How to Cite | Publication History | PlumX Article Matrix
Biodegradable Polycaprolactone Nanoparticles Based Drug Delivery Systems: A Short Review
Ranjith Ramanujam1, Balraj Sundaram1, Ganesh Janarthanan1, Elamparithi Devendran2, Moorthy Venkadasalam2 and M.C. John Milton1
1Research Department of Advanced Zoology and Biotechnology, Loyola College, Chennai, Tamil Nadu, India.
2Department of Biotechnology, Annai College of Arts and Science, Kumbakonam, Tamil Nadu, India.
Corresponding Author E-mail: biotechres17@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2676
ABSTRACT: Nanoparticles based drug delivery systems showing greater potential in various biomedical applications to deliver the drugs/bioactive molecules in controlled manner to the targeted site. Polycaprolactone, biodegradable polyester, owing its tailorable properties, various forms of polycaprolactone are used as drug carrier for a range of biomedical applications. Nanoprecipitation is a simple method to prepare the polycaprolactone nanoparticles to improve the bioavailability and therapeutic potential of various drugs/bioactive molecules. This short review focused on the preparation of polycaprolactone nanoparticles using nanoprecipitation method, nanoparticles-drug formulations and its use in various drug delivery applications.
KEYWORDS: Controlled Drug Release; Drug Delivery; Nanoprecipitation; Polycaprolactone Nanoparticles
Download this article as:| Copy the following to cite this article: Ramanujamv R, Sundaram B, Janarthanan G, Devendran E, Venkadasalam M, Milton M. C. J. Biodegradable Polycaprolactone Nanoparticles Based Drug Delivery Systems: A Short Review. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Ramanujamv R, Sundaram B, Janarthanan G, Devendran E, Venkadasalam M, Milton M. C. J. Biodegradable Polycaprolactone Nanoparticles Based Drug Delivery Systems: A Short Review. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31040 |
Introduction
Currently, pharmaceutical drugs are under study against various diseases which encounters the limitations of instability and fast degradation before reaching the targeted site.1 Usually, to reach the targeted site, the administered drug has to cross several biological barriers, where it can degrade or expresses unwanted influence with the tissues or organs which are not involved in pathological process.2 As a consequence, it necessitates high drug dosage to achieve the preferred concentration at the targeted site resulting cytotoxic and other side effects.3 These adverse effects can be conquer by employing polymeric drug delivery systems providing targeted drug delivery.1,4,5
Polymer based nanoparticles has gathered more attention in biomedical applications due to a range of potential advantages including the ability to promote targeted drug delivery with controlled release rate.6 The nanoparticles–drug formulation helps to improve the solubility, drug efficacy, bioavailability, tolerability and retention time of a range of water soluble/insoluble bioactive molecules and drugs, also it reduces the risks of toxicity and patient expenses.7 These nanoparticles–drug formulation protects the premature degradation of the encapsulated drugs and also it enhances the absorption into a targeted site and intracellular penetration.8
The nanoparticles based drug delivery systems can be evaluate by various essential performance metrics such as size of particles, drug entrapment efficiency, surface properties, drug content and drug release properties.9,10 Therefore, understanding the factors controlling the nanoparticles performance metrics is most important in designing the nanoparticles based drug delivery systems.9 Many different drugs and bioactive molecules are effectively encapsulated into a variety of natural and synthetic polymers to improve the bioavailability, bioactivity and controlled release of the encapsulated drugs.11–13 Synthetic biodegradable polymers has gathered more attention in biomedical applications due to its various advantages such as biologically inert, batch-to-batch uniformity and their structure and composition can be tailored for specific applications, devoid of several drawbacks associated with the natural polymers.14,15
Polycaprolactone
Polycaprolactone is Food and Drug Administration approved biodegradable semi-crystalline aliphatic polyester, use as an implantable biomaterial in various biomedical applications.14,16,17 In addition, it has been used as carrier for sustained delivery of several therapeutic molecules.18,19
Physiochemical Properties of Polycaprolactone
Polycaprolactone (Fig. 1), synthesized in the 1930s by ring-opening polymerization of ε-caprolactone by using various anionic or cationic catalysts or by using a free radical ring opening polymerization of 2-methylene-1- 3-dioxepane.16,20–22 It has a low melting temperature (59ºC to 64ºC) and a glass transition temperature (Tg) of about -60°C.23 At room temperature, polycaprolactone is soluble in dichloromethane, benzene, chloroform, 2-nitropropane, carbon tetrachloride and cyclohexanone; slightly soluble in ethyl acetate, 2-butanone, acetonitrile and dimethylformamide; insoluble in diethyl ether, petroleum ether, alcohol and water.24 Polycaprolactone has capability to form miscible blends by copolymerization with a range of polymers, this copolymerization alters chemical properties of polycaprolactone and also it could indirectly modify/affect some other properties such as solubility, degradation pattern and crystallinity resulting a tailored polymer with intended drug delivery properties.14,16 Due to its slow degradation rate, it has been used as a long-term implantable device and also it facilitates high permeability to various drugs.20,23
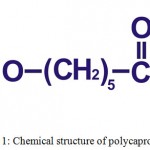 |
Figure 1: Chemical structure of polycaprolactone.
