Manuscript accepted on : 11 December 2017
Published online on: --
Azmi Naqvi and Dinesh C. Sharma
Department of Zoology, Km. Mayawati Government Girls P.G. College, Badalpur, Gautam Buddha Nagar (U.P.), India – 203207.
Corresponding Author E-mail: azminqv@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2592
ABSTRACT: Herein, we describe a quick method of immobilization of invertase onto photoreactive long chain alkylamine controlled pore glass (LCAA-CPG). Photorective LCAA-CPG which is prepared using microwave radiation holds at least one photoactivable functional group capable of forming a covalent bond with the biomolecule in a photochemical reaction. Invertase is subsequently immobilized onto Photorective LCAA-CPG using photochemical reaction.
KEYWORDS: Controlled Pore Glass 1-Fluoro-2-Nitro-4-Azidobenzene;Invertase; Immobilization;
Download this article as:| Copy the following to cite this article: Naqvi A, Sharma D. C. Rapid and Efficient Method of Immobilization of Invertase on Long Chain Akylamine Controlled Pore Glass. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Naqvi A, Sharma D. C. Rapid and Efficient Method of Immobilization of Invertase on Long Chain Akylamine Controlled Pore Glass. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28497 |
Introduction
Demand for immobilization of biomolecules onto organic or inorganic polymer has increased dramatically in recent years, particularly in the post genome era. Immobilized biomolecules have versatile applications in clinical laboratories, biosensors, membrane bioreactors, diagnostics, genomics, proteomics, drug screening, affinity chromatography and many other related fields.1-2 There are different approaches to immobilize a biomolecule onto an inert surface such as (i) to activate the polymer surface, (ii) to activate the biomolecule or (iii) activate both prior to immobilization. From the practical point of view, approach (i) is a better choice as in most of the cases the biomolecules are precious and sensitive towards chemicals and during activation some of them may be lost.
There are various methods available for immobilization of biomolecules onto the polymer surfaces. Covalent immobilization may results in better biomolecule activity, reduced nonspecific adsorption, and greater stability.3 Glucose oxidase, uricase, xanthine oxidase and penicillin acylase were immobilized on alkylamine glass or silica gel through glutaraldehyde coupling method.4 Glucose oxidase, cholesterol oxidase and uricase immobilized on controlled pore glass were applied for determining glucose, cholesterol and urea respectively in serum. Thionyl chloride-activated succinamidopropyl-controlled-pore glass was used to covalently immobilize rabbit anti- bovine IgG.5 Enzymes such as acetylcoenzyme A synthetase was immobilized on CNBr-activated controlled pore glass. Activation of inert organic polymer surface such as polystyrene, polypropylene or polyethylene has been reported to occur through radiation graft polymerization or gaseous plasma technique.6-7 However, all these methods suffer from one or more’ shortcomings such as (i) they are tedious (ii) they are time consuming, (iii) they require hazardous chemicals, or (iv) require harsh reaction conditions. The photolinker- mediated technique permits covalent binding of a biomolecule to solid surface under gentle reaction condition. This method is usually based on a photolinker having atleast two functional groups, one of which is essentially a photoactivable group.
However, in most of these photolinker- mediated procedures, surfaces were activated by a photochemical reaction whereas biomolecules were immobilized by a thermochemical reaction. Hence, biomolecule must have one or more functional group (s) for reaction with the activated surface resulting covalent linkage. In contrast, in photochemical immobilization technique, biomolecule can be immobilized irrespective of their functional group. There are very few reports on photochemical immobilization of biomolecule. Sigrist, H. et al immobilized biomolecule on an inert surface by using a photolinker polymer made by reaction of BSA and 3-(triethylamine)-3-(m-isothiocyanophenyldiazirine). Photolinker polymer was then used to bind streptavidin as well as polystyrene surface simultaneously by exposing to light.8 However, preparation of this photolinker itself is a time consuming multistep procedure.
The main object of the present work is to provide a simple and quick method of immobilization of invertase on to photoreactive LCAA-CPG. Photorective LCAA-CPG is prepared using microwave radiation. Prepared LCAA-CPG holds at least one photoactivable functional group capable of forming a covalent bond with the biomolecule in a photochemical reaction. Invertase is subsequently immobilized onto the prepared Photorective LCAA-CPG using photochemical reaction.
