Manuscript accepted on : 22 December 2017
Published online on: --
Mohd Tasleem1,2 , Mamta Baunthiyal1 and Gohar Taj2
, Mamta Baunthiyal1 and Gohar Taj2
1Department of Biotechnology, G. B. Pant Institute of Engineering and Technology, Pauri Garhwal, India.
2Department of Molecular Biology and Genetic Engineering, College of Basic Science and Humanities, G. B. Pant University of Agriculture and Technology, Pantnagar, India.
Corresponding Author E-mail: mohdtasleem99@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2593
ABSTRACT: Alternaria brassicae causes a highly destructive disease in Brassica juncea (Rapeseed mustard) resulting in significant yield losses. Studies of MAPK machinery components in Arabidopsis thaliana have indicated that MPK3, MPK4, & MPK6 are involved in defense response and provide resistance against various bacterial and fungal pathogens. In this study, we analyzed the expression level of MPK3, MPK4 & MPK6 in overexpressed MPK3 transgenic (BjV5) Brassica juncea at different stages of Alternaria brassicae inoculation.Expression study revealed that MPK3/MPK6 was involved in early defense response and MPK4 in late defense response. These results suggested that BjMPK3 positively regulate SA mediated defense response, which might play an important role in resistance to Alternaria brassicae in Brassica juncea.
KEYWORDS: Alternaria Brassicae; Brassica Juncea;MPK3; MPK4; MPK6 and BjV5
Download this article as:| Copy the following to cite this article: Tasleem M, Baunthiyal M, Taj G. Induction of MPK3, MPK6 and MPK4 Mediated Defense Signaling in Response to Alternaria Blight in Transgenic Brassica Juncea. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Tasleem M, Baunthiyal M, Taj G. Induction of MPK3, MPK6 and MPK4 Mediated Defense Signaling in Response to Alternaria Blight in Transgenic Brassica Juncea. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28514 |
Introduction
Indian mustard [Brassica juncea (L.) Czern & Coss] is an important rabi oilseed crop in India.As indicated by USDA report, the aggregate production of rapeseed mustard in 2015-2016 is diminished by 6.35% in the World wide and 4.91% in India as contrast with earlier year 2014-2015.Somewhat diminished oil seed production is because of biotic and abiotic factors. In India, more than 30 diseases are known to happen on brassica crops.1 Alternaria blight is one of the most important diseases of mustard that leads to major yield losses as well as deterioration in quality of oil seeds. Symptom of this disease is characterized by formation of black/brown spots on leaves, stem and siliquae.2 The pathogen of Alternaria brassicae delivers a chlorotic toxin known as destruxin B that plays an important role in signal transduction prompting to programmed cell death.3 Plant immune system is activated by recognition of common microbial components (MAMPs, microbe-associated molecular patterns), for example, bacterial flagellin or the fungal cell wall component chitin.4,5 MAMP recognition invigorates to intracellular calcium influx, generation of reactive oxygen species, initiation of mitogen-activated protein kinases (MAPKs), and the production of salicylic acid.6,7 Investigation of plant pathogen interaction has been accounted for defense responses against microbes/pathogens which are modulated by a complex network of interconnecting signaling pathways in which the plant signal molecules salicylic acid (SA), jasmonate (JA), and ethylene (ET) plays an important role against biotic/ abiotic stresses.8,9,10
Mitogen Activated Protein Kinase (MAPK) pathway has a vital role in plant defense against both bacterial and fungal pathogen.11,12 MAPK action is controlled by successive phosphorylation by the receptor itself, intermediate bridging factors or interlinking of kinase proteins.13,14 These conserved signalling cascades are generally composed of MAPKKK (MAPK kinase kinase), MAPKK (MAPK kinase) and MAPK, also it functionally translates extracellular signal into intracellular responses.15 MAPKs are serine/ threonine kinases that are phosphorylated by various substrates like transcription factors, protein kinases and cytoskeleton associated proteins.16 Among every one of the components of MAPKs, MPK3, MPK4 and MPK6 are best considered cases in disease resistance.17 A study reported that on the basis of both loss-of-function and gain of function, specific receptor FLS2 acts upstream of the MAPKKK/At MEKK1, which activates the two highly conserved MAPKK/At MKK4 and At MKK5, thus phosphorylates and activates MPK3 and MPK6, leading to the expression of early-defense response genes for providing resistance to disease.15 AtMPK3 and AtMPK6 work together in a single MAPK cascade because they share common upstream kinases andare functionally redundant.11,18,19 Ta MPK3 and Ta MPK6 are differentially regulated at different stages of pathogenesis of fungal pathogen Mycosphaerella graminicola.20 In another study it was revealed that MPK4 has an essential role in plant defense against biotic stresses.21 Therefore, the present investigation analyzed the expression level of MPK3, MPK4 and MPK6 in transgenic (BjV5) and wild Brassica juncea (var.) varuna.
