Manuscript accepted on : 25 November 2017
Published online on: --
Palaq1, Seema Langer1, Fayaz Ahmad2 and Nighat –Un- Nissa2
1Department of Zoology, University of Jammu, 180006.
2Department of Zoology, University of Kashmir, 190006.
Corresponding Author E-mail: cytogeneticsrocks18@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2576
ABSTRACT: The study describes the effect of pathological manifestation of an acanthocephalan parasite Neoechinorhynchus sp. in the intestines of fish Labeo rohita of river Tawi (Chenab) of Jammu. For the histopathological investigations, tissue samples from the infected fish were fixed in Bouin’s fixative for 24 hours, processed and 6-8 micron thick sections were prepared using standard microtomy techniques. This parasite caused severe histological changes in the fish intestine like damaged villi, shortening of villi, inflammation, hyperplasia, erosion of the normal structure, widening of the intestinal lumen and increase in the number of mucus cells. In case of serious infection, damage occurs to both mucosal and submucosal layers. Compression and absence of intestinal villi was also evident.
KEYWORDS: Hyperplasia; Labeo Rohita; Neoechinorhynchus; Tawi
Download this article as:| Copy the following to cite this article: Palaq P, Langer S, Ahmad F, Nissa N. U. Histolopathological Alterations in the Intestines of Labeo Rohita Infected with Acanthocephalan Parasite Neoechinorhynchus Sp. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Palaq P, Langer S, Ahmad F, Nissa N. U. Histolopathological Alterations in the Intestines of Labeo Rohita Infected with Acanthocephalan Parasite Neoechinorhynchus Sp. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28399 |
Introduction
Fish parasite infection constitutes one of the most important problems and challenges throughout the world. Various pathological reports of the acanthocephalan parasites on the digestive tract and other associated organs of fishes have been elaborated. Parasitism in fishes has become a major constraint in aquaculture (Bondad- Reantaso et al., 2005). In addition to the direct losses, parasites adversely affects the growth, making them more susceptible to predation thus affecting marketability and also paves way for secondary infections (Lorn and Dykova, 1992; Scholz,1999; Woo, 2006). Management of parasitism has become a major limiting factor in finfish aquaculture in terms of profitability and environmental health (Mustafa et al., 2001; Costello, 2009; Burridge et al., 2010).
Both fresh and marine water fish harbor a wide variety of endoparasitic helminthes including acanthocephalans. They involve arthropods as intermediate hosts and vertebrates as definitive hosts and also cause many diseased conditions in finfishes (Nickol, 2006). The insertion of proboscis deep into the tissue adversely affects the tissue architecture thus leading to diseased conditions. The absorptive efficiency of the animal is greatly affected by the damage to the intestinal tissue and formation of capsules associated with immune response of the host. Parasites have been known to migrate to certain uncommon locations and cause heavy infections thus causing occlusion of the gut (Nickol, 2006). In addition to the loss of valueable nutrients, release of toxins and localized toxemia have also been reported(Holloway, 1966; Schmidt et al., 1974).
Fresh water fishes including carps constitute an important part of the human diet across the world. Needless to say that in J & K state also, Labeo rohita is one of the most relished and most commonly consumed fish by the fish eating section of the population. This study aims to examine the histopathological changes in the intestines of Labeo rohita caused by acanthocephalan parasite Neoechinorhynchus sp.
Material and Methods
The examined fish species Labeo rohita were collected throughout the year from river Tawi. Total length and weight of the fishes were measured and examined for the parasitic infections in the intestine. Besides, site of attatchment, orientation and number of parasites in each fish were recorded. For histological procedure, the tissue samples of infected intestines were fixed in Bouin’s fixative for 24 hours, dehydrated using graded series of alcohol, then infilterated and embedded in paraffin wax. By using rotary microtome, 6-8 micron thick sections were prepared and stained in haematoxylin-eosin. These sections were mounted in Canada balsam. Finally microphotographs were taken with the help of Olympus microscope.
