Manuscript accepted on : 19 September 2017
Published online on: --
Plagiarism Check: Yes
Nooshin Barikrow1, Naser Amirizadeh2, Nasim Hayati Roodbari1 and Mahin Nikougoftar2
1Department of Biology, Science and Research Branch, Islamic Azad University, Tehran, Iran.
2Blood Transfusion Research center, High institute for education and research in Transfusion Medicine, Tehran, Iran.
Corresponding Author E-mail: n.amirizadeh@ibto.ir
DOI : http://dx.doi.org/10.13005/bbra/2526
ABSTRACT: Because of insufficient number of umbilical cord blood hematopoietic stem cells (UCB-HSCs), expansion of these cells seems to be important for clinical application in adults. The aim of this study was to co-culture of UCB-HSCs with the amniotic membrane derived mesenchymal stem cells (AMMSCs) as a feeder layer in order to expand hematopoietic stem cells (HSCs). UCBs and amniotic membrane were collected from concern mothers. Ex vivo culture of UCB-HSCs were performed in four culture conditions: cytokine cocktail with MSCs feeder layer, cytokine cocktail, stem cell factor, and co-culture with MSCs without any cytokine. The number of total nucleated cells (TNC) was counted by hemocytometer. The HSC count and immunophenotyping of Mesenchymal stem cells (MSCs) and expanded HSC were evaluated by flow cytometry. Colony forming unit (CFU) assay was used to evaluate the potential of expanded HSCs for production of different lineage colonies. The mean fold changes of total nucleated cells (TNC) and CD34+ cells in the cytokine culture with feeder layer were higher than the cytokine culture without MSCs. However, in the co-culture system without cytokine, TNC and CD34+ cell numbers were increased up to 8 folds, but cell viability was more than 80% and differentiation rate was low. Our results demonstrated that we could increase the number of CD34+ cells of UCB that were used as primary HSC for transplantation.
KEYWORDS: Amnion; Cord Blood Stem Cell Hematopoietic Stem Cells; Mesenchymal Stromal Cells;Tissue Expansion; Transplantation
Download this article as:| Copy the following to cite this article: Barikrow N, Amirizadeh N, Roodbari N. H. Nikougoftar M. Expansion of Cord Blood Hematopoietic Stem Cells on the Amniotic Membrane Derived Mesenchymal Stem Cells. Biosci Biotech Res Asia 2017;14(3). |
| Copy the following to cite this URL: Barikrow N, Amirizadeh N, Roodbari N. H. Nikougoftar M. Expansion of Cord Blood Hematopoietic Stem Cells on the Amniotic Membrane Derived Mesenchymal Stem Cells. Biosci Biotech Res Asia 2017;14(3). Available from: https://www.biotech-asia.org/?p=27664 |
Background
Hematopoietic stem cells (HSCs) have been known as primitive, undifferentiated cells that have self- renewability and differentiation into all blood cell types.1,2 Their progeny, referred to hematopoietic progenitor cells (HPC), contain cells with a limited capacity to self-renew.3,4 The functioning of hematopoietic stem cells (HSCs) related to internal regulators,1,5 which are adjusted by external signals such as cytokines and extracellular matrix produced by stromal and auxiliary cells. Together, stromal and auxiliary cells and their products organize a complex structural and functional network known as the hematopoietic microenvironment (HM).6,7 During the last two decades, another source for HSCs, except bone marrow, was described such as peripheral blood cells and umbilical cord blood (UCB). HSCs have become a central issue in the study of hematopoiesis, playing a key role in the development of novel strategies for HSC transplantation and have become an important source of HSCs for myeloablative and non-myeloablative therapies.7