|
Biodegradation of Polycaprolactone
Polycaprolactone is degraded through the hydrolytic cleavage of ester groups in physiological conditions, thus it has gained more attention to be used as an implantable biomaterial.16,25 Based on literature presented about the degradation of polycaprolactone, it can be concluded that, polycaprolactone undergoes a two discrete stage of degradation process, (i) non-enzymatic hydrolysis of ester linkages, which attributed to random chain scissions, caused decrease in molecular weight; (ii) it go through intracellular degradation when the polycaprolactone has a low molecular weight (less than 3000) and more highly crystalline.16,20,26 Polycaprolactone, homopolymer, has a degradation time of two to three years, which depends on the starting molecular weight of the device/implant.20,27 The rate of degradation of polycaprolactone can be tailored by blending or copolymerization with lactones or glycolides/lactides.16 Wu et al., fabricated the porous biocomposite scaffold composed of polycaprolactone and magnesium phosphate and it’s in vitro degradation rate was evaluated, the results showed the faster degradation rate compared to pure polycaprolactone scaffolds.28 At present, polycaprolactone based nanocarriers has gathered more interest in various drug delivery and biomedical applications.14,29
Nanoprecipitation Method for Polycaprolactone Nanoparticles Preparation
The nanoprecipitation method, also called as solvent displacement, adopted to prepare the polycaprolactone nanoparticles (Fig. 2).7 It is a simple and reproducible method, has been extensively used to prepare the nanoparticles from various polymers, especially biodegradable polyesters.30 This method has numerous advantages such as simplicity, good reproducibility, ease of scalability, helps to avoid high volumes of toxic solvents and also supports to achieve submicron particles.30,31 To prepare the nanoparticles, requires solvent phase (organic phase) and non-solvent phase (aqueous phase).30,32 In this need, first the polycaprolactone is dissolved in an appropriate organic solvent, the organic phase is introduced slowly into an aqueous phase containing surfactant under continuous magnetic stirring, evaporate the solvent (by a rotavapor or at ambient temperature with continuous stirring), then centrifuge and lyophilize, in order to prepare the polycaprolactone nanoparticles.29,30,33–35 Generally, the aqueous phase is water, but various excipients (natural or synthetic hydrophilic surfactants) could be added to prevent aggregation of the particles, few of aqueous solutions of various surfactants used for polycaprolactone nanoparticles are listed in Table 1.30 Further, various operating conditions (polymer amount, molecular weight of the polymer, amount of surfactant, stirring rate etc) of this method could influence on the size of the nanoparticles.30
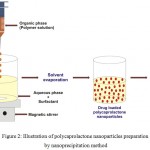 |
Figure 2: Illustration of polycaprolactone nanoparticles preparation by nanoprecipitation method.
|
Table 1: List of few various aqueous solution of surfactants used for polycaprolactone nanoparticles preparation.
| Aqueous solution of surfactants | References |
| Pluronic® F-68 | 36–38 |
| Polyvinyl alcohol | 39 |
| Tween® 80 | 40 |
| Cremophor EL | 41 |
| Polysorbate 80 | 42 |
The polycaprolactone nanoparticles prepared by nanoprecipitation method has been utilized for the encapsulation of various drugs/bioactive molecules, few of them are listed in Table 2.7,35,43 To encapsulate the drug into nanoparticles, dissolve the drug in suitable solvent and add to the prepared polycaprolactone solution prior to addition of organic phase into aqueous phase.43 Basically, this technique helps to encapsulate various hydrophobic drugs/molecules; however, several hydrophilic drugs/molecules are encapsulated effectively by dissolving the hydrophilic drugs/molecules in an external aqueous phase or use of co-solvent or dissolution of minimum quantity of hydrophilic drugs/molecules in the organic phase.7,30
Table 2: List of few drugs/bioactive molecules have been loaded into polycaprolactone nanoparticles.