Results and Discussions
Parasitism is responsible for causing heavy mortality both in wild and cultured fish. Smaller sized fishes are more susceptible to parasitism because they cannot support a parasitic burden and succumb to death earlier than the large sized fish (Krist and Lively, 1998). Weaker and stressed fish are always more susceptible and provide an ideal place for parasites to live and flourish. In the present study, since the intensity of infection is not that large, therefore apparently there were no external morphological symptoms regarding the presence of parasites but the parasites have caused major histological changes in fish intestines. The helminth parasites not only affect the morphology of the infected organ but also interferes with the nutrition and metabolism, disturb the movements and secretory functions of the alimentary canal and also effects the glands of internal secretion. All these adverse changes may lead to the disease or death of the host. The degree of pathogenicity and the damage caused, however depend upon the toxins released by the parasite and the xtent of the proboscis inserted in the tissue layers.The main pathological changes in the host include abnormal increase or decrease in the layers of intestine and haemorrhage of cells.
Presently, the intestinal tissue of Labeo rohita has revealed some interesting histopathological changes. The tissue damage is quite prominent showing the degeneration of intestinal folds. Heavy parasitic infilteration caused flattening of villi, flaking of their apices and abrasion of the epithelium. The shrinkage of villi is quite evident causing the widening of the intestinal lumen (Fig 1). Heavy infections, as recorded presently not only cause morphological changes but also promotes fish mortality that adversely affects the production in nature as well as in captivity. Though this type of parasitic infestations usually occur in farmed fish mostly due to overcrowding and thereby bring the fish under stress thus making them susceptible to many parasitic infestations but parasitic outbreaks are also known to occur under natural conditions (Irshadullah et al 2009).
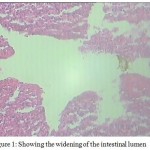 |
Figure 1: Showing the widening of the intestinal lumen
|
The other histopathological changes observed in the intestine are fibrosis associated with hyperplasia and metaplasia, vacuolation of submucosal cells and proliferative changes which lead to degeneration of various layers of the intestine. The pathological changes include an increase in the thickness and the damage to serosa, mucosa and submucosa layers. The decrease in the thickness of the muscular layer occurs as a consequence of helminthic infection.
Acanthocephalans mostly cause adverse effects on the intestinal layers due to deeper penetration of proboscis which causes excessive mucus secretion which may be a consequence of host’s defense mechanism against parasitic invasion that acts as a physical barrier for the micro organisms, parasites and their toxins (Lamont, 1992 and Bosi et al., 2005) therefore preventing secondary infection. A survey of literature reveals that the nature of helminth infections in vertebrates has been studied extensively but information on the pathogenic effects particularly in the fresh water fishes of Jammu has remained completely meagre. Present study has therefore been undertaken to ascertain the histopathological changes in the tissues of host fish L. rohita collected from Tawi river of Jammu region.
The gross pathological examination of intestine revealed heavy infection of acanthocephalans. It was found that the infected intestinal tissue gets broken down due to penetration of the hooks deep inside the tissue. Also the histopathology shows that acanthocephalan parasites are attatched to the tissue due to their boring action, causing shortening, flattening and damage of villi, destructed mucosa, submucosa of intestine by penetration of hooks. The histology of intestine of infected fish showed complete change in the architecture of villi. The number and the type of of parasites greatly influences the extent of the damage caused to the tissue (Bauer, 1941). When parasites are in abundance, they often cause gross pathological changes and damage to the host (Heckman, 1966; Hoffman, 1998). Though in the present study the intestines was not overcrowded with parasites, instead they were found at localized positions in the intestines, but the extent of damage was quite significant due to the boring action of the acanthocephalans.
In the present findings, histopathological studies revealed that parasite caused extensive damage to all the layers of intestine accompanied with disintegration of villi, erosion and loss of normal structure (Fig 2).