Experimental
Material and Methods
Invertase, long chain alkyl amine-controlled pore glass (Normal diameter 500 A°, Mesh size: 80-120) were purchased from Sigma, USA. Sodium chloride, di-sodium hydrogen orthophosphate, sodium dihydrogen ortho phosphate, citric acid, sucrose, benedict solution were of analytical grade purchased from Merck, India. FNAB was prepared from 4-fluoro-3-nitroaniline by diazotization reaction as reported earlier.9 Microwave mediated reactions were carried out in BPL Sanyo microwave oven operating at a frequency of 2450 Hz with a power output of 700 watts. Photo-immobilization was carried out at a wavelength of 365 nm in an U.V stratalinker; model 2400, (Stratagene, USA) fitted with five 15-watt tubes. All the solutions were freshly prepared in triple-distilled water before use. Phosphate Buffered Saline (PBS) was prepared by mixing 0.85 % NaCl to 0.01 M phosphate buffer (pH 7.2). Wash buffer was prepared by adding (0.1% Tween 20 in PBS).
Optimization of amount of FNAB for the preparation of photoreactive LCAA-CPG controlled pore glass
50 mg FNAB dissolved in 500 µl of methanol was mixed with 50 mg of LCAA-CPG beads in the petridish. After thorough mixing, the beads were dried in air in dark. The dried FNAB coated beads were exposed to microwave radiation for 60 seconds. Support was then washed with methanol and dried to get photoreactive LCAA-CPG. The 48 mg of activated LCAA-CPG was then mixed with Invertase (48 µg/ 80 µl of 0.01 M, pH 7.4 phosphate buffer) and exposed to photoradiation for 60 min in UV stratalinker. After washing, 100 µl of 70 % sucrose solution was added onto the activated support and incubated at 28°C for 1 h. Benedict solution (200 µl) was added to each of the activated support and the change in color was recorded colorimetrically. Amount of FNAB was optimized by performing the same experiment with different amount of FNAB (12.5, 25 and 75 mg respectively) for the activation of 50 mg of controlled pore glass. Control experiment was carried out without using FNAB (FNAB: 0 mg)
Optimization of exposure time for microwave irradiation for making photoreactive LCAA- CPG.
Microwave exposure time for making photoreactive LCAA-CPG was optimized by changing microwave exposure time (30, 50, 60 and 70 seconds) in the reaction of 50 mg LCAA-CPG with 50 mg FNAB as described in above experiment. Photoreactive surface thus prepared in each reaction was checked by irradiating 48 μg of enzyme with 48 mg support and assaying the immobilized enzyme as described in above.
Optimization of photoirradiation time for enzyme immobilzation onto the photoreactive LCAA- CPG
Immobilization time was optimized by mixing 50 mg photoreactive LCAA- CPG with invertase (48 μg) and irradiating it by UV in different time (2, 10, 20, 40 and 60 minutes) for each reaction. Immobilized enzyme was then assayed colorimetrically after addition of substrate as described above.
Optimization of enzyme concentration for its immobilization onto microwave- activated LCAA- CPG.
Optimization of Invertase concentration for 50 mg photoreactive LCAA-CPG was carried out by taking different amount of enzyme (6, 12, 24, 48 and 96 μg) for each reaction and irradiating it for 20 minutes by UV light. Immobilized enzyme was then assayed after addition of substrate as described in above experiments.
Result and Discussion
There are few methods reported for the immobilization of biomolecules onto the LCAA-CPG surfaces. However, the reported methods are tedious and time consuming procedure. In this paper, we report a simple and mild method for activation of LCAA-CPG using 1-fluoro-nitro-4-azidobenzene and immobilization of invertase onto it. Schematic representation of the thermoactivation of PVC and immobilization of protein ligand is shown in figure 1.
