning Electron Microscopy) and zeta potential studies were carried out for characterization.
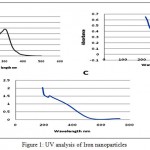 |
Figure 1: UV analysis of Iron nanoparticles
|
Figure:1 A: nanoparticles synthesized by Syzygium aromaticum Figure B: nanoparticles synthesized by Azadirachta indica Figure C: nanoparticles synthesized by Camellia sinensis
The UV visible is in spectroscopy of the synthesized nanoparticles were in the range of 216-300 nm. Syzygium aromaticum , Azadirachta indica and Camellia sinensis synthesize iron nanoparticles by the indication of suitable surface Plasmon resonance (SPR) with high band intensities and peaks under visible spectrum.
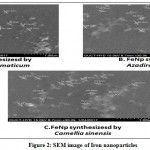 |
Figure 2: SEM image of Iron nanoparticles
|
Figure:2 A: nanoparticles synthesized by Syzygium aromaticum Figure B: nanoparticles synthesized by Azadirachta indica Figure C: nanoparticles synthesized by Camellia sinensis
 |
Figure 3: FTIR analysis
|
FTIR measurement was done to identify iron ions and capped plant compounds which could account for the reduction of iron chlorides into iron oxide nano particles. The sample was mixed with KBr. Thin sample pellet was prepared by pressing with the Hydraulic Pellet Press and subjected to FT-IR analysis.
FTIR measurement was carried out to identify the biomolecule involved in capping and stabilization of the metal nanoparticles synthesized from the selected plant extracts. FT-IR analysis showed that the biosynthesized iron oxide nano particles were capped with bimolecular compounds which were responsible for reduction of iron oxide,the bands seen at 3413.7 cm-1 indicates –OH Stretching, 1622.48 cm-1 indicates H-O-H bond , 1401.7cm-1, 1174.4cm-1 indicates plant materials, 707.8cm-1 and 609.2 cm-1 were assigned to the Fe-O stretching
 |
Figure 4: Zeta potential studies
|
The results obtained from the Zeta potential are shown in the figure 4. The particles with more positive than +30 mV or more negative than -30 mV are normally considered stable, the synthesized Iron nanoparticles were found to be stable. This stability of the nanoparticles is due to the capping action of plant based compounds.
MIC And MCB Concentration Studies
To study the relative effect of combination of FeNP with other antimicrobial agents, the bacterial sample was subjected to the tree combinations of treatment i.e. simple aqueous plant extract, plant extract + FeNp and plant extract + FeNp + amoxicillin.
The MIC and MCB values for the treatment given are depicted in the table bellow (Table. 1). The results observed clearly showed enhanced activity of the plant extract and the antibiotics when in combination of FeNP. The Azadirachta indica extract showed greater activity against the Streptococcus mutans and the antibacterial activity was enhanced when the extracts were combined with the Green synthesized iron nanoparticles. In general the application of nanoparticles with the other antimicrobial agents has shown enhancement of antimicrobial its activity.
Table 1: Minimum inhibitory concentration and Minimum bactericidal concentration studies.
| Sample no. | Treatment | Syzygium aromaticum, | Azadirachta indica, | Camellia sinensis |
| MIC µg/ml | MIC µg/ml | MIC µg/ml | ||
| 1 | Plant extract | MIC:250
MCB:8 |
MIC:270
MCB:5 |
MIC:295
MCB:25 |
| 2 | Plant extract + FeNp | MIC:50
MCB:5 |
MIC:40
MCB:3 |
MIC:75
MCB:12 |
| 3 | Plant extract + FeNp + amoxicillin | MIC:0.28
MCB: 0 |
MIC:0.25
MCB: 0 |
MIC:0.30
MCB:5 |
Zone of Inhibition Studies
The antimicrobial activity of the prepared combination wer further tested with the zone of inhibition studies. The obtained results are listed below (Table 2). It was found that among all three extract Azadirachta indica extract exhibited high zone of inhibition around 29±0.57mm which was enhanced further to 50±0.73 with combination FeNP. The treatment combination of plant extract, antibiotics and FeNP has superior impact on the bacterial growth which was observed to be 85±0.78 and was also recorded to be highest among other sample treatment.
Table 2: Zone of Inhibition studies of combinations of plant extract and Iron nanoparticles
| Treatment | Syzygium aromaticum, | Azadirachta indica, | Camellia sinensis |
| IZ mm | IZ mm | IZ mm | |
| FeNP | 0 | 0 | 0 |
| Plant extract | 23±0.4 | 29±0.57 | 15±0.40 |
| Plant extract + FeNp | 40±0.55 | 50±0.73 | 29±0.84 |
| Plant extract + FeNp + amoxicillin | 70±0.69 | 85±0.78 | 46±0.58 |
IZ: Inhibition Zone (in mm).
Values are mean of triplicate reading (mean ± S.D.).