Clinical trials have basically shown that UCB transplantation causes less graft versus host disease (GVHD) and graft failure.8 Recipients seem to be less sensitive to HLA mismatching when UCB is used as a source of HSCs/HPCs. One clinically interesting fact is that UCB myeloid progenitor cells are relatively chemo-resistant (9). The major limiting factor to UCB usage is the low cell dose available for transplantation. It is shown that the total nucleated cell (TNC) dose transplanted per kg of body weight of the recipient correlates with results. Patients with a total body weight of at least 45 kg who receive only a single unit of UCB have been shown to have a significant delay in time to neutrophil and platelet engraftment, as well as higher risk of engraftment failure.1,5,6,10,11 For this reason different experimental systems was designed for expansion HSC and two major aims are followed in the near future: first, the generation of HSCs that retain multipotentiality and self-renewal and the second is, production of such cells in sufficient numbers for their clinical usage.12
Currently, several ways exist for ex vivo HPCs expansion. A combination of several cytokines is typically used for ex vivo culture of HSCs/HPCs. Although there are not the exact combination of cytokines, but it usually consists of several cytokines such as Flt3/Flk2 ligand,6,13 interleukin- 3 (IL-3),14,15 IL-6,6,16 and thrombopoietin.6,17-19
Primary clinical trials using CD34+ or CD133+ selected UCB cells expanded in an ex-vivo expansion system have used for transplantation.20 This stimulated our interest in strategies that might improve the expansion capability of HSCs that generated with co-cultures system. The majority of static liquid culture ex vivo expansion systems first require the isolation of CD34+ or CD133+ HPCs from fresh or frozen hematopoietic tissue.21,22 Clinically, liquid ex vivo expansion of CD34+ UCB cells was reported to increase TNC by 56- fold and the total number of CD34+ cells by 4 fold.23 One kind of cells that exists in microenvironment of HSCs is mesenchymal stem cell (MSCs). MSCs have some sources such as BM, adipose tissue, placenta and amniotic membrane.
Objectives
In current research we use amniotic derived MSCs (AMMSCs) for the first time as microenvironment for co-culture HSCs. In our current UCB expansion protocols, CD34+ cells are isolated before culture. Thus, we thought to develop a UCB expansion method, which would increase the expanded cell dose available for transplantation.
Materials and Methods
Isolation of HSCs
HSCs were collected from fresh human UCB. The blood were taken from normal full-term pregnant women after written informed consent and according to guidelines approved by the Ethics Committee at the Iranian Blood Transfusion Organization. Mononuclear cells (MNCs) were isolated by density gradient centrifugation on Ficoll-Hypaque (Bio sciences, Sweden). The MNCs were incubated with anti-human CD34+ conjugated with MicroBeads, (Miltenyi Biotec, USA) for 30 min at 4 °C. CD34+ cells were isolated using an affinity column.
Isolation and Cultivation of AMMSCs
The human amnion was mechanically peeled from the chorion of a placenta obtained from an un-complicated cesarean section with informed consent. AMMSCs were isolated by sequential trypsin and collagenase digestion as described previously.24 Briefly, the amnion was washed in phosphate- buffered saline (PBS) and then cut into the pieces. The resulting minced amnion was digested with 0.2% trypsin (Sigma-Aldrich, Germany) and collagenase in Dulbecco’s Modified Eagle’s Medium (DMEM) (Sigma, UK). After incubation at 37°C with stirring at 100 rpm for 30 min, the medium was removed. The tissue was minced again and incubated at 37°C in DMEM with trypsin and stirred at 400-600 rpm for 30 min. The mixture was then poured over gauze to separate the dispersed amnion cells from the tissue pieces. The dispersed cells were collected by centrifugation and suspended in DMEM supplemented with heat-inactivated 10% FCS (Gibco, USA) and 1% antibioticantifungal solution (Gibco, USA) and then seeded into culture dishes (Greiner Bio-One, Germany) at a concentration of 3.0 × 104 cells /cm2. Incubation was carried out at 37°C under 5% CO2 in air. The MSCs were characterized by flow cytometric analysis using monoclonal antibodies against CD105, CD44, CD166, CD29,CD73 and CD34,CD45 (Dako, Denmark). Differentiation potential of MSCs was investigated by osteogenic differentiation and adipogenic differentiation.
