How to Cite | Publication History | PlumX Article Matrix
An Efficient Protocol for Shoot Organogenesis and Plant Regeneration of Polygonummultiflorum
Woo Tae Park1, Sang Un Park1, Sun Kyung Yeo,1 Md Romij Uddin2, Haeng Hoon Kim3
1Department of Crop Science, Chungnam National University, 99 Daehak-ro, Yuseong-gu, Daejeon, 305-764, Korea 2Department of Agronomy, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh. 3Department of Well-being Resources, Sunchon National University, 413 Jungangno, Suncheon, Jeollanam-do, 540-742, Korea
ABSTRACT: We developed a rapid protocol for efficient shoot organogenesis and plant regeneration from stem node cultures of Polygonummultiflorum. Stem node explants were cultured with varying concentrations of cytokinins, then with 0.5 mg/l of 6-benzylaminopurine (BAP) supplemented with various concentrations of different auxins, and regeneration capacity and growth of shoots were assessed. Among the cytokinins, BAP induced shoot initiation with the highest efficiency, whereas zeatin showed higher efficacy for stimulation of shoot growth after 6 weeks in culture. BAP with indole-3-acetic acid (IAA) was highly effective in stimulating shoot initiation. Among the treatment combinations, 0.5 mg/l BAP with 1.0 mg/l IAA proved optimal yielding the highest number of shoots (3.1 per explant). This system can produce approximately 4 plantlets in vitro from one stem node explant culture of P. multiflorum, and the system can be used at any time of the year.
KEYWORDS: plant regeneration; Polygonummultiflorum; shoot organogenesis
Download this article as:| Copy the following to cite this article: Park W. T, Park S. U, Yeo S. K, Uddin M. R, Kim H. H. An Efficient Protocol for Shoot Organogenesis and Plant Regeneration of Polygonummultiflorum. Biosci Biotech Res Asia 2015;12(2) |
Introduction
Polygonummultiflorum(Family: Polygonaceae) is a flowering plant commonly used for medicinal and ornamental purposes. The tuberous roots of P. multiflorumare used as a tonic and in many remedies in traditional Chinese medicine1,2. The plant has been used clinically for anti-allergic, anti-tumor, antibacterial, hemostatic, spasmolytic, and analgesics purposes in Korean traditional medicine3.Several classes of secondary metabolites have been isolated from Polygonummultiflorumsuch as phenolic compounds, flavonoids, anthraquinones, stilbenes, and tannins4, including a number of anthraquinones in the stilbene class such as (E)-2,3,4′,5-tetrahydroxystilbene-2-β-D-glucoside, rhein, emodin, aloe-emodin, chrysophanol, physcion, and derivatives thereof5,6.
Although most plants normally reproduce sexually, some species capable of vegetative propagation. Micropropagation, a method of vegetative propagation, is the practice of rapidly multiplying stock plant material to produce a large number of progeny plants, using in vitro plant regeneration systems based on tissue culture7,8.
Propagation of P. multiflorumis achieved either by division of the tuberous roots or by planting seeds. Seed propagation is difficult because of the low propagation rate and the delay implicit in root harvesting. Thus, this species is propagated conventionally through the division of roots9.Although in vitromicropropagation of P. multiflorumby shoot organogenesis4 has been reported, plant regeneration methods have not been described. An efficient method for the regeneration of intact plants from tissue culture is essential for establishment of a micropropagation system and a genetic transformation protocol. In this study, we developed a method for plant regeneration and micropropagation from stem node cultures of P. multiflorum.
Material and Methods
Seed sterilization and germination
Seeds of P. multiflorum were purchased from Aram Seed Company, Seoul, Korea and stored at 4°C. Seedswere surface-sterilized with 70% (v/v) ethanol for 30 s and 2% (v/v) sodium hypochlorite (Sigma, St. Louis, Mo.USA) solution for 10 min, then rinsed three times in sterilized water. Seven seeds were placed on 25 ml of agar-solidified culture medium in Petri dishes (100 × 15 mm). The basal medium consisted of MS10salt and vitamin medium (Sigma, St. Louis, Mo.USA) solidified with 0.7% (w/v) agar. The MS salt and vitamin medium was adjusted to pH 5.8 before agar addition and was then sterilized by autoclaving at 121°C for 20 min. Seeds were germinated after 2 weeks culture in a growth chamber at approximately 70-80% humidity and 25°C, under standard cool white fluorescent tubes with a flux rate of 35 µmol s-1 m-2 and a 16-h photoperiod.
