Manuscript accepted on : 05 December 2017
Published online on: --
Antioxidant Activities and Phytochemical Screening of Martynia Annua Fruit Extract
Zarina Arshad , Sumayya Saied and Shaista Naz
, Sumayya Saied and Shaista Naz
Department of Chemistry, University of Karachi, Karachi, Pakistan.
Corresponding Author E-mail: zarinaarshad37@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2581
ABSTRACT: Martynia annua (cat’s claw, bichu) is part of Martyniaceae family. For centuries, extracts of leaves, roots, stems, fruits and seeds of M. annua have been used to cure eplilepsy, inflammation, tuberculosis, skin infections etc. Present investigations revealed qualitative phytochemical screening and bioactivites of fruit extracts of M.annua in solvents of different polarties. The qualitative phytochemical analysis exhibited the presence of alkaoids, flavonoids, glycosides, saponins, tannis, anthocyanins, steroids, amino acids and phenols. DPPH (1.1-diphenyl-2-dipicryl hydrzal free radical scanvenging, reducing power assay and lipid peroxidation inhibition assay likability in different solvents were explored which revealed that with increase in concentration of extracts resulted increase in degree of reduction. The outcome of the present studies revealed that the fruit extracts of M. annua have eminent antioxidant activity
KEYWORDS: Antioxidant; DPPH;Lipid Martynia Annua; Phytochemical Screening; Peroxidation Inhibition; Reducing Power Assay
Download this article as:| Copy the following to cite this article: Arshad Z, Saied S, Naz S. Antioxident Activities and Phytochemical Screening of Martynia Annua Fruit Extract. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Arshad Z, Saied S, Naz S. Antioxident Activities and Phytochemical Screening of Martynia Annua Fruit Extract. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28368 |
Introduction
For thousands of years, plants have been good source of medicine to treat illness and maintain health. Mostly fruits, flowers, leaves, stem, barks and seeds of plants are rich in secondary metabolites that produce definite pharmacological effects on human body. M. annua is an upright short-lived herbaceous plant. The young fruits are oblong and green with long beak(claw), but when dry, becomes woody with two sharp hairy curved hooks.1 M. annua belongs to family Martyniaceae. It is native to Mexico, Central America, mostly naturalized in northern Australia and South Eastern Asia. It is commonly known as cat’s claw, devil’s claw and bichu. The fruits and leaves are biologically active parts of this plant.2 It is used for the treatment of epilepsy, inflammation and tuberculosis.3 The leaves of the M. annua are edible and used as antiepileptic, antiseptic and applied locally to tuberculosis glands of the neck.4 The juice of the leaves is used as a gargle for sore throat and the leaf paste for wounds of domestic animals.5 The unripe fruits of M.annua found to have antioxidant activity3 and the ash of fruits mixed with coconut oil are used to cure burns.4 The fruits are also used as local sedative and antidote to scorpion stings.6 Seed oil is used for abscesses and treating itching and skin infections. The seeds of M.annua are used for prevention of graying of hair.7The whole plant is used for fever, hair loss, scabies and abscess on the back.8
An antioxidant is a substance that prevents or delays oxidation of other molecules. Free radicals are produced during oxidation which can be trapped by antioxidants. In plants, natural exogenic antioxidant substances are present i.e. vitamins phenolic acids, flavonoids, phenolic diterpenes, oils and plant pigments like anthocyanins scavenge free radicals such as peroxide, hydroperoxide or lipid pexoxidation. Free radical and reactive oxygen species(ROS) are basically the main causes of several disorders in humans like cancer, heart disease, ageing, diabetes, Alzheimer’s, Parkinson’s diseases9 by inhibiting a reaction cycle. Different methods are used to assess the antioxidant and free radical scavenging activity. In vitro antioxidant activity is mostly measured by DPPH method developed by Biols (1958), hydrogen peroxide scavenging assay,10 nitric acid scavenging activity,11 ferric reducing antioxidant power assay12 and reducing power method.13 Present investigation reports DPPH, lipid peroxidation and ferric reducing power assay activities of the fruit extracts of M.annua.
