How to Cite | Publication History | PlumX Article Matrix
Cloning, Sequencing and Phylogenetic Analysis of Stn Gene of Salmonella Typhimurium
Yashpal Singh1, Apoorv Tiwari1, Rajesh Kumar2 and M. K. Saxena3
1Department of Molecular Biology and Genetic Engineering, G.B. Pant University of Agriculture and Technology,Pantnagar 263145, India.
2Department of Veterinary Microbiology, G.B. Pant University of Agriculture and Technology, India.
3Animal Biotechnology Center, Department of Veterinary Physiology and Biochemistry, G.B. Pant University of Agriculture and Technology, Pantnagar 263145, India.
Corresponding Author E-mail mumtesh@rediffmail.com
DOI : http://dx.doi.org/10.13005/bbra/2583
ABSTRACT: Salmonella Typhimurium is an important pathogen having broad host range . Several genes contribute in virulence of Salmonella. Stn is an important virulent gene which code for Salmonellatoxin, increases the level of c-AMP in the host, and ultimately results into diarrhoea and vomiting. In present study stn gene was cloned, sequenced and on basis of sequence information of stn gene,phylogenetic relation was deduced between different serovars of Salmonella Typhimurium. Genomic DNA was isolated from field isolate of Salmonella Typhimurium (isolate No-A201) and stn gene was amplified using gene specific primer and cloned in pJET vector by the positive selection system. Amplification of stn gene yielded a product of approximately 750 bp. Subsequently gene was sequenced and a complete ORF of 750 bp was obtained. The sequence was submitted to NCBI Genbank and allotted the Accession No KF032246 was allocated.Sequence was further used for bioinformatics analysis of Stnprotein, which exhibited two major domains and one amino acid substitution at 609residue. On phylogenetic analysis, S.Typhimurium exhibited 99% similarity with Salmonella enterica subsp. entericaserovar Newport.Our findings indicate that stn is an important toxin gene, which is conserved among many serovars of Salmonella. Therefore, it may be proven as a suitable candidate for development of sub-unit vaccine against Salmonella.
KEYWORDS: Gene; Protein; Salmonella; Vaccine
Download this article as:| Copy the following to cite this article: Singh Y, Tiwari A, Kumar R, Saxena M. K. Cloning, Sequencing and Phylogenetic Analysis of Stn Gene of Salmonella Typhimurium. Biosci Biotech Res Asia 2017;14(4). |
| Copy the following to cite this URL: Singh Y, Tiwari A, Kumar R, Saxena M. K. Cloning, Sequencing and Phylogenetic Analysis of Stn Gene of Salmonella Typhimurium. Biosci Biotech Res Asia 2017;14(4). Available from: https://www.biotech-asia.org/?p=28558 |
Introduction
Gastrointestinal diseases of infectious origin usually arise uponingestion of contaminated foods or water. Many pathogenic bacterium can cause gastrointestinal infection, among them, the genus Salmonella is one of the most important for developing countries, where the hygienic conditions are not very good (Kozak et al.,2013; Vojdaniet al.,2008; Ansari et al.,2012 ;Kabir et al., 2012; Kariuki et al,. 2006). In whole of the World, several outbreaks attributed to different Salmonella serovars are reported each year indicating the presence of organism globally (Kozak et al.,2013; Vojdani et al.,2008; Ansari et al., 2012; Kabir et al.,2012; Kariuki et al,. 2006). Salmonella Typhimurium causes gastroenteritis in human being but in few cases it may cause serious health problems like Endocarditis, Meningitis etc.( Mc Clelland et al.,2001; Everest et al.,1999). In poultry, S. Typhimurium has been isolated from broilers, breeders, and commercial egg-laying flocks (Chima et al., 1998; Jamshidi et al., 2009). The backyard poultry practices are very common in India so there is always a possibility of transferring of Salmonella Typhimurium from poultry to human populations where it can cause serious health problems (Morenoet al., 2000; Swe et al., 2008; Saxena et al., 2006). Hence, there is an urgent need for to develop vaccine against poultry salmonellosis for reducing the infection caused by this organism.