Materials and Methods
Plant Material and Growth Conditions
The seeds of transgenic (BjV5) and wild Brassica juncea (var.) varuna were collected from plant stress lab and crop research center (CRC), GB Pant University of agriculture and technology, Pantnagar.Plants were grown on an autoclaved mixture of soil, vermicompost and sand (2:1:1) in a transgenic glass house with appropriate condition of 22 ±1°C, 16/8 hours (light/dark) photoperiod. Experiments were performed with 45 days old and unstressed plants exhibiting uniform appearance.
Plant Infection with Pathogen
Pure Alternaria brassicae spores were collected from CRC, Pantnagar and were further suspended in sterile distilled water at a concentration of ~1 x 104 spores/ml. Spores suspension were inoculated on 45 days old transgenic and wild plants with 80-90% relative humidity at temperature 20± 2οCfor the development of the symptoms.Inoculated leaves were sampled at control, 15 min, 1hr, 6hr post inoculation (hpi),1 day post inoculation (dpi), 5 dpi(early), 8 dpi (middle) and 11 dpi (late).
RNA Isolation and Real Time PCR
For RT and real time PCR reaction, total RNA of transgenic and wild B. juncea leaves were extracted from the homogenate using RNA extraction kit (Himedia, India). Samples of RNA were first treated with D Nase (Fermentas, U.S.A.).RT reaction was done in a 20 μl reaction containing 2000 ng of RNA, 1 μl of (100 μM) oligodT primer, 200 units of reverse transcriptase (Fermentas, USA), 2 μl of 10mM dNTPs, 20 unit of R Nase inhibitor and 4μl of 5X RT buffer for 1 h at 42°C. Quantitative real time PCR was performed using One Plus Real Time PCR Systems (ABI, USA). Quantification of the threshold cycle (CT) values in quantitative real time PCR analysis was achieved by using the 2(–ΔΔC(T) method.22 B. juncea actin was used as an internal control to develop a standard. The reaction mixture of 12 μl contained 1X Maxima SYBR Green q PCR master mix, 2.5 mM Mgcl2, 0.25 μl of forward and reverse primer and 1:10 diluted cDNA. The amount of product was determined at the endof each cycle by Step One™ Software and Applied Biosystems™. In analyzing gene expression of MPK3, MPK4 and MPK6 (Table. 1),amplification was conducted for 40 cycles, with initial denaturation of 95οC for 10 min, then 95οC for 15 sec, 58οC for 30 sec, 72οC for 30 sec followed by one cycle each of 95°C for 15 sec, 60°C for 15 sec and 95°Cfor 15 sec.
Table 1: The list of primers for detection of target gene transcripts
| Gene name | Primer sequence (5′-3′) | Amplicon length | Tm |
| Actin F
Actin R |
5´GAATCCACGAGACGACTTACAAC3´
5´CGATCCAGACACTGTACTTCCTC3´ |
200 bp | 50-60οC |
| MPK3 F
MPK3 R |
5´GATGTGGTTCCTCCACCACT3´
5´AGTTGGCGTTCAGGAGAAGA3´ |
210 bp | 58οC |
| MPK4 F
MPK4 R |
5´GCTCTAACCAACCCTTAACTG3´
5´GTACCAGCGTGTAACAACGTA3´ |
228 bp | 59οC |
| MPK6 F
MPK6 R |
5´CCGAGAGTGACTTCATGACTG3´
5´CTATGAGCTCCATGAGCAAAC3´ |
200 bp | 57 οC |
Statistical Analysis
All experiments were performed in a completely randomized design with triplicates. Relative gene expression was expressed as mean ±SE. The statistical analysis of the results was conducted by analysis of variance (ANOVA)at 5% probability level (p≤0.05) using GraphPad Prism version 5.01.
Results
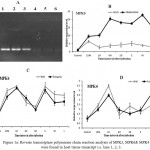 |
Figure 1a: Reverse transcriptase polymerase chain reaction analysis of MPK3, MPK6& MPK4 bands were found in host tissue transcript i.e. lane 1, 2, 3.
|
No band was seen in fungal transcript i.e. lane 4, 5 & 6. (B,C&D). Response of MPK3, MPK6 & MPK4 to Alternaria brassicae pathogen. Relative expression level of MPK3, MPK6 & MPK4 in both transgenic (BjV5) and wild Brassica juncea (var.) varuna were determined by real time polymerase chain reaction at control, 15m, 1h, 6h, 1d, early, middle & late after Alternaria brassicae inoculation. All values were mentioned in mean of triplicate. Error bars were indicates standard deviations and Statistical significance was determined by using an analysis of variance (p≤ 0.05).