In vitro plant regeneration
Stem nodes of P. multiflorum were cut into pieces approximately 0.7 cmin size, from plants grown in vitro that had been cut aseptically at the ends. Explants (7 per dish) were placed on medium (approximately 25 ml) in 100 × 25 mm Petri dishes. The basal medium consisted of MS medium, adjusted to pH 5.8 before solidification with 0.7% (w/v) Phytagar (Sigma, St. Louis, MO., USA). Media were sterilized by autoclaving at 1.1 kg cm-2 (121 °C) for 20 min. For shoot regeneration from stem node explants, the MS medium was supplemented with 0, 0.1, 0.5, 1, 2, and 4 mg/l BAP (6-benzylaminopurine), kinetin (N6-furfuryladenine), TDZ (1- phenyl-3-(1,2,3-thiadiazol-5-yl) urea; thidiazuron), or zeatin. For improvement of shoot regeneration, the medium was optimized by testing the effects of different concentrations (0.0, 0.1, 0.5 and 1.0 mg/l) of IAA (indole-3-acetic acid), IBA (indole-3-butyric acid), and NAA (naphthalene acetic acid) on shoot formation and growth. Plant hormones were purchased from Sigma (St. Louis, MO, USA). Cultures were maintained at 25±1 °C in a growth chamber with a 16-h photoperiod under standard cool white fluorescent tubes (35 mmol s-1 m-2) for 6 weeks.
Rooting of regenerated shoots
Regenerated shoots (approximately 1 cm long; 4 shoots per vessel) were placed in MS medium solidified with 3g/lGelrite (Sigma, St. Louis, MO., USA) and dispensed at 30 ml per culture vessel. Regenerated shoots were incubated for 5 weeks in a growth chamber as described above. After five weeks, rooted plants were washed with sterile water to remove Gelrite, transferred to pots containing autoclaved vermiculite, and covered with polyethylene bags for one week to maintain high humidity. The plants were then transferred to soil and maintained in a growth chamber with a 16-h photoperiod, and a night/day temperature of 18/20°C for 2 weeks. These hardened plants were then transferred to the greenhouse.
Statistical analysis
Data are expressed as mean ± standard deviation from 50 meristems tested.
Results and Discussion
Effect of cytokinins
We developed a simple and effective protocol for the in vitro plant regeneration and micropropagation of P.multiflorum. We investigated the effects of different cytokinins on the efficiency of shoot organogenesis. Shoot development from stem node cultures was successful under all cytokinin treatments. The shoot number and shoot length varied significantly among different concentrations of the cytokinin BAP. At 0.5 mg/l, BAP produced the highest number of shoots (2.0) per explant among all cytokinin treatments. Interestingly, with increasing BAP concentration, the number of shoots and shoot length decreased (Table 1). The tallest shoot was observed using the lowest concentration (0.1 mg/l). In contrast with BAP, for the cytokinin kinetin, the trend was reversed for number of shoots initiated and shoots length. With increasing concentrations of kinetin, the number of shoots and shoot length increased in most cases. A similar trend was seen TDZ treatment. With increasing concentrations of TDZ, the number of shoots and shoot length increased up to 2 mg/l TDZ and then declined. The highest shoot number and shoot length were observed at 2 mg/l TDZ, and the shoot length was higher than any induced by BAP or Kinetin. The trend for zeatin-induced shoot initiation was almost identical to that for BAP, but for shoot length, zeatin’s effects were similar to those of kinetin and TDZ. With increasing zeatin concentrations above 0.5 mg/l, shoot initiation decreased, whereas shoot length increased up to 2 mg/l and then declined. Zeatin at 2.0 mg/l resulted in the tallest shoot (29.3 mm) among all cytokinin treatments (Table 1).
When P.multiflorum stem nodes were cultured on MS solid media supplemented with 0.5 mg/l BAP, the various stages of shoot organogenesis were clearly observed. During the initial stage (up to 2 weeks of incubation), some expansion and proliferation of cells at the cut surface were observed, but callus growth was limited. After enlargement of the cut end of the stem node explant (within 4 weeks), formation of shoot primordia and small elongated shoots adjacent to the cut surface were noted (Fig. 1A). We observed that cells of the epidermis proliferated to produce shoots directly (without an intervening callus phase). Regenerated shoots had developed from shoot primordia within 4-5 weeks. After 6 weeks of culture, an average of 2.0 fully developed shoots at least 21.4 mm long were produced, presumably from the meristem explant (Fig. 1B).