Salmonella enterotoxin (Stn) is a virulence factor and contributes in diarrhea in host (Chopraet al.,1994;.Chopraet al.,1999). It has been observed that the stn gene is specifically present only in Salmonella . irrespective of their serovars (Dinjuset al., 1997; Makino et al., 1999; Mooreet al.,2007). Biological activities of Stn are important to Salmonella virulence, especially in enteric phase of disease (Chopra et al.,1999). As other functions of Stn are not completely deduced, it is possible that Stn may play important role in patho-physiology of Salmonella. To explore more information about Stn protein in the present study stn gene of Salmonella Typhimurium was cloned, sequenced and phylogenetic analysis was done by using bioinformatics approach.
Materials and Methods
Bacterial Strains
Salmonella Typhimurium field isolate (A201) was used in this study which had been characterized by Salmonella specific PCR, biochemical characterization and serotyped at National Salmonella Centre, IVRI Izatnagar as Salmonella Typhimurium. Escherichia coli DH5α used in cloning experiment was obtained from Animal Biotechnology Center and grown in LB broth. Blunt cloning vector pJET 1.2, blunting enzyme, and T4 DNA ligase were procured from Fermentas, USA. The antibiotics (Ampicillin (100 μg/mL) and Kanamycin (50 μg/mL)) used for selection of recombinants were procured from Himedia, India.The cultures were maintained in LB agar slants and their purity was tested by biochemical tests and Salmonella specific PCR.
Cloning of stn gene
Genomic DNA was isolated by CTAB method. Primers were designed for Stn gene of Salmonella Typhimurium using sequence information available on NCBI and using gene tool software.
Stn 1 (Forward): 5’ GGATCC TTG TTA ATC CTG TTG TCT CGC TAT 3’
Stn 2 (Reverse): 5’ AAGGTT TTA CTG GCG TTT TTT TGG CAT 3’
50 µl of PCR reaction mixture was set up containing 20 ng of genomic DNA (template), 200 µM dNTPs, 20 picomoles of each primer, 3 unit of JumpstartTMAccu TaqTMLA (SIGMA) with 5 ul of 10X Accu TaqTM LA PCR buffer. Final volume to 50 ul was made up by using sterilised water. The gene was amplified by PCR using the following program,that is, initial denaturation at 94°C for 5 min., followed by 30 cycles of denaturation at 94°C for 1 min, annealing at 51°C for 2 min, and elongation at 68°C for 1 min. fallowed by final elongation at 68°C for 5 min.
PCR product was loaded on 1.5% Agarose gel and the size of the amplicon was measured by comparing with standard molecular weight marker. To remove PCR dimer, PCR product was eluted from agarose gel using Qiagen Mini Elute Gel extraction kit. PCR product was cloned in pJET vector by blunt end cloning. After blunting of PCR product, 1 µl of pJET vector and 1 µl of T4 DNA ligase were added and ligation was carried out at 22ºC for 4 hours. 5 ul of ligated product was checked on 1.5 % Agarose gel. Ligated product was transformed into DH5α cells using Calcium chloride method (Sambrook et al., 1989). Recombinants were screened by selecting Ampicillin resistant colonies and analyzing the presence of insert by colony PCR. Plasmids were isolated from selected clones and insert was released by double digestion with Bam H1 and Hind III. The size of insert was measured by comparing with standard molecular weight marker. Selected clones were sent to University of Delhi South Campus for sequencing and obtained sequence was analyzed for presence of open reading frame. The sequence was submitted to NCBI.
GC Content Analysis
DNA/RNA GC Content Calculator was used to calculate the percentage of GC content in stn gene. (http://www.endmemo.com/bio/gc.php).
Open Reading Frame Analysis
ORF analysis of stn gene of Salmonella Typhimurium (750 bp) was performed by GENE TOOL software.
Conserved Domain Search
Conserved domain analysis of the protein sequence was performed using CD Search tool of NCBI.
Sequence Similarity and Phylogenetic Analysis
The sequence obtained was subjected to homology search using BLASTn (http://www.ncbi.nlm.nih.gov/). The sequences showing maximum similarity with stn gene was subjected to multiple sequence alignment and a phylogenetic tree was constructed based on the comparative analysis of related sequences using MEGA(Molecular Evolution Genetics Analysis)tool at nucleotide level.Analysis was performed on the default values of the MEGA software and Neighbour-joining statistical method at 1000 bootstrap replication was used for tree construction.
Results and Discussion
The purity of the culture was checked by biochemical characterization and Salmonella-specific PCR. The culture was found to be MR+, VP-, Urease- biochemically, which is characteristic of Salmonella Typhimurium.