Expression of defense response genes MPK3, MPK6 and MPK4 in response to Alternaria brassicae infection was analyzed on leaves of both transgenic (BjV5) and wild Brassica juncea (var.) varuna. Pathogen inoculated leaves were used forinvestigation of defense responsive genes using quantitative real time PCR.Total RNA was isolated form host tissues. The fungal growth increased in the host tissue during pathogenesis of Alternaria brassicae. For limiting the biasness of fungal RNA in the host tissue RNA, all leaves were cleaned by sterilized tissue paper at the time of sampling.For further confirmation of host gene specificity, fungal RNA was used as a template for RT PCR. No amplification was found in fungal transcript as compared with positive bands of host MPK3/4/6 genes on agarose gel (Fig. 1A).Brassica junceaacting gene was used as an internal control for standardization of RNA samples. To check the expression of MPK3 at various stage of infection, MPK3 was constitutively expressed at all stages of infection.MPK3 expression was significantly high up to later stage in transgenic (BjV5) plant than the wild brassica (Fig. 1B).The highest expression level of MKP3 might be indicates its role in early responsive defense against Alternaria blight.At later stage of infection, the transcript of MPK3 was observed to be decreased in both transgenic and wild brassica.The expression pattern of MPK6 gene was approximately same in wild and transgenic Brassica juncea. The induction of MPK6 was found to increase up to 1 hour after inoculation and afterwardtranscript level was induced (3.5 fold) up to early stage of infection (Fig. 1C). This indicate that MPK3 and MPK6 might be an early responsive for defense against Alternaria blight.The transcript level of MPK4varied notably in different stages of infection.Expression of MPK4 was upregulated in the later stage of infection in both wild and transgenic (BjV5) plants.MPK4 expression was increased up to 4 fold in transgenic then wild brassica (Fig. 1D).
Discussion
Plant Mitogen Activated Protein Kinase (MAPKs) is involved in cell growth, differentiation, cell cycle and stress response.23 The pathophysiology of Brassica juncea and Alternaria blight fromthe relative gene expression level of MPK3, MPK4 and MPK6 revealed that all of them might play a role in induction of defense mechanism. During the time of pathogenesis at early stage of infection, overexpression of MPK3 restricts pathogen growth and provides resistance against pathogen of Alternaria brassicae.In this study, MPK4 was also positively recorded in induction of defense at later stage of infection.It was also studied that overexpression of BnMPK4 significantly enhance disease resistance to Sclerotiniasclerotiorumin oilseed rape.24 MPK3 and MPK6 are also involved in reactive oxygen species (ROS) generation and hyper sensitive response like cell death.18 Mizogeuchiet al.(1993)25 reported that AtMPK6 involved in pathogen induced signalling for induction of defense response against pathogen.Our results indicated that overexpression of MPK3 gene in transgenic (BjV5) plants significantly induced downstream components of MAPK machinery and significantly enhances the resistance to Alternaria brassicae pathogen. Our result also suggested that overexpressed MPK3 in transgenic lines (BjV5) positively regulated salicylic acid (SA)-mediated defense response(Fig. 2). Therefore, SA mediated immune response is essential for the establishment of systemic acquired resistance (SAR) and provide rapid activation of defense responses.
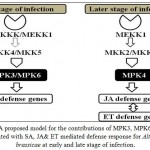 |
Figure 2: A proposed model for the contributions of MPK3, MPK6 & MPK4 associated with SA, JA& ET mediated defense response for Alternaria brassicae at early and late stage of infection.
|
Conclusion
In brief, this study exhibits that overexpressing BjMPK3 gene in transgenic Brassica juncea (BjV5 line) significantly triggers the downregulated components of MAPK module and provide resistance to Alternaria brassicae. MPK3/MPK6 was found more important in early defense and MPK4 in late defense response.
Acknowledgements
This work was supported by grants from the Department of Biotechnology, New Delhi under the Project of programme mode support (PMS) in agricultural biotechnology.
Conflicts of Interest
The authors declared no conflict of interest.
Funding Source
Department of Biotechnology (DBT), New Delhi
References
- Saharan G.S. Management of rapeseed and mustard diseases. Advances in oilseeds research. 1992:152-188.
- Meena P.D, Awasthi R.P, Chattopadhyay C, Kolte S.J, Kumar A. Alternaria blight: a chronic disease in rapeseed-mustard. Journal of Oilseed Brassica. 2010;1(1):1-11.
- Taj G, Kumar A, Bansal K.C, Garg G.K. Introgression of osmotin gene for creation of resistance against Alternaira blight by perturbation of cell cycle machinery. Indian Journal of Biotechnology. 2004;3(2):291-298.