Study Protocol
DPPH Assay
By using stable free radical, α,α-dipheny-β-picryl hydrazyl, the odd electron of nitrogen in DPPH is reduced by receiving hydrogen from antioxidents to corresponding hydrazine.14
Reducing Power Assay
In this method potassium ferrocynide, ferric chloride and trichloroacetic acid form a colored complex with antioxidant compound increase in absorbance of reaction mixture indicates the increase in antioxidant activity.
The present study revealed the qualitative phytochemical analysis and in vitro antioxident activities of ethanolic fruit extract partitioned in different solvents (ethanol, methanol, n-hexane, ethyl acetate, water) by scavenging effect on 1,1-diphenyl-2-picryldrazyl, ferric reducing power and lipid peroxide inhibition assay to protect the oxidative damage.
Material and Methods
The fruits of M.annua were collected from local market in december 2012 and identified by Dr. Rubina Dawar,Plant Taxonomist, Department of Botany, University of Karachi.The fruits of M.annua were dried (20kg) crushed and soaked in 30L of ethanol for 3 weeks. The extraction with ethanol was repeated thrice at room temperature. The extract was evaporated in rotary evaporator under pressure to yield a gum (1789 g). This gum was suspended in water (1L) and extracted with different solvents yielding n-hexane soluble fraction(910g), ethyl acetate soluble fraction(364g) and n-butanol(386 g). All the chemicals used were of analytical grade(Merck) and BDH.
Phytochemical Analysis
Qualitative phytochemical analysis of fruit extracts of M.annua in (Ethanol, ethyl acetate ,n-Hexane ,n-Butanol, water) for the identification of alkaloids, flavonoids, terpenoids, saponins, tannins, anthocyanins, steroids and cardiac glycosides were done by prescribed methods.15-17
Test for Alkaloids
Dragendorff’s Test: 5 mg of each extract was mixed with 1% HCl (5 mL) and heated on water bath for 5 minutes. After filtration, 2mL filtrate was treated with solution of potassium bismuth iodide (Dragendroff’s reagent). Formation of red ppt indicated the pressence of alkaloids.
Mayer’s Test: 2 mL of filtrate was treated with 1 mL potassium mercuric iodide solution (Mayer’s reagent). Formation of cream coloured ppt indicated the pressence of alkaloids.
Test for Flavonoids
Alkali Test: 5mg of each extract was dissolved in water. Filtrate was treated with 2mL of 10% NaOH To yield yellow coloured solution formed. On addition of HCl it became colorless showing the presence of flavonoids.
Filtrate was treated with 2 mL of dilute ammonia solution. Yellow color disappear after addition of 1 mL sulphuric acid indicated the presence of flavonoids. c. Lead acetate test: 2 mg of each extract (ethanol, ethyl acetate, n.hexane, n-butanol, water) was treated with few drops of lead acetate solution. Formation of yellow solution showed the presence of flavonoids.
Test for Terpenoids and Steroids
Salkowski’s Test: 2mg of each extract was mixed with 2 mL of chloroform then 3 mL of sulphuric acid was added through the walls of test tube to form a layer. A reddish brown colour of interface showed the existance of terpenoids or steroids.
Test for Saponins
Foam Test: 5 mg of each extract was shaken with 2 mL of water. Foam produced persisted for ten minutes proving the presence of saponins.
Test of Tannins
Ferric chloride test: 5 mg of each extract was boiled with 10 mL of water then filtered. Few drops of 0.1% ferric chloride were added in the filtrate. Bluish green ppt indicated the presence of tannins.
Potassium dichromate test: Filtrate(above) was treated with few drops of potassium dichromate,dark colour is developed showed presence of tannins.
Test for Phenols
2 mg of each extract weremixed with few drops of ferric chloride. Bluish black coloured appearance showed the presence of phenols.
Test for Carbohydrate
Molish Test: 2 mg of each of extract was disslolved in water then ethanol was added followed by few drops of α-napthol. Sulphuric acid was added through the walls of test tube. Violet ring appeared at the junction of two layers indicating the presence of carbohydrates in the fruits.