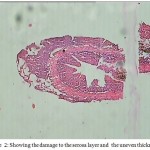 |
Figure 2: Showing the damage to the serosa layer and the uneven thickness.
|
These results are in accordance with the findings of Sanil et al. (2011) who also witnessed a similar pattern of erosion of intestinal villi in fishes. The helminth parasites when lodge in the intestinal wall with the help of the proboscis, cause damage to the villi and other layers of the intestine Benarjee and Reddy 2006 & 2008, Reddy and Benarjee, 2014. In our findings, pathological symptoms include hyperplasia of intestinal villi and lamina propia , curling of the villi (Fig 3) which is primarily host response against parasitic infection.
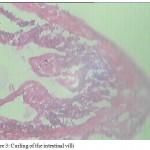 |
Figure 3: Curling of the intestinal villi
|
Distortion and separation of muscular layer and hyperplasia of goblet cells along with an increase in the number of mucus cells (Fig 4) are other pathological conditions associated with parasitic invasion.The mucus layer protects the intestinal mucosa acting as a barrier against various damages caused by the parasites (Bosi and Dezfuli, 2015).Arellano et al. (2001) correlated such functions with the production of acid mucins. Salah et al. (2012) has reported a rise in the number of goblet cells and hyperplasia.
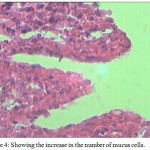 |
Figure 4: Showing the increase in the number of mucus cells.
|
Intestinal folds at the site of parasite attatchment caused the erosion of tips (Fig 5) along with the hyperplasia of the intestinal villi and lamina propia. Moreover,degeneration and necrosis of the mucosal epithelium are also clearly evident in the figure and in many areas they are totally eroded. The uneven thickness of the layers of intestines is quite evident in this study which drastically reduces the absorptive area thus interfering with the absorptive functions of the animal.
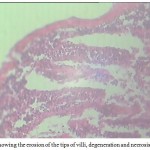 |
Figure 5: Showing the erosion of the tips of villi, degeneration and necrosis, hyperplasia
|
These results are in agreement with the results of Bullock (1963), Chaicharn and Bullock (1967), Sanil et al. (2010), Khurshid and Fayaz (2012). Flattening of villi and deep lesions in the intestinal wall were observed which might impair the digestion and absorption of food as well as water and electrolytes as it is suggested that intestinal helminthes often provoke structural modifications of host tissue and induce alterations in normal intestinal physiology (Hoste, 2001). Studies pertaining to the effect of fish parasites on fish morphology and physiology is already on record (Gussev, 1976; Amin, 1986; Zaman et al., 1986; Jha and Sinha 1990; Banu et al., 1993; Shomorendra et al., 2005; Bhuiyan et al., 2007; Shomorendra et al., 2007). In India reported cases of helminth in L.rohita are already on record (Malhotra and Chauhan, 1984; Tripathi, 1959). Similar cases were reported by Bilqees and Khan (1990) in Pakistan wherein they have reported that parasitic invasions in fish intestines also cause the disappearance of the epithelial cells and eating up cell stage (Fig. 6).
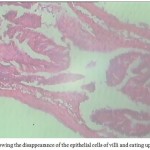 |
Figure 6: Showing the disappearance of the epithelial cells of villi and eating up cell stage
|
Parasites often affect their hosts through the diversion of resources either directly by using up energy and nutrients or indirectly by enhancing the immune response (Wedekind 1992, Deerenberg et al., 1997; Candolin and Voigt 2001). Parasites may also affect the food preferences of the host which in turn also results in delayed growth and stunting. Such observations however, are seen only in laboratory conditions and not in the wild (Karvonen and Sepala, 2008; Tops et al., 2009). This may be due to the fact that parasite infected stunted fish may not survive in the wild and are more susceptible to predation.
Acknowledgements
One of the author, Ms. Palaq is thankful to University Grants Commission for financial assistance through NET JRF. The author is also thankful to the Head, Deptt. of Zoology, University of Jammu for providing the necessary facilities in the laboratory. The author is equally thankful to Prof. Fayaz Ahmed , Deptt. of Zoology,University of Kashmir for taxonomic identification of parasites.
References
- Amin O.M. Acanthocephala from lake fishes in Wisconsin: host and seasonal distribution of species of genus. Neoechinorhynchus Hamann, Parasitol. 1986;72(1):111–118.