Table 1: Effect of different concentrations of cytokinins on shoot regeneration and growth from meristem cultures of Polygonummultiflorum after 6 weeks of culture
| Cytokinin (mg/l) | Shoots per explants | Shoot length (mm) | |
| BAP | 0.1 | 1.8 ± 0.08 | 23.0 ± 1.05 |
| 0.5 | 2.0 ± 0.12 | 21.4 ± 0.84 | |
| 1.0 | 1.4 ± 0.11 | 21.6 ± 1.17 | |
| 2.0 | 1.2 ± 0.09 | 20.4 ± 1.17 | |
| 4.0 | 1.3 ± 0.12 | 17.9 ± 1.10 | |
| Kinetin | 0.1 | 0.6± 0.05 | 18.1 ± 0.57 |
| 0.5 | 0.5 ± 0.05 | 17.8 ± 0.92 | |
| 1.0 | 0.8 ± 0.08 | 20.5 ± 0.85 | |
| 2.0 | 0.8 ± 0.09 | 22.6 ± 0.84 | |
| 4.0 | 1.1 ± 0.06 | 22.2 ± 0.92 | |
| TDZ | 0.1 | 0.9 ± 0.06 | 19.9 ± 0.99 |
| 0.5 | 0.8 ± 0.06 | 17.9 ± 0.99 | |
| 1.0 | 0.9 ± 0.07 | 20.7 ± 0.68 | |
| 2.0 | 1.4 ± 0.10 | 24.4 ± 0.84 | |
| 4.0 | 1.2 ± 0.06 | 24.0 ± 0.82 | |
| Zeatin | 0.1 | 1.3 ± 0.07 | 24.4 ± 0.97 |
| 0.5 | 1.4 ± 0.07 | 27.6 ± 0.84 | |
| 1.0 | 1.2 ± 0.08 | 28.2 ± 0.79 | |
| 2.0 | 0.9 ± 0.07 | 29.3 ± 1.16 | |
| 4.0 | 0.7 ± 0.07 | 27.7 ± 0.82 | |
BAP, 6-benzylaminopurine; TDZ, 1- phenyl-3-(1,2,3-thiadiazol-5-yl) urea
Combined effects of cytokinin (BAP) and different concentrations of auxins
BAP at 0.5 mg/l produced the highest number of shoots from each explant among the cytokinin treatment. Shoot number is the most important factor in micropropagation; therefore, 0.5 mg/l BAP was selected for evaluation of the combined effects of cytokinin and auxins. Explants from stem nodeswere grown for 6 weeks in 0.5 mg/l BAP supplemented with varying concentrations of the different auxins (IAA, IBA, and NAA) to observe regeneration and shoot-growth capability from excised stem node cultures. In every case, the combination of BAP and auxin produced more and longer shoots from the explants, compared to control (BAP alone). The optimal conditions for shoot number (3.1 per explant) were obtained with BAP and 1.0 mg/l IAA (Table 2). The other combinations of BAP and auxins varied little with regard to shoot initiation and differed little compared to the control. In contrast, shoot length varied widely among the different combinations. BAP with 1 mg/l NAA, induced the maximum shoot length (62.1 mm). Moreover, BAP with other concentrations of NAA produced longer shoots compared to IAA and IBA.
Table 2: Combined effect of BAP at 0.5 mg/L and different concentrations of auxins on shoot regeneration and growth from meristem cultures of P. multiflorum after 6 weeks of culture.
| Auxin (mg/l) | Shoots per explant | Shoot length (mm) | ||
| BAP 0.5
mg/l |
Control | 0.0 | 2.1±0.10 | 22.3±0.92 |
| IAA | 0.1 | 2.2±0.10 | 24.5±1.16 | |
| 0.5 | 2.4±0.12 | 23.8±1.22 | ||
| 1.0 | 3.1±0.14 | 22.1±0.99 | ||
| IBA | 0.1 | 2.0±0.11 | 26.3±1.85 | |
| 0.5 | 2.3±0.12 | 35.3±2.45 | ||
| 1.0 | 2.4±0.13 | 48.6±2.47 | ||
| NAA | 0.1 | 2.2±0.12 | 51.4±2.61 | |
| 0.5 | 2.1±0.09 | 56.7±2.86 | ||
| 1.0 | 1.9±0.09 | 62.1±3.12 | ||
IAA, indole-3-acetic acid; IBA, indole-3-butyric acid; NAA, naphthalene acetic acid
Plant micropropagation
Regenerated shoots (~1 cm) were transferred to MS medium without any exogenous plant hormone. After 5 weeks, the regenerated shoots induced roots (Fig. 1C). The rooted plants were transferred to pots containing autoclaved vermiculite and were covered with polyethylene bags for 1 week to maintain high humidity. The regenerated plants were hardened and transferred to soil (90% survival rate) where they grew normally in a greenhouse.
The importance of traditional skills for vegetative propagation of plant species must not be underestimated; at one time, these were the only practices for regeneration of some plant species. However, regeneration techniques based on tissue culture of economically important plants is a relatively new development in the last decade11,12..In vitro techniques expand the potential of these approaches by the application of nutritional and hormonal systems under aseptic conditions. Plant regeneration by these means is known as “micropropagation” referring to miniature shoots or plantlets from which plants are initially derived13-15. There are several strategies for the regeneration of whole plants from excised plant parts. Among these, two are predominant; generation through shoot organogenesis and regeneration by somatic embryogenesis16,17.