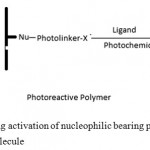 |
Figure 1: Scheme showing activation of nucleophilic bearing polymer for the immobilization of biomolecule
|
Solid surface nucleophilic group reacts with 1-fluoro-2-nitro-4-azidobenzene thermally to produce an activated support. Protein is then immobilized onto the activated surface by UV radiation at 365 nm
Quick method for preparation of photoreactive polymers and immobilization of invertase onto it is developed. Particularly, this work is related to a microwave-mediated rapid and efficient method for the preparation of photoreactive polymer having atleast one photoactivable functional group capable of forming a covalent bond with the biomolecule in a photochemical reaction. Any matrix either organic or inorganic having a nucleophilic group preferably, amino, hydroxyl or thiol group can be modified by this method. Immobilization of biomolecules onto the photoreactive polymers was carried out by UV radiation. The method has applications for immobilization of bio-molecules irrespective of their functional groups in the fields of molecular biology, proteomics, genomics, diagnostics, chemical or biochemical industry and other related fields.
Concentration of photolinker plays an important role in preparing photoreactive polymer. Best result was obtained when the ratio of the photolinker (FNAB) and polymer is 1:1 (w/w). Further increase in FNAB did not increase the photoreactive group in a polymer. When untreated surface (FNAB: 0 mg) was used, there was practically no immobilization of biomolecule onto it. Microwaves were found an excellent tool for making photoreactive surface. Thus, photoreactive LCAA-CPG prepared in 70 second of microwave irradiation was found to give best results. The optimum time for photoimmobilization of enzyme on photoreactive LCAA-CPG was found as 20 minutes. However, increase in UV irradiation time beyond 20 minutes did not increase immobilization. Enzyme concentration is also an important factor for immobilization. 2 μg Invertase / 48 mg photoreactive LCAA-CPG was found optimum for the immobilization on PVA and further increase did not appreciable increase its immobilization. However, optimum amount of HRP was 4 μg/ 48 mg support for immobilization on photoreactive LCAA-CPG.
Table 1: Optimization of enzyme required for its optimum immobilization onto the photoreactive polymer
| S.No | Enzyme Concentration (µg) | Optical Density | |
| Unactivated polymer | Activated Polymer | ||
| 1 | 6 | 0.15 | 0.42 |
| 2 | 12 | 0.21 | 1.81 |
| 3 | 24 | 0.31 | 1.85 |
| 4 | 48 | 0.33 | 1.88 |
| 5 | 96 | 0.35 | 1.85 |
Enzyme required for immobilization onto photoreactive Controlled pore glass was determined by taking 6, 12, 24, 48, 96 µg enzyme separately followed by UV irradiation for 20 min. Absorbance was recorded after addition of substrate to the immobilized enzyme.
Conclusion
LCAA-CPG was activated for immobilization of enzymes, invertase. Immobilized invertase can be used for the production of invert syrup extracting from beet or cane sugar and for prevention of crystallization of sugar in confection by hydrolysis of sucrose (glucose and fructose) in chocolate coated candies with soft centers.
References
- Neděla O al. Surface Modification of Polymer Substrates for Biomedical Applications. Materials (Basel). 2017;10(10);1115.
CrossRef - Kim D al. Protein immobilization techniques for microfluidic assays. Biomicrofluidics. 2013;7(4);041501.
CrossRef - Ahmad al. Enzyme immobilization: an update. J. Chem Biol. 2013;6(4); 185–205.
CrossRef - Jung D et. al. Covalent Anchoring of Chloroperoxidase and Glucose Oxidase on the Mesoporous Molecular Sieve SBA-15. Int. J. Mol. Sci. 2010;11:762-778.
CrossRef - Casale E.S al. Anti-IgG immobilized controlled-pore glass. Thionyl chloride-activated succinamidopropyl-glass as a covalent immobilization matrix. Stabel T .J. Appl Biochem Biotechnol. 1992;36(2);87-96.
- Abednejad A.S al . Surface modification of polypropylene membrane by polyethylene glycol graft polymerization. Mater Sci Eng C Mater Biol Appl. 2014;42:443-50.
CrossRef - Asadinezhad A et. al. Recent Progress in Surface Modification of Polyvinyl Chloride. 2012;5(12).
- Collioud A. et.al. Oriented and covalent immobilization of target molecules to solid supports: synthesis and application of a light-activatable and thiol-reactive cross-linking reagent. Bioconjug Chem. 1993;4(6):528-36.
CrossRef - Naqvi A. et. al. Introduction of functional groups onto polypropylene and polyethylene surfaces for immobilization of enzymes. Anal Biochem. 2002;1;306(1):74-8.

This work is licensed under a Creative Commons Attribution 4.0 International License.