Ex vivo Expansion of CD34+ Enriched Cells
CD34+ enriched cells were cultured in 4 well plates (Nunc, Denmark) for 10 days in the serum-free medium (Stem cell technology, Denmark) at 37 °C under 5% CO2 humidified air in the four culture conditions:
Cytokines culture: supplemented with SCF (100 ng/mL), TPO (100 ng/mL) and FLT3L (50 ng/mL) (Stem Cell Technology, Denmark)
Co-culture with MSCs feeder layer and above mentioned cytokines
Co-culture with AMMSCs without any cytokines
Culture with only SCF as negative control
Colony Forming Cell Assays
Fresh CD34+ cells and expanded cells at 5th and 10th days of culture were seeded in semisolid culture (Stem cell technology, Denmark) following the manufacturer’s instruction. Methylcellulose-based media were aliquted in 35-mm2 petri dishes and incubated at 37 °C, 5% CO2 and humidified incubator. After 10 days of culture, the number of colony was counted under the inverted microscope.
Proliferative and Phenotypic Analysis
Cell viability was determined at 0, 7th, 10th days of culture by counting HSCs using trypan blue staining method (Stem Cell Technologies, Denmark), and analyzed for stem cell by flow cytometric analysis (Partec, Germany). Flow cytometric analysis was performed by incubating harvested cells with CD34 and CD38 fluorescent conjugated monoclonal antibodies at 4 °C for 30 minutes. The cells were washed in PBS and fixed with 2% paraformaldehyde (Sigma-Aldrich, Germany). Isotype controls were used in every experiment.
Statistical Analysis
Results obtained from multiple experiments are expressed as the mean and standard deviation (SD). The data were analyzed by the Student’s t-test using SPSS version 16.0 (Chicago, USA). P values < 0.05 defined significant differences between test points.
Results
AMMSCs Characterization and Immunophenotyping
MSCs were positive for the following adhesion molecules: CD44, CD166, CD73, CD105, and CD29 which were considered as markers for MSCs. As shown, the MSCs were negative for hematopoietic lineage markers, namely, CD34, and CD45 (Figure 1).
 |
Figure 1: Flow Cytometeric Analysis of Mesenchymal Stromal Cells
|
Osteogenic and Adipose Differentiation Assay
Osteogenic differentiation potential of AMMSCs was assayed by alizarian red staining and evaluation of alkaline phosphatase activity. Also, the ability of MSCs to adipose cells differentiation was assayed with oil Red O staining. All staining showed a positive reaction (Figure 2).
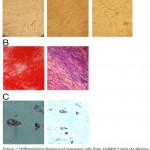 |
Figure 2: Differentiation diagnosis of osteogenic cells from AMMSCs with (A) alkaline phosphatase staining and (B) alizarin red staining. Differentiation to adipose cells was determined by oil Red O staining (C)
|
Ex vivo Expansion of CD34+ Enriched Cells
Purity of separated CD34+ cells, determined by flow cytometric analysis, was 92.69 ± 14.00 %. Moreover, 6.81 ± 7.00% of them were positive for the CD38 marker (n=3) (figures 4). Ex vivo expansion of human cord blood CD34+ enriched cells in serum-free medium supplemented with SCF, TPO and FLT3L was evaluated either with or without feeder layer using flow cytometric analysis (Figure 4). Figures 3 show the fold increase in TNC and number of CD34+ cells during 10-day liquid cytokine culture in the presence and absence of human MSCs and in the co-culture system without cytokine. Increase in culture with only SCF is lower than the other conditions. Maximum expansion was observed at 7th day of culture in all the four culture conditions. In the co-culture system without cytokine, the mean fold change of TNC was 8.40 ± 2.30, in cytokine culture without MSCs feeder layer was 20.00 ± 8.96, in the co-culture system with cytokines was 25.50 and in the culture with SCF was 6 at 7th of culture. The mean fold change of CD34+cells was 20.10 ± 2.30 in cytokines supplemented culture and 25.56 ± 24.83 in the co-culture system with cytokines. In the co-culture without cytokine the fold increase in CD34+ was 8.43 and in the culture with SCF was 4.84 (n = 6) (Figure 3a). Expansion of CD34+ selected UCB cells at 10th day of both cytokine culture and co-culture with cytokines was decreased, and relative differentiation was also observed. So the mean fold change of TNC and CD34+ cells at 7th day in the co-culture system with cytokines was higher than the cytokine culture without MSCs feeder layer and co-culture system without cytokines significantly (n = 6, P < 0.05). However, in the co-culture system without cytokine TNC and CD34+ cell numbers were increased up to 8 folds, the cell viability was remained 80% after 10 days (Figure 3 a and b).