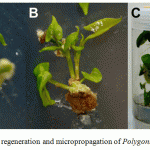 |
Figure 1: In vitro plant regeneration and micropropagation of Polygonummultiflorum |
Conclusion
We developed and optimized a rapid protocol for efficient shoot organogenesis and normal plant regeneration from stem node cultures of P. multiflorum. The continuous production of P. multiflorum regenerated plants without the loss of morphogenetic capacity is important, as this technique has potential in micropropagation systems and regeneration of transgenic plants. Every year, conventional cultivators remove some tuberous roots from the harvest for the purpose of future mass propagation; however, our system has a higher throughput: it can produce approximately 4 plantlets in vitro from 1 stem-node explant culture of P. multiflorum irrespective of season.
Acknowledgements
This work was carried out with the support of “Cooperative Research Program for Agriculture Science & Technology Development (Project No. PJ906938)” Rural Development Administration, Republic of Korea.
References
- Chen,S.Y., Li, F. Clinical Guide to Chinese Herbs and Formulae.Churchill Livingston, Madrid, 1993;75 p.
- Huang, K.C. The Pharmacology of Chinese herbs. CRC Press, Boca Raton, 1993;101-102pp.
- Kim,H.K., Choi, Y.H.,Choi, J.S., Choi, S.U., Kim, Y.S., Lee, K.R., Kim, Y.K., Ryu, S.Y. A new stilbene glucoside gallate from the roots of Polygonummultiflorum. Arch. Pharmacal Res., 2008;31:1225-29.
- Lin, L.C., Nalawade, S.M., Mulabagal, V., Yeh, M.S., Tsay, H.S. Micropropagation of Polygonummultiflorum THUNB and quantitative analysis of the anthraquinonesemodin and physcion formed in in vitro propagated shoots and plants. Biol. Pharmaceut. Bull., 2003; 26:1467-71.
- Yao, S., Li Y., Kong, L. Preparative isolation and purification of chemical constituents from the root of Polygonummultiflorumby high-speed counter-current chromatography. J. Chromatography A., 2006;1115: 64-71.
- Yi, T., Leung, K.S., Lu, G.H.,Zhang, H., Chan, K. Identification and determination of the major constituents in traditional Chinese medicinal plant PolygonummultiflorumThunb by HPLC coupled with PAD and ESI/MS. Phytochem. Anal., 2007;18:181-87.
- Debnath, M.,Malik, C.P., Bisen, P.S. Micropropagation: a tool for the production of high quality plant-based medicines. Curr. Pharmaceut. Biotechnol., 2006;7:33-49.
- Chandra, S.,Bandopadhyay, R.,Kumar V., Chandra, R.Acclimatization of tissuecultured plantlets: from laboratory to land. Biotechnol. Lett. 2010; 32:1199-1205.
- Shinju, H., Higuchi,M., Okada, M.Studies on cultivation of Polygonummultiflorum Thunberg (Part 1) on the methods of vegetative propagation. J. Natural Med., 1994;48:126-30.
- Murashige, T.,Skoog, F. A revised medium for rapid growth and bioassays with tabacco tissue cultures. Plant Physiol., 1962; 15:473-97.
- Chaturvedi, H.C., Jain, M., Kidwai, N.R.Cloning of medicinal plants through tissue culture-a review. Indian J. Exp. Biol., 2007;45:937-48.
- Park, S.U., Park, N.I., Kim, Y.K.,Suh, S.Y., Eom, S.H., Lee, S.Y. Application of plant biotechnology in medicinal plant, RehmanniaglutinosaLiboschitz. J. Med. Plants Res.,2009; 3:1258-63.
- Ducos, J.P., Terrier, B., Courtois, D. Disposable bioreactors for plant micropropagation and mass plant cell culture. Adv. Biochem. Engin./ Biotechnol., 2010;115: 89-115.
- Gago, J., Pérez-Tornero, O., Landín, M., Burgos, L., Gallego, P.P. Improving knowledge of plant tissue culture and media formulation by neurofuzzy logic: a practical case of data mining using apricot databases.J. Plant Physiol., 2011; 168:1858-65.
- Naik, S.K., Chand, P.K. Tissue culture-mediated biotechnological intervention in pomegranate: a review. Plant Cell Rep., 2011;30:707-21.
- Phillips,G.C.,Hubstenberger, J.F. Micropropagation by proliferation of axillary buds. In: Gamborg, Phillips (eds) Plant cell, tissue and organ culture: fundamental methods, Springer-Verlag, Berlin Heidelberg, 1995; 81-90pp.
- Vizel, M., Loya, Y., Downs, C.A., Kramarsky, E.A novel method for coral explant culture and micropropagation. Marine Biotechnol., 2011; 13:423-32.

This work is licensed under a Creative Commons Attribution 4.0 International License.