Test for Glycosides
Keller-Killiant Test: 1 mg of each of extract was mixed 5 mL of water. 2 mL of glacial acetic acid with few drop of ferric chloride were added. The sulphuric acid was then poured through the wall of test tube. Formation of brown ring at the interface proved the existance of glycosides.
Test for Anthocyanins
2 mg of each extract was treated 2 mL(2N) HCl. Reddish pink colour apperared which was changed to purple after addition of ammonia. proving the existance of anthocyanins.
Antioxidant Activity
DPPH Free Redical Scavenging Activity
Determination of DPPH radicals scavenging activity was estimated by method given by Ishikawa18 and with slight modification byAyoola GA, Sofidiya T (2006).19
The radical scavenging activites of different concentration of fruit extracts of Martynia annua in water, ethanol, n-hexane, n-butanol and ethyl acetate against DPPH were determined by spectrophotometer at 517nm. The stock solution (1M) of DPPH was prepared in methanol. The 0.01 mM solution of DPPH was prepared by adding 90 mL of methanol to10 mL of stock soluion. Vitamin C (Ascorbic acid) was used as an antioxident standard . The concentrations of standard and fruit extracts prepared were 10, 20, 30, 40, 50 and 60 mg/mL. The solutions of fruit extracts were treated with stable DPPH radical. The reaction mixture was prepared by adding 3 mL of 0.1mM solution of DPPH in 1 mL each of the different concentration of fruit extracts of M.annua and allowed to stand for 30 minutes in the dark. The inhibition of DPPH radical by the reaction of antioxidant was observed by change in colour from deep voilet to light yelllow. A blank solution was prepared containing same amount of DPPH and methanol. Tests were carried out in triplicate and the absorbance was recorded at 517 nm by using double beam UV/Visible spectrophotometer.. The percent inhibition was calculated by following formula.
% Inhibition=100 x (Ab – Aa /Ab).
where Ab is absorbance of blank and Aa is absorbance of extract.
% inhibition of both samples and blanks was calculated for each concentration. Graphs were plotted against % inhibition and concentration.
Ferric Reducing Antioxedent Power Assay(frap)
The reductive power capacity of water, n-butanol, ethanol, ethyl acetate and n-hexane extracts of M. annua fruit(method used was carried out by Oyaizu et al 1986). The 1 mL of each extract of increasing concentration (60, 40, 30, 2, 10 mg/mL) was mixed with phosphate buffer (0.2 M, 6.6 pH) and 2.5 mL potassium ferrocynate (1w/v) .The reaction mixtures were incubated at 50oC for 20 mints. 10% trichloroacetic acid (2.5mL) was added to each solution and centrifuged at 3000 rpm for 10 minutes. Supernatant of each solution was mixed with water (2.5 mL) and 0.1% of FeCl3 (0.5 mL). The absorbance was recorded at 700 nm using double beam UV/Visible spectrophotometer. The antioxidant activity of extracts was measured by the intensity of absorbance.
Lipid Peroxidationinhibition Assay
Lipid peroxidation is a chemical process in which unsaturated fatty acids of lipids are damaged by free radicals and oxygen under lipoperoxide formations . Lipoperoxides are unstable and decompose to form reactive carbonyl compounds, that damage cells by binding with free amino groups of proteins. One of oldest method for determiniation of lipid peroxides is iodometric method.20-21 The principle of this method is based upon ability of lipid peroxidases to oxidize iodide (I–) to iodine(I2), which is further titrated using sodium thiosulphate solution in the presence starch solution as indicator. The procedure used to measure the peroxide value was prescribed by Hortwitz(2002). The 60 mg/mL of each mustard oil treated with fruit extract (ethanol, ethyl acetate ,n.hexane ,n-butanol and water). were incubated at 60± 5oC for 4, 8, 12, 16, 20 and 24 days.22
After incubation, 0.5mL of freshly prepared solution of potassium iodide was added followed by the addition of 30 mL distilled water. The reaction mixture was titrated with 0.1 N sodium thiosulphate in the presence of starch solution. Peroxide value was measured as milliequivalent peroxide per kg oil.