- Boller T, Felix G. A renaissance of elicitors: perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annual review of plant biology. 2009;60:379-406.
CrossRef - Macho A.P, Zipfel C. Plant PRRs and the activation of innate immune signaling. Molecular cell. 2014;54(2):263-272.
CrossRef - Tsuda K, Glazebrook J, Katagiri F. The interplay between MAMP and SA signaling. Plant signaling & behavior. 2008;3(6):359-361.
CrossRef - Tsuda K, Katagiri F. Comparing signaling mechanisms engaged in pattern-triggered and effector-triggered immunity. Current opinion in plant biology. 2010;13(4):459-465.
CrossRef - Dong X, SA J.A. ethylene, and disease resistance in plants. Current opinion in plant biology. 1998;1(4):316-323.
CrossRef - Feys B.J, Parker J.E. Interplay of signaling pathways in plant disease resistance. Trends in Genetics. 2000;16(10):449-455.
CrossRef - Glazebrook J, Chen W, Estes B, Chang H.S, Nawrath C, Métraux J.P, Katagiri F. Topology of the network integrating salicylate and jasmonate signal transduction derived from global expression phenotyping. The Plant Journal. 2003;34(2):217-228.
CrossRef - Asai T, Tena G, Plotnikova J, Willmann M.R, Chiu W.L, Gomez-Gomez L, Sheen J. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature. 2002;415(6875):977-983.
CrossRef - Zhang S,Klessig D.F. MAPK cascades in plant defense signaling. Trends in plant science. 2001;6(11):520-527.
CrossRef - Madhani H.D, Styles C.A, Fink G.R. MAP kinases with distinct inhibitory functions impart signaling specificity during yeast differentiation. Cell. 1997;91(5):673-684.
CrossRef - Widmann C, Gibson S, Jarpe M.B, Johnson G.L. Mitogen-activated protein kinase: conservation of a three-kinase module from yeast to human. Physiological reviews. 1999;79(1):143-180.
CrossRef - Meng X, Zhang S. (2013). MAPK cascades in plant disease resistance signaling. Annual Review of Phytopathology. 2013;51:245-266.
CrossRef - Nakagami H, Pitzschke A, Hirt H. Emerging MAP kinase pathways in plant stress signalling. Trends in plant science. 2005;10(7):339-46.
CrossRef - Frey F.N, Garcia A, Bigeard J, Zaag R, Bueso E, Garmier M, Hirt H. Functional analysis of Arabidopsis immune-related MAPKs uncovers a role for MPK3 as negative regulator of inducible defences. Genome biology. 2014;15(6):87.
CrossRef - Dongtao R, Yang H, Zhang S. Cell death mediated by MAPK is associated with hydrogen peroxide production in Arabidopsis.Journal of Biological Chemistry. 2002;277(1):559-565.
CrossRef - Xu J, Li Y, Wang Y, Liu H, Lei L, Yang H, Ren D. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt stress in Arabidopsis. Journal of Biological Chemistry. 2008;283(40):26996-27006.
CrossRef - Rudd J.J, Keon J, Hammond-Kosack K.E. The wheat mitogen-activated protein kinases TaMPK3 and TaMPK6 are differentially regulated at multiple levels during compatible disease interactions `with Mycosphaerellagraminicola. Plant Physiology. 2008;147(2):802-15.
CrossRef - Berriri S, Garcia A.V, dit Frey N.F, Rozhon W, Pateyron S, Leonhardt N, Montillet J.L, Leung J, Hirt H, Colcombet J. Constitutively active mitogen-activated protein kinase versions reveal functions of Arabidopsis MPK4 in pathogen defense signaling. The Plant Cell. 2012;24(10):4281-93.
CrossRef - Livak K.J, Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(–ΔΔC(T)) method. 2001; 25:402-408.
- Lu K, Guo W, Lu J, Yu H, Qu C, Tang Z, Li J, Chai Y, Liang Y. Genome-wide survey and expression profile analysis of the mitogen-activated protein kinase (MAPK) gene family in Brassica rapa. PLoS One. 2015; 10(7):e0132051.
CrossRef - Wang Z, Mao H, Dong C, Ji R, Cai L, Fu H, Liu S. Overexpression of Brassica napus MPK4 enhances resistance to Sclerotiniasclerotiorum in oilseed rape. Molecular plant-microbe interactions. 2009;22(3):235-44.
CrossRef - Mizoguchi T, Hayashida N, Yamaguchi-Shinozaki K, Kamada H, Shinozaki K. ATMPKs: a gene family of plant MAP kinases in Arabidopsis thaliana. FEBS letters. 1993;336(3):440-4.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