CrossRef - Banu A.N.H, Hossain M.A and Khan M.H. Investigation into the occurance of parasites in carps, catfish and tilapia. Prog Agric. 1993;4:11-16.
- Bauer O.N. Contributions to the knowledge of fish parasites of the river Khatanga. Inta. Pol. Zeml. Ser. Prom. Khaz. 1941;16:84-103.
- Benarjee G and Laxma B.R. Histopathological and histochemical changes in the liver of Clariasbatrachus due to trematode hetreostomum. J. Natcon. 2006;18(2):251-259.
- Benarjee G and Laxma B.R. Pathological and histochemical changes on the liver of freshwater murrel infected with trematode parasite. Ecotoxicol Environ. Monitor. 2008;18(6):565-572.
- Bhuiyan A.S, Akther S and Musa G.M. Occurrence of parasites in Labeo rohita (Hamilton) from Rajshahi. Univ J. Zool Rajshahi Univ. 2007;26: 31–34.
- Bilqees F.M and Khan A. A new trematode Laciotocus rohtai (Trematoda :Monorchidae) from the fish Labeo rohita of Kalri Lake , Sind, Pakistan. Pak .J. Zool. 1990;22(4):323-327.
- Reantaso B.M.G, Subasinghe R.P, Arthur J.R, Chinabut O.S, Adlard R.T and Shariff Z.M. Disease and health management in Asian aquaculture . Parasitology. 2005;132:249-272.
CrossRef - Bosi G, Arrighi S, Giancamilo D.A and Domeneghini C. Histochemistry of glycol conjugates in mucous cells of Salmo trutta uninfected and naturally parasitized with intestinal helminthes. Disease of Aquatic Organism. 2005;64:45-51.
CrossRef - Bosi G and Dezfuli S. Responses of Squalius cephalus intestinal mucous cells to Pomphorhynchus laevis. Parasitol Int. 2015;64:167–172.
CrossRef - Bullock W.L . Intestinal histology of some salmonid fisheswith particular reference to the histopathology of acanthocephalan infections. Journal of Morphology. 1963;112:23-44.
CrossRef - Burridge L, Weirs J.S and Bostick K. Chemical use of salmon aquaculture, a review of ament practices and possible environmental effects. 2010;306:7-23.
- Candolin U and Voigt H.R. No effect of a parasite on reproduction in stickleback males:a laboratory artifact? 2001;122:457-464.
- Chaicharn A and Bullock W.L. The histopathology of acanthocephalan infections in suckers with observation on the intestinal histology of two species of catostomid fishes. Acta Zoologica. 1967;48:19-42.
CrossRef - Costello M.J. The global economic cost of sealice to the salmonid farming industry. Journal of fish diseases. 2009;32:115-118.
CrossRef - Deerenberg C, Apanius V, Daan S and Bos N. Reproductive effort decreases antibody responsiveness. Proceedings of the Royal Society of London. B. 1997;264:1021-1029.
CrossRef - Gussev A.V. Fresh water Indian Monogenoidea. Principles of systematic, analysis of the worldfaumas and their evolution . Indian J Helminth. 1976;25(26):1-124.
- Heckman R . Protozoan parasites of fish, Part 1. Mag. 1996;22(3):44-57.
- Hoffman G.L. Lesions due to internal helminthes of freshwater fishes. In the pathology of fishes (W.E Ribelin & G Higaki, eds.)The university of Wisconsin Press. Madison. Wisconsin . 1967;151-186.
- Hoffman G.L. Parasites of North American freshwater fishes. Second edition. Univ. California Press London.
- Holloway H.L. Prosthorhynchus formosum in song birds with notes on acanthocephalans as potential parasites of poultry in virginia. Virginia journal of science. 1966;17:149-154.
- Irshadullah M, Nizami W.A and Macpherson C.N.L. Observations on the suitability and importance of the domestic intermediate hosts of. echinococcus granulosus. in Uttar Pradesh India. 2009;63(1):39-45.
- Jha A.N and Sinha P. The occurrence of helminth parasites in relation to size of fish. Bio J. 1990;2(11):311–316.