Milliequuivalent of peroxide/Kg of oil = S × N × 1000/ Wt of sample
S = Volume of sodium thio sulphate used.
N = Normality of sodium thiosulphate.
Result and Discussion
Phytochemical Analysis
The qualitative phytochemical screening of fruit extracts of Martynia annua (Table 1) revealed the pressence of secondary metabolites such as flavonoids, terpenoids, saponins, tannins, anthocyanins, steroids and cardiac glycosides. Flavonoids and Tannins are major group of compounds that act as a primary antioxedants or free radical scanvengers.
Table 1: Pytochemical Analysis of Fruit Extract of Martynia Annua
| Compounds | Aqueous | Ethanol | n-Hexane | Ethyl Acetat | n-Butanol |
| Alkaloids | – | + | + | – | – |
| Flavonoids | + | + | + | + | + |
| Terpenoids | + | + | – | + | _ |
| Saponins | + | – | + | + | + |
| Steroids | – | + | + | + | – |
| Tannins | + | + | + | + | + |
| Phenols | + | + | – | + | + |
| Glycosides | + | – | – | + | + |
| Anthocyanins | + | – | – | + | _ |
DPPH Radical Scavenging Activity
Figure 1: shows the DPPH scavenging activity of different concentration of fruit extract of Martynia annua in water, ethanol, n-hexane, ethyl acetate and n-butanol. DPPH is nitrogen centered free radical having an odd electron that reacts with a compound that has a H-donating ability. It is reduced to diphenyl picryl hydrazine. After the reaction, the solution changes the colour from dark-purple to yellow which gives a strong absorbance at 517nm. This analysis showed an increase in DPPH scavenging activity with increase in concentration of extracts. The percentage of inhibition was maximum in a extract having 60 mg/mLconcentration. n-Hexane extract exhibited 28.06% inhibition, water extract 40.69%, ethyl acetate extract 60.53%, n-butanol extract 63.49% and ethanol showed 83.43% inhibtion with reference of ascorbic acid (60mg/mL) which revealed 95.8%. Among all, ethanolic fractions possessed highest antioxidant activity.
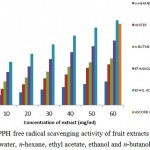 |
Figure 1: DPPH free radical scavenging activity of fruit extracts of Martynia annua in water, n-hexane, ethyl acetate, ethanol and n-butanol fractions.
|
Reducing Power Assay
Figure 2 showed: The reducing power capacity of water, n-butanol, ethanol, ethyl acetate and n– hexane extracts of M.annua fruits. Antioxidant components present in the extracts converted Fe+3 into Fe+2 (Benzie, Strain 1996). The results revealed that increase in reducing power activity with an increase in antioxidant .i.e. increase in concentration of fruit extracts. Among all, ethanolic fractions were found to possess highest antioxidant capability.
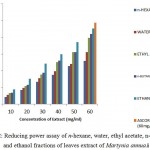 |
Figure 2: Reducing power assay of n-hexane, water, ethyl acetate, n-butanol, and ethanol fractions of fruit extract of Martynia annua.
|
Lipid Peroxidation Inhibition Assay
Fig 3 showed the lipid peroxidation inhibition assay of fruit extracts of Martynia annua in water, ethanol, n-hexane, ethyl acetate and n-butanol. The results exhibited significant protective efficiency of fruit extracts to protect the tissue from oxidative damage. Ethanol extract showed the highest LPOI activity.
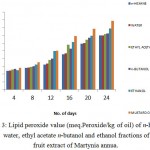 |
Figure 3: Lipid peroxide value (meq.Peroxide/kg of oil) of n-hexane, water, ethyl acetate n-butanol and ethanol fractions of fruit extract of Martynia annua.
|
Conculsion
The present investigation revealed phytochemical screening of the fruit extracts of Martynia annua. The qualitative analysis exhibited the presence of secondary metabolites such as flavonoids, alkaloids, steroids, tannins, saponins and glycosides. The fruit extracts also exhibited antioxidant potential which indicated that it can help to improve immune system. Antioxident activities measured by different methods like DPPH free radical scavaning, ferric reducing power and oxidative stress mechanism by lipid peroxidative assays. The ethanolic fraction showed maximum extent but in water these activities were also significant. The phenolic compounds and flavonoids are responsible of antioxidant activities.