 |
Figure 3: Mean Fold Change of TNC Cells (A) and CD34+ cells (B) in Cytokine-Supplemented Culture with and without MSC and Co-culture with MSCs alone
|
 |
Figure 4: Flow Cytometric Analysis of Fresh CD34+ Enriched Cells (A) and expanded cells in the co-culture system without cytokine (B) cytokine culture without MSCs (C) with MSCs (D) and without cytokine and MSCs(E):
|
Specific staining was performed with anti-CD34- PE antibodies and anti-CD38-, FITC FL1: CD38, FL2: CD34. (A) CD34: 92.69 CD38:6.81%; (B) CD34: 91.64% CD38:8.36%; (C) CD34: 97.87% CD38:2.14%; (D) CD34: 95.85% CD38:4.16%, (E) CD34: 72.06% CD38:27.96%.
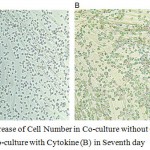 |
Figure 5: Increase of Cell Number in Co-culture without Cytokine (A) and in Co-culture with Cytokine (B) in Seventh day
|
Colony Forming Cell Assays
The highest CFU fold change was also observed in cytokine culture with MSCs at 10th day (125 ± 24) (Figure 6). The CFU increase in cytokine culture without MSCs at 10th day was 91 ± 13, in co-culture with MSC was 52 ± 9 and in the culture without cytokine was 39. hence the mean fold change of CFU at 10th day in both cytokine cultures with and without MSCs feeder layer was higher than co-culture system without cytokines significantly (n = 3, P < 0.05), but there was no significant differences between two cytokine cultures with and without MSCs feeder layer (Figure 4).
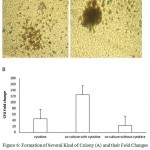 |
Figure 6: Formation of Several Kind of Colony (A) and their Fold Changes (B) in Co-culture Conditions with or without Cytokine
|
Discussion
This study compared the supporting effects of AMMSCs alone or in combination with cytokines in the proliferation and differentiation of HSCs. Ex vivo expansion of UCB-HSCs at different conditions of culture recombinant stimulatory cytokines is one way to increase the number of CD34+ cells and UCB-HSCs.25 The number of CD34+ cells of UCB that were used as primary HSCs for transplantation can increase significantly (26). Data presented here demonstrate that the ex vivo co-culture CD34+ cells with MSCs generated superior TNC and HPC expansion. The improved TNC and HPC expansion observed during the CD34+ MSC co-culture would be consistent with the observation that ex vivo contact with stromal components of the HM preserves HSCs activity.27,28 Moreover, in this study, UCB-CD34+ cells expansion with hematopoietic cytokines in the absence or presence of human AMMSCs feeder layer and in co-culture and culture system without cytokine was performed. The mean fold changes of TNC and CD34+ cells in the cytokine culture with MSCs were higher than the cytokine culture without MSCs. However, in the co-culture system without cytokine TNC and CD34+ cell numbers were increased up to 8 folds, but cell viability was more than 80% and differentiation rate was low.29
In the present study, maximum expansion was observed at 7th day of culture in the two cytokine supplemented cultures. Moreno et al (2010) reported that UCB derived CD34+ cells showed significant proliferation after 14 days in the cytokine culture and maximum expansion was observed at 7th day of culture.30 Also, Walenda et al (2010) showed that MSCs and cytokines have synergistic effect on HSCs expansion.31 Robinson et al (2006) reported that MSCs co-culture with UCB cells without the need for an initial positive selection step (and the associated loss of HPC) might produce a UCB graft with better engraftment potential.32 Our mean fold change of UCB-CD34+ with cytokines and without feeder layer was 20.1 that is similar to results of Mohamed (2006). They reported 25 fold change in CD34+ cells in the culture with cytokines.33