PCR Amplification and Cloning
The PCR amplification with stn specific primers was conducted with genomic DNA, which resulted in a product of approximate size 750 bp (Figure 1a). The desired product was successfully purified using QIA quick gel extraction kit and cloned in pJET 1.2 blunt cloning vector (Fermentas, USA) and transformed into chemically competent E.coli DH5α cells. Recombinant clones were selected by colony PCR (Figure 1b). The insert was sequenced and complete cdsof 750 bp was obtained. The sequence was submitted to NCBI Gene bank and accession no. KF032246 was allocated by NCBI.
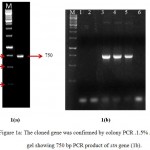 |
Figure 1a: The cloned gene was confirmed by colony PCR .1.5% Agarose gel showing 750 bp PCR product of stn gene (1b). Lane (1,2): PCR of false recombinants, Lane (3) and (4) corresponds to positive clones of stn.
|
A corresponding band of 750 bp was seen for Salmonella isolate A201 that acted as a positive control (lane 5), DH5-α as negative control (lane 6), M: 1kb ladder.
GC Content Analysis
GC content is found to be variable with different genes but evidence of GC ratio with that of length of the coding region of a gene has shown that the length of the coding sequence is directly proportional to higher G+C content. This has been pointed to the fact that the stop codon has a bias towards A and T nucleotides, and, thus, the shorter the sequence the higher the AT bias and in this case as shown in the graph (Fig 2) the average GC content is 53.3 % i.e. a good prediction.
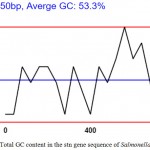 |
Figure 2: Total GC content in the stn gene sequence of Salmonella Typhimurium
|
Open Reading frame Analysis
 |
Figure 3: Open reading frame analysis of stn gene
|
The sequence when subjected to ORF analysis using GENE TOOL software showed a complete ORF of 750 bp. (Fig 3)
Conserved Domain Search
 |
Figure 4: The sequence was screened for the presence of conserved regions that may exist within a family of gene
|
Nucleotidesequence (750bp) was used to predict the domain region in the sequence. Histidine kinase-like ATPases (25-342bp) and Histidine Kinase A (478-672bp), two domains were found in the stn gene sequence of Salmonella. Histidine kinase-like ATPases family includes several ATP-binding proteins for example: histidine kinase, DNA gyrase B, topoisomerases, heat shock protein HSP90, phytochrome-like ATPases and DNA mismatch repair proteins.
Histidine Kinase A dimers are formed through parallel association of 2 domains creating 4-helix bundles; usually these domains contain a conserved Histidine residue and are activated via trans-autophosphorylation by the catalytic domain of the histidine kinase. They subsequently transfer the phosphoryl group to the Asp acceptor residue of a response regulator protein. Two-component signalling systems, consisting of a histidine protein kinase that senses a signal input and a response regulator that mediates the output, are ancient and evolutionarily conserved signaling mechanisms in prokaryotes and eukaryotes.
Substitution of Amino Acids at Nucleotide Level
Sequence alignment of stn gene of Salmonella Typhimurium (nucleotide level) was performed by BLAST tool of NCBI to find the similar homologous sequence. In the Pairwise sequence alignment at nucleotide level the stn gene showed maximum similarity with Salmonellaenterica subsp. Enteric serovar Newport andat amino acid level substitution was found at position 609bpwith Cysteine to Tyrosine. (Fig 5)
 |
Figure 5: Substitution of amino acid at nucleotide level in the stn gene of Salmonella Typhimurium with the homologous sequences
|
Phylogenetic analysis of S. Typhimurium stn gene at nucleotide level
The NCBI BLASTn search of the stn gene showed maximum homology (99%) with strain Salmonella enterica subsp. enterica serovar Newport and Salmonella enterica subsp. entericaserovar Dublin complete genomeat nucleotide level. (Fig 6). Though thesequence was shared by many serovars of Salmonella like S. Choleraesuis, Sinfantis, SAnatum, SEnteritidis, S Dublin etc
 |
Figure 6: Dendogram for Phylogenetic analysis at nucleotide level of stn gene marked with red square box with otherclosely related sequences. A is the collapse group of the sequences.