Conclusion
Development of antibiotic resistance in microbial strain due to overexploitation of antibiotics is alarming, even the conventional medications used in the health care are not effectively eliminating the chance of infection due to the resistance developed in the pathogens; answerer to this problem can be the safe application of nanotechnology, as it works on the mechanical – cidal action.
The dental pleacktonic formation is a continuous process by different form of invading microbes; nanoparticulate metals have grabbed the attention because of their high surface to volume ration and small size. The nanoparticle facilitates the mechanical damage to the inter-linkage build by the pathogens as well at the cellular levels and this opportunity is taken up by the other antimicrobial agents to kill them rapidly. Due to the mechanical action of killing there is no chance of resistance development at the molecular level making this FeNp a promising option in the treatment of this stubborn dental pleacktonic which results in tooth decay.
Moreover C. Lee et. al. 2008 suggested that ROS includes superoxide radicals, hydroxyl radicals, hydrogen peroxide, and singlet oxygen that may cause chemical damage to proteins and DNA in bacteria. Thus the main mechanism could be related to oxidative stress generated by ROS.
The promising results from in vitro studies provide for a new and urgently needed strategy for the treatment of complex and notorious dental pleacktonic, giving us the opportunity to conduct in-vitro and in-vivo pharmacological activities which are useful for mankind.
Conflict of Interest
The authors have no conflict of interest.
Acknowledgement
We are grateful to Dr. SS.Vutukuru for his valuable suggestions. We would also like to thank department of Biotechnology, Sreenidhi Institute of science and Technology for providing us infrastructure for the project.
References
- Hall-Stoodley L., Costerton J. W., Stoodley P. Bacterial biofilms: from the natural environment to infectious diseases. Nature reviews. Microbiology. 2004;2(2):95.
CrossRef - Lebeaux D., Ghigo J. M., Beloin C. Biofilm-related infections: bridging the gap between clinical management and fundamental aspects of recalcitrance toward antibiotics. Microbiology and Molecular Biology Reviews. 2014;78(3):510-43.
CrossRef - Koo H., Falsetta M. L., Klein M. I. The exopolysaccharide matrix: a virulence determinant of cariogenic biofilm. Journal of dental research. 2013;92(12):1065-73.
CrossRef - Takahashi N., Nyvad B. The role of bacteria in the caries process: ecological perspectives. Journal of dental research. 2011;90(3):294-303.
CrossRef - Ishida H., Ishida Y., Kurosaka Y., Otani T., Sato K., Kobayashi H. In vitro and in vivo activities of levofloxacin against biofilm-producing Pseudomonas aeruginosa. Antimicrobial agents and chemotherapy. 1998;42(7):1641-5.
- Sharma P., Busby M., Chapple L., Matthews R., Chapple I. The relationship between general health and lifestyle factors and oral health outcomes. British dental journal. 2016;221(2):65-9.
CrossRef - Liakos I., Grumezescu A. M., Holban A. M. Magnetite nanostructures as novel strategies for anti-infectious therapy. Molecules. 2014;19(8):12710-26.
CrossRef - Yoo D., Guk K., Kim H., Khang G., Wu D., Lee D. Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. International journal of pharmaceutics. 2013 Jun 25;450(1):87-94.
CrossRef - Stevens P. D., Fan J., Gardimalla H. M., Yen M., Gao Y. Super paramagnetic nanoparticle-supported catalysis of Suzuki cross-coupling reactions. Organic letters. 2005 May 26;7(11):2085-8.
CrossRef - JunáPark Y. Demonstration of a magnetic and catalytic Co@ Pt nanoparticle as a dual-function nanoplatform. Chemical communications. 2006(15):1619-21.
- Lee S., Oh H. M., Lim W. B., Choi E. J., Park Y. N., Kim J. A., Choi J. Y., Hong S. J., Oh H. K., Son J. K., Lee S. H. Gene induction by glycyrol to apoptosis through endonuclease G in tumor cells and prediction of oncogene function by microarray analysis. Anti-cancer drugs. 2008 Jun 1;19(5):503-15.
CrossRef - Rao D. S., Penmatsa T., Kumar A. K., Reddy M. N., Gautam N. S., Gautam N. R. Antibacterial activity of aqueous extracts of Indian chewing sticks on dental plaque: An in vitro study. Journal of pharmacy & bioallied sciences. 2014 Jul;6(1):S140.
CrossRef - Badr A. E., Omar N., Badria F. A. A laboratory evaluation of the antibacterial and cytotoxic effect of Liquorice when used as root canal medicament. International endodontic journal. 2011 Jan 1;44(1):51-8.
CrossRef - Thomas A., Venkataramana J. Antimicrobial activity of TiO2 nanoparticles against Microbial Isolates causing dental plaques. International Journal of Bioassays. 2014 May 31;3(06):3106-10.
- Donlan R. M. Biofilms microbial life on surfaces. Emerging infectious diseases. 2002 Sep;8(9):881.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