McNiece et al. showed that UCB-MSCs co-culture does not require the isolation of CD34+ or CD133+ cells before expansion, minimizing manipulation and loss of HPC. Also, they have demonstrated 10 to 20-fold increase in TNC, 7 to 18-fold increase in committed progenitor cells (colony forming cells), 2 to 5-fold increase in primitive progenitor cells (high proliferative potential colony-forming cells) and 16 to 37-fold increase in CD34+ cells, using an MSC co-culture expansion technique.34 In agreement with our results, Jang et al showed that proliferation capacity of HSCs in the recombinant cytokine culture with UCB-MSCs feeder layer was higher than the cytokine culture without MSCs, and in the co-culture system without cytokine CD34+ cell numbers were increased up to 8 folds.35 Also, Silvaa et al (2005) cultured UCB-CD34+enriched cells in serum-free medium supplemented with SCF, LIF, and FLT-3, in the presence or absence of stroma, and observed that the stromal layers would support process of expansion without the exhaustion of the more primitive stem cells and differentiation induction.36 However, present study suggests that MSC co-culture of a UCB unit will yield approximately 5 × 106 TNC (and approximately 4.7 × 106 CD34+ cells). This yield would be sufficient to provide a transplant dose for a > 75 kg recipient. This procedure would therefore potentially permit the transplantation of patients > 75 kg who are currently precluded from receiving UCB units because single UCB units with acceptable cell doses are not available. On the other hand, comparison of the results of the present study with others showed that the percentage of CD34+ was higher than the others up to 90% that maybe related to the different source for feeder of culture. Based on our results, use of amniotic membrane for extraction of MSCs is suggested.
In conclusion expression of CD34+ cells in HSCs were increased during ex vivo expansion. Also, among several studies investigating different types of feeder layer-based culture systems, amniotic derived MSCs are more effective than stroma-free culture system (supplemented with cytokines). Previous studies showed that CD34+ cells derived from cord blood are capable of functional hemato-endothelial development. However, the relationship between CD34+ and embryonic stem cell-derived MSC remains unknown. Future studies must be focused on the developmental hierarchy of CD34+ and MSCs, the functional engraftment of present CD34+ cells and AMMSCs progenitor cells and the potential clinical application for adult and children disorders.
References
- Stanevsky A./, Goldstein G., Nagler A. Umbilical cord blood transplantation: pros, cons and beyond. Blood Rev. 2009;23(5):199-204. PMID: 19282073.
- Copelan E. A. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813-26. PMID: 16641398
- Dahlberg A., Delaney C., Bernstein I. D. Ex vivo expansion of human hematopoietic stem and progenitor cells. Blood. 2011;117(23):6083-90. PMID: 21436068.
- Hofmeister C. C., Zhang J., Knight K. L., Le P., Stiff P. J. Ex vivo expansion of umbilical cord blood stem cells for transplantation: growing knowledge from the hematopoietic niche. Bone Marrow Transplant. 2007;39(1):11-23. PMID: 17164824.
- Bornstein R., Flores A. I., Montalban M. A., del Rey M. J., de la Serna J., Gilsanz F. A modified cord blood collection method achieves sufficient cell levels for transplantation in most adult patients. Stem Cells. 2005;23(3):324-34. PMID: 15749927.