|
Salmonella is an important human and animal pathogen. It causes two types of diseases in human being i.e. Typhoid fever caused by S. Typhi and Non-typhoidal salmonellosis caused by S. Typhimurium. Both of the forms of disease are dreaded and cause high mortality in human being at global level (Kemalet al., 2014).Antibiotic drug resistance is a major concern in case of Salmonella as it has been reported in most of the part of the world (Chandane et al.,2017). Vaccination is the only possible viable option but in both the cases (Typhoidal and Non-typhoidal salmonellosis) effective vaccines are not available as in case of Typhoid presently available Vi Polysachharide vaccine has serious limitations such as it cannot be used in pregnant ladies, high cost of production and a debatable immune response in children. (Ochiai et al.,2014) Though to overcome from these problems new adjuvant like Calcium phosphate nano-particles (Tamuly and Saxena 2012) which can produce better immune response. By the use of molecular techniques efforts have been made to differentiate the pathogen at molecular level (Shivachandra et al.,2006; Zheng et al., 2014) to assess the genetic variation among isolates to resolve the problem of vaccination failure. A conserved and immunogenic protein may be a suitable target for various vaccine development against Salmonella (Jha et al., 2015). Stn has been considered as putative virulence factor of Salmonella and causative agent of diarrhoea (Chopra et al., 1999) but in the later stages it was deduced that the mutation in stn gene did not resulted into reduction of virulence of Salmonella (Watson et al., 1998) but it is an interested fact that stn gene is distributed only in Salmonella sp (Moore et al., 2007) and in our study it has exhibited higher degree of homogeneity among the various serovars of Salmonella. On amino acid sequencing it has shown some similarity with active site of cholera toxin (Dinjus et al., 1997). Therefore, from the findings of the workers and our study we could conclude that though Stn is a protein which may play role in virulence of Salmonella more over is a conserved proteins among the serovars of Salmonella. Therefore, like other toxoid vaccine like tetanus (Moreira et al., 2016) it may be a suitable target for the development of toxoid vaccine against salmonellosis.
Acknowledgements
Authors are highly acknowledged to Department of Biotechnology, Govt. of India for providing financial support.
Conflict of Interest
The authors declare that they have no conflict of interests.
References
- Kozak G.K, Macdonald D, Landry L, Farber J.M. Foodborne outbreaks in Canada linked to produce: 2001 through 2009. J. Food Prot. 2013;76:173–183.
CrossRef - Vojdani J.D, Beuchat L.R, Tauxe R.V. Juice-associated outbreaks of human illness in the United States, 1995 through 2005. J. Food Prot. 2008;71:356–364.
CrossRef - Ansari S, Sherchand J.B, Parajuli K, Mishra S.K, Dahal R.K, Shrestha S, Tandukar S, Pokhrel B.M. Bacterial etiology of acute diarrhea in children under five years of age. J.Nepal Health Res. Counc. 2012;10:218–223.
- Kabir M.R, Hossain M.A, Paul S.K, Mahmud C, Ahmad S, Mahmud N.U, Sultana S, Yesmin T, Hoque S.M, Habiba U, Rahman M.A, Kobayashi N. Enteropathogens associated with acute diarrhea in a tertiary hospital of Bangladesh. Mymen singh. Med. J. 2012;21:618–623.
- Kariuki S, Revathi G, Kariuki N, Kiiru J, Mwituria J, Hart C.A. Characterisation of community acquired non-typhoidal Salmonella from bacteraemia and diarrhoeal infections in children admitted to hospital in Nairobi, Kenya. BMC Microbiol. 2006;6:101.
CrossRef - McClelland M, Sanderson K.E, Spieth J, Clifton S.W, Latreille P, Courtney L. Complete genome sequence of Salmonella enterica serovar Typhimurium LT2. Nature. 2001;413:852-856.
CrossRef - Everest P, Ketley J, Hardy S, Douce G, Khan S, Shea J. Evaluation of Salmonella Typhimurium mutants in a model of experimental gastroenteritis. Infect Immun. 1999;67:2815–2821.
- Chima J, Ogbogu D.A. Chronic fowl typhoid infection in a commercial poultry farm. Niger Vet J. 1998;19:1-4.
- Jamshidi A, Bassami M.R, Afshari-Nic S. Identification of Salmonella spp. and Salmonella Typhimurium by a multiplex PCR-based assay from poultry carcasses in Mashhad. Iran Int J Vet Res. 2009;3:43-48.