Acknowledegements
The authors are thankful to, The chairman Department of chemistry, University of Karachi for generous assistance and financial support of higher education commission government of Pakistan.
Reference
- Manandhar N.P, Manandhar S. Plants and People of Nepal, Portland: Timber Press, Inc. 2002;311.
- Chopra R.N, Nayer S.L. Glossary of Indian Medicinal Plants, National institute of Science Communication, New Dehli. 1996;162.
- Kenwat P, Martynia annua. UK Journal of pharmaceutical and Biosciences. 2013;1(1):7-10.
- Babu B, Mohana L.S. Studies on phytochemical and anticonvulsant property of Martynia annua. International journal of Phytopharmacology. 2010;1(2):82-86.
- Singhai L.AK. Preliminary pharmacological evaluation of Martynia annua Asian Pacific journal of Tropical Biomedicine. 2011;1(6):421-427.
CrossRef - Ashwani D, Bhawan C, Sanjeet K.M. Martynia anuua: A Review on its Ethnobotany and Pharmacological Profile. Journal of Pharmacognosy and Phtochemistry. 2013;1( 6):135-138.
- Khare C.P. Encyclopedia of Indian Medicinal Plants. (1956-2007 updated). 2007;44(10):772-773.
- Kirtikar B. Indian Medicinal Plants, II.in 1730-38. Kokate KC, 4th ed. Delhi: Vallabh Prakashan,. Practical pharmacognosy. 1994;218.
- Young I.S. Woodside Antioxedent in health and diseases. Clinical.Pathology. 2001;54:176-186.
- Ruch R.J, Cheng S.J, Klaunig J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis. 1989;10:1003-1008.
CrossRef - David S. Bredt, Endogenous nitric oxide synthesis: biological function and pathophysiology. Free Radic. Res. 1999;31(6):577-596.
CrossRef - Virginia L.E, Sarah J.S, Rachel T, Joesh E.A. Mitochondril nitric oxide synthase: Role in pathophysiology. IUBMB Life. 2003;55(10):599-603.
- Benzie I, Strain J. The ferric reducing ability of plasma (FRAP) as measure of antioxidant power. Analytical Biochemistry. 1996;239:70-76.
CrossRef - Contreras-Guzman E.S, Strong F.C. Determination of tocopherols (Vitamin E) by reduction of cupric ion. JAOAC. 1982;65:1215–1222.
- Harborne J.B. Pytochemical Methods: A Guide to modern Techniques of plant analysis, London: 1973;1(10):100-117.
- Trease E and Evans W.C. Pharmacognosy. 13th Edition,London. 1989; 345-6(535-6):772-3.
- Sofowora A. Medicinal plants and Traditional medicine in Africa. Spectrum books Ltd, Nigeria. 1993;289.
- Kapoor S.L, Shirsvatava S.N. (1969), Indian medicinal plants for saponins, alkaloids and flavonoids. 1977;32:297-302.
- Ayoola G.A, Coker H, Adesegun S.A. Phytochemical screening and antioxident activities of some medicinal plants used for malaria therapy in Nigeria. Tropical Journal of Pharmaceutical Research. 2008;7(3):1019-1024.
- Halliwell B, Chirico S. Lipid peroxidation –its mechansm measurement, and siginificance. J.Clin.Nutr. 1993;57:715-725.
CrossRef - Takagi T, Mitsuno Y, Masumura M. Determination of peroxide value by colorimetric iodine method with protection of iodide as cadmium complex. Lipids. 1978;13:147–151.
CrossRef - Hortwitz W.(Ed), Peroxide Value of Oils and fats.Official Methods of Analysis of AOAC International. 2002;17:965798.

This work is licensed under a Creative Commons Attribution 4.0 International License.