- Ueda T., Tsuji K., Yoshino H., Ebihara Y., Yagasaki H., Hisakawa H., et al. Expansion of human NOD/SCID-repopulating cells by stem cell factor, Flk2/Flt3 ligand, thrombopoietin, IL-6, and soluble IL-6 receptor. J Clin Invest. 2000;105(7):1013-21. PMID: 10749580.
CrossRef - Rofani C., Luchetti L., Testa G., Lasorella R., Isacchi G., Bottazzo G. F., et al. IL-16 can synergize with early acting cytokines to expand ex vivo CD34+ isolated from cord blood. Stem Cells Dev. 2009;18(4):671-82. PMID: 19006448.
CrossRef - Laughlin M. J., Barker J., Bambach B., Koc O. N., Rizzieri D. A., Wagner J. .E, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344(24):1815-22. PMID: 11407342.
CrossRef - Gluckman E., Broxmeyer H. A., Auerbach A. D., Friedman H. S., Douglas G. W., Devergie A., et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321(17):1174-8. PMID: 2571931.
CrossRef - Ballen K. K., Spitzer T. R., Yeap B. Y., McAfee S., Dey B. R., Attar E., et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13(1):82-9. PMID: 17222756.
CrossRef - Barker J. N., Wagner J. E. Umbilical-cord blood transplantation for the treatment of cancer. Nat Rev Cancer. 2003;3(7):526-32. PMID: 12835672.
CrossRef - Jones R. J., Collector M. I., Barber J. P., Vala M. S., Fackler M. J., May W. S., et al. Characterization of mouse lymphohematopoietic stem cells lacking spleen colony-forming activity. Blood. 1996;88(2):487-91. PMID: 8695796.
- Gabbianelli M., Pelosi E., Montesoro E., Valtieri M., Luchetti L., Samoggia P., et al. Multi-level effects of flt3 ligand on human hematopoiesis: expansion of putative stem cells and proliferation of granulomonocytic progenitors/monocytic precursors. Blood. 1995;86(5):1661-70. PMID: 7544638.
- Bryder D., Jacobsen S. E. Interleukin-3 supports expansion of long-term multilineage repopulating activity after multiple stem cell divisions in vitro. Blood. 2000;96(5):1748-55. PMID: 10961873.
- Rossmanith T., Schroder B., Bug G., Muller P., Klenner T., Knaus R., et al. Interleukin 3 improves the ex vivo expansion of primitive human cord blood progenitor cells and maintains the engraftment potential of scid repopulating cells. Stem Cells. 2001;19(4):313-20. PMID: 11463951.
CrossRef - Tajima S., Tsuji K., Ebihara Y., Sui X., Tanaka R., Muraoka K., et al. Analysis of interleukin 6 receptor and gp130 expressions and proliferative capability of human CD34+ cells. J Exp Med. 1996;184(4):1357-64. PMID: 8879208.
CrossRef - Schipper L. F., Brand A., Reniers N. C., Melief C. J., Willemze R., Fibbe W. E. Effects of thrombopoietin on the proliferation and differentiation of primitive and mature haemopoietic progenitor cells in cord blood. Br J Haematol. 1998;101(3):425-35. PMID: 9633882.
- Ohmizono Y., Sakabe H., Kimura T., Tanimukai S., Matsumura T., Miyazaki H., et al. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia. 1997;11(4):524-30. PMID: 9096693.
- Petzer A. L., Zandstra P. W., Piret J. M., Eaves C. J. Differential cytokine effects on primitive (CD34+CD38-) human hematopoietic cells: novel responses to Flt3-ligand and thrombopoietin. J Exp Med. 1996;183(6):2551-8. PMID: 8676076.
- Kobari L., Giarratana M. C., Pflumio F., Izac B., Coulombel L., Douay L. CD133+ cell selection is an alternative to CD34+ cell selection for ex vivo expansion of hematopoietic stem cells. J Hematother Stem Cell Res. 2001;10(2):273-81. PMID: 11359674.
- Kurtzberg J., Laughlin M., Graham M. L., Smith C., Olson J. F., Halperin E. C., et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335(3):157-66. PMID: 8657213.