- Karvonen A and Seppala O. Effect of eye fluke infection on the growth of white fish (Coregonus lavaretus)-An experimental approach. 2008;279:6-10.
- Khurshid I and Fayaz A. A short note on heavy infection of acanthocephalan worm (Pomphorhynchus kashmiriensis) in the alimentary canal of naturally infected snow trout, Schizothorax niger (Haeckel).International Journal of Advanced Life Sciences. 2012;5(1):79-84.
- Krist A.C and Lively C.M. Experimental exposure of juvenile snails (Potamopyrgus antipodarum) to infection by trematode larvae (Microphallus ) infectivity, fecundity compensation and growth. Oecologia. 1998;116:575-582.
CrossRef - Lamont J.T. Mucus: the front line of intestinal mucosal defense. Annual New York Academic Science. 1992;664:190-201. Acta Zoologica. 1992;48:19-42.
- Lorn J and Dykova I . Protozoan parasites of fishes. Developments in aquaculture and fisheries sciences. 1992;26:315.
- Malhotra S.K and Chauhan R.S . Distribution of cestodes in the digestive tract of Indian hill-stream fishes. Korean J.Parasitol. 1984;22(2):238-241.
CrossRef - Mustafa A, Rankaduwa W and Campbell P. Estimating the cost of sea lice to salmon aquaculture in eastern Canada .The Canadian veterinary journal. 2001;41:54-56.
- Nickol B.B. Fish diseases and disorders second edition :Protozoan and metazoan parasites.CAB International, Wallingford UK. 2006;444-465.
- Reddy B and Benarjee G. Histopathological changes induced by cestode parasite in fresh water murrel. 2014;2(1):324-328.
- Salah Y.M.H, Idris O.F and Sabahelkhier M.K. Pathological studies on the small intestine of wild rabbit fish infectd with helminth parasite (Procamellus sp.) in Red sea coast area, Sudan. Journal of Asian Scientific Research. 2012;2(3):132-140.
- Sanil N.K, Asokan P.K, Jhon K.K and Vijayan K.K. Pathological manifestations of of the acanthocephalan parasites Tenuiproboscis In the mangrove red snapper, a candidate spcies for aquaculture from Southern India. Aquaculture. 2010;3(10):3-4:259-260.
- Schmidt G.D, Walley H.D and Wijek D.S. Unusual pathology in a fish due to the acanthocephalan. Acanthocephalus jacksoni. The Journal of Parasitology. 1974;60:730-773.
CrossRef - Scholz T. Parasites in cultured and feral fish.Veterinary Parasitology. 1999;84(3-4):317-335.
CrossRef - Shomorendra M, Jha A.N and Kumar P. Seasonal occurrence of helminth parasites in fishes of Loktak lake, Manipur. Uttar Pradesh .J. Zool. 2005;25(1):23–27.
- Shormorendra M, Jha A.N and Kumar P. Seasonal occurance of helminth parasites in fishes of Loktak lake, Manipur. Uttar Pradesh J Zool. 2007;25(1):23-27.
- Tops S, Hartikainen L and Okamura B. The effect of infection by Tetracapsuloides bryosalmonae (Myxozoa) and temperature on Fredericella sultana (Bryozoa).International Journal of Parasitology. 2009;9:1003-1010.
CrossRef - Tripathi Y.R . Monogenetic trematodes from fishes of india. J. Helminth. 1959;9(1-2):1-149.
- Wedekind C. Detailed information about parasites revealed by sexual ornamentation. Proceedings of the Royal Society of London .B. 1992; 247:169-174.
CrossRef - Woo P.T.K. Fish diseases and disorders 2ndedition . Protozoan and metazoan infections, CAB International , Wallingford UK. 2006;808.
CrossRef - Zaman Z, Leong T.S and Hamida K.A. Effects of length (equal age) of Clarias on the abundance of parasites. Bangladesh J. Zool. 1986; 14(2):171–178.

This work is licensed under a Creative Commons Attribution 4.0 International License.