- Moreno G, Moar C, Roman F, Perez M.R, Lopez D, Letona J. M “Salmonella Endocarditis presenting as cerebral hemorrhagez”. European Journal of Internal Medicine. 2000;11:96–97.
CrossRef - Swe K.N, Westhuizen V.D .G.M and Hoosen A. A. “Salmonella Typhimurium Meningitis in an adult patient with AIDS”. Journal of Clinical Pathology. 2008;61:138–139.
CrossRef - Chopra A. K, Peterson J.W, Chart P and Prasad R. Molecular characterization of an enterotoxin from Salmonella Typhimurium. Microb. Pathog. 1994;16:85–98.
CrossRef - Chopra A. K, Huang J. H, Xu X.J, Burden K, Niesel D.W, Rosenbaum M.W, Popov V.L and Peterson J.W. Role of Salmonella enterotoxin in overall virulence of the organism. Microb. Pathog. 1999;27:155–171.
CrossRef - Dinjus U, Hänel I, Müller W, Bauerfeind R and Helmuth R. Detection of the induction of Salmonella enterotoxin gene expression by contact with epithelial cells with RT-PCR. FEMS Microbiol. Lett. 1997;146:175–179.
CrossRef - Makino S, Kurazono H,Chongsanguam M, Hayashi H, Cheun H, Suzuki S and Shirah ata T. Establishment of the PCR system specific to Salmonella spp. and its application for the inspection food and fecal samples. J. Vet. Med. Sci. 1999;61:1245–1247.
CrossRef - Moore M.M and Feist M.D. Real-time PCR method for Salmonellaspp. targeting the stn gene. J. Appl. Microbiol. 2007;102:516–530.
CrossRef - Sambrook J,Fritsch E.F,Maniatis T. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York. 1989.
- Kemal J. A Review on the public health importance of Bovine Salmonellosis. J. Veterinary Sci and Tech. 2014;5:175.
CrossRef - Chandane P, Gandhi A, Bowalekar S. Study of antibiotic susceptibility pattern of Salmonella Typhi in children suffering from enteric fever. Ann Trop Med Public Health. 2017;10:440-443.
CrossRef - Leon O.R, Khan I.M , Soofi B,Sur S, Kanungo D, You S , Young A, Atif H.M , Muhammad S.S, Byomkesh M, Shanta D, Camilo A.J, Mohammad A, Bhattacharya K. Bhutta S.A , Clemens Z,John D.Immune responses to Vi Capsular Polysaccharide Typhoid vaccine in children 2 to 16 years old in Karachi, Pakistan and Kolkata, India. Clinical and Vaccine Immunology. 2014;21:661-666.
CrossRef - Tamuly S and Saxena M.K. Preparation of calcium phosphate nanoparticles and evaluation of their effect on muscle cells of rats. Current science. 2012;102(4):610-612.
- Shivachandra S.B,Kumar A.A,Gautam R,Joseph S,Saxena M.K,Chaudhuri P,Srivastava S.K. Characterization of avian strains of Pasteurella multocida by restriction endonuclease and amplified fragment length polymorphism. Research in Veterinary Science. 2006; 81:8-18.
CrossRef - Jie Z, James P, Errol S, Allard W, Ahmed M, Zhao R, Brown S, Eric W. Genetic diversity and evolution of Salmonella enterica serovar Enteritidis strains with different phage types. Journal of Clinical Microbiology. 2014;52:1490-1500.
CrossRef - Jha R, kumar A, Saxena A,Pandey M,Kumar R,Saxena M.K. Heterogenous expression and functional evaluation of in silico characterized recombinant OmpC of Salmonella Typhimurium as a functional poultry vaccine to eradicate zoonotic transmission. African journal of Biotechnology. 2015;14:2862-2870.
CrossRef - Watson P.R, Galyov E.E, Paulin S.M. Mutation of invH, but not stn, reduces Salmonella-induced enteritis in cattle. Infect and Immunity. 1998;66:1432-1438.
- Moreira C.G,Russell R,Mishra A.A,Naraynan S,Ritchie J.M,Waldor M.K. Bacterial adrenergic sensors regulate virulence of enteric pathogens in the gut. Scientific Reporter. 2016.

This work is licensed under a Creative Commons Attribution 4.0 International License.