- McKenna H. J., de Vries P., Brasel K., Lyman S. D., Williams D. E. Effect of flt3 ligand on the ex vivo expansion of human CD34+ hematopoietic progenitor cells. Blood. 1995;86(9):3413-20. PMID: 7579445.
- Abboud M. R., Xu F., Payne A., Laver J. Effects of recombinant human Steel factor (c-kit ligand) on early cord blood hematopoietic precursors. Exp Hematol. 1994;22(4):388-92. PMID: 7512047.
- Suzuki A., Zheng Y., Kondo R., Kusakabe M., Takada Y., Fukao K., et al. Flow-cytometric separation and enrichment of hepatic progenitor cells in the developing mouse liver. Hepatology. 2000;32(6):1230-9. PMID: 11093729.
- Petersen B. E., Bowen W. C., Patrene K. D., Mars W. M., Sullivan A. K., Murase N., et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284(5417):1168-70. PMID: 10325227.
- Oh S. H., Miyazaki M., Kouchi H., Inoue Y., Sakaguchi M., Tsuji T., et al. Hepatocyte growth factor induces differentiation of adult rat bone marrow cells into a hepatocyte lineage in vitro. Biochem Biophys Res Commun. 2000;279(2):500-4. PMID: 11118315.
- Davila J. C., Cezar G. G., Thiede M., Strom S., Miki T., Trosko J. Use and application of stem cells in toxicology. Toxicol Sci. 2004;79(2):214-23. PMID: 15014205.
- Wang N. D., Finegold M. J., Bradley A., Ou C. N., Abdelsayed S. V., Wilde M. D., et al. Impaired energy homeostasis in C/EBP alpha knockout mice. Science. 1995;269(5227):1108-12. PMID: 7652557.
- Kasahara H., Usheva A., Ueyama T., Aoki H., Horikoshi N., Izumo S. Characterization of homo- and heterodimerization of cardiac Csx/Nkx2.5 homeoprotein. J Biol Chem. 2001;276(7):4570-80. PMID: 11042197.
- Alvarado-Moreno A., Chavez-Gonzalez A., Cerbulo A., Arriaga L., Mayani H. In vitro cell cycle dynamics of primitive hematopoietic cells from human umbilical cord blood. Hematology. 2010;15(1):11-20. PMID: 20132657.
- Walenda T., Bork S., Horn P., Wein F., Saffrich R., Diehlmann A, et al. Co-culture with mesenchymal stromal cells increases proliferation and maintenance of haematopoietic progenitor cells. J Cell Mol Med. 2010;14(1-2):337-50. PMID: 19432817.
- Robinson S. N., Ng J., Niu T., Yang H., McMannis J. D., Karandish S., et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37(4):359-66. PMID: 16400333.
- Mohamed A. A., Ibrahim A. M,. El-Masry M. W., Mansour I. M., Khroshied M. A., Gouda H. M., et al. Ex vivo expansion of stem cells defining optimum conditions using various cytokines. Lab Hematol. 2006;12(2):86-93. PMID: 16751136.
- McNiece I., Harrington J., Turney J., Kellner J., Shpall E. J. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy. 2004;6(4):311-7. PMID: 16146883.
- Jang Y. K., Jung D. H., Jung M. H., Kim D. H., Yoo K. H., Sung K. W., et al. Mesenchymal stem cells feeder layer from human umbilical cord blood for ex vivo expanded growth and proliferation of hematopoietic progenitor cells. Ann Hematol. 2006;85(4):212-25. PMID: 16391912.
- da Silva C. L., Goncalves R., Crapnell K. B., Cabral J. M., Zanjani E. D., Almeida-Porada G. A human stromal-based serum-free culture system supports the ex vivo expansion/maintenance of bone marrow and cord blood hematopoietic stem/progenitor cells. Exp Hematol. 2005;33(7):828-35. PMID: 15963859.

This work is licensed under a Creative Commons Attribution 4.0 International License.