| Drugs/Bioactive molecules | References |
| Aripiprazole | 34 |
| Camptothecin | 36 |
| Isradipine | 38 |
| 5-amino salicylic acid | 39 |
| Griseofulvin | 40 |
| Indomethacin | 42,44 |
| Cyclosporin A | 45 |
| Docetaxel | 46 |
| Levofloxacin | 47 |
| Ibuprofen | 44 |
| α-tocopherol | 48 |
| Etoposide | 49 |
| Ellagic acid | 50 |
| Ftorafur | 51 |
| Diclofenac sodium | 51 |
| Triclosan | 52 |
| Quercetin | 53 |
| Olanzapine | 54 |
| Meropenem | 55 |
Polycaprolactone Based Nanoparticles in Drug Delivery Applications
Polycaprolactone, a promising biomaterial has been widely used in various drug delivery applications to achieve an excellent therapeutic potential owing to a high permeability to various drugs, excellent biocompatibility and also it has ability to be excreted totally from the body, once bioresorbed.20,56 Polycaprolactone nanoparticles based drug delivery systems has shown efficiency of controlled and targeted drug delivery, high capability to cross various physiological barriers and reduced systemic side effects.57 In a study, Kasinathan et al., has studied the effect of various processing parameters of curcumin loaded polycaprolactone nanoparticles, and the results showed that the homogenization speed and polyvinyl alcohol has showed high impact compared to other parameters.58 Alex et al., formulated the carboplatin loaded polycaprolactone nanoparticles and used to treat glioma anticipating improved brain delivery by the nasal route; results showed, no severe damage observed on the integrity of nasal mucosa.3
Polycaprolactone can be blend or copolymerized with other polymers to achieve fascinating properties for an effective drug loading and releasing characteristics.29,59 Shenoy et al., prepared tamoxifen (hydrophobic anti-cancer drug) loaded polyethylene oxide-modified polycaprolactone nanoparticles, the results showed that increased level of drug accumulation within tumor with time.60 Damge et al., synthesized the biodegradable polycaprolactone and a polycationic non-biodegradable acrylic polymer blended nanoparticles for oral delivery of insulin and its antidiabetic effect was examined in vivo, the results showed an increased serum insulin level with improved glycemic response for prolonged time period.61 Choi et al., used poly(N-isopropylacrylamide)-b-poly(3-caprolactone) as block copolymers to encapsulate clonazepam (hydrophobic drug); the poly(N-isopropylacrylamide) layer on the nanoparticles surfaces act as an additional diffusion barrier to the encapsulated drug, which results delayed in release profile and also these barriers helps to modulate or achieve the desired drug release pattern.62 Bakre et al., prepared curcumin loaded polycaprolactone-montmorillonite nanoclay modified with n-cetyl-N,N,N,-trimethylammonium bromide nanoparticles to enhance its antitumor activity.63 Prabu et al., prepared vinblastine loaded polycaprolactone grafted dextran copolymeric nanoparticles and its in vitro anticancer activity evaluated with breast cancer cell line, the results showed an efficient cellular uptake of the prepared nanoparticles.64
Conclusion and Future Perspectives
Nanoparticles based drug delivery systems are showing greater potential in improving the pharmacological and therapeutic potential of various bioactive molecules. The encapsulation of drugs/bioactive molecules into nanoparticles protects degradation of the drugs/bioactive molecules and also supports controlled release for targeted drug delivery. In addition, it minimizes the toxicity and other adverse effects in normal tissues. Polycaprolactone, synthetic biodegradable polymer has studied extensively in different formulations as a carrier for various drug delivery applications. Here, this short review focused primarily on the recent approaches in various drugs/bioactive molecules loaded polycaprolactone nanoparticles using nanoprecipitation method. In future perspective, encapsulation of various drugs/bioactive molecules into polycaprolactone based nanoparticles facilitates sustained and targeted delivery in various drug delivery and biomedical applications for an effective therapeutic potential.
References
- Ponnappan N., Chugh A. Nanoparticle-Mediated Delivery of Therapeutic Drugs. Pharmaceut. Med. 2015;29:155–167.
CrossRef - Torchilin V. P. Drug targeting. Eur. J. Pharm. Sci. 2000;11:81–91.
CrossRef - Alex A. T., Joseph A., Shavi G., Rao J. V., Udupa N. Development and evaluation of carboplat in-loaded PCL nano particles for intranasal delivery. Drug Deliv. 2014:1–10.
CrossRef - Kumar A., Sawant K. Encapsulation of exemestane in polycaprolactone nano particles optimization characterization and release kinetics. Cancer Nanotechnol. 2013;4:57–71.
CrossRef - Ibrahim K. E., Bakhiet A. O., Khan A., Khan H. A. Recent Trends in Biomedical Applications of Nanomaterials. Biosci. Biotechnol. Res. Asia. 2018;15:235–243.
CrossRef - Guilherme M. R., Mauricio M. R., Tenório-Neto E. T., Kunita M. H., Cardozo-Filho L., Cellet T. S. P., Pereira G. M., Muniz E. C., da Rocha S. R. P., Rubira A. F. Polycaprolactone nano particles containing encapsulated progesterone prepared using a scCO2 emulsion drying technique. Mater. Lett. 2014;124:197–200.
CrossRef - Kumari A., Yadav S. K., Yadav S. C. Biodegradable polymeric nano particles based drug delivery systems. Colloids Surfaces B Biointerfaces. 2010;75:1–18.
CrossRef - Alexis F., Pridgen E., Molnar L. K., Farokhzad O. C. Factors Affecting the Clearance and Biodistribution of Polymeric Nano particles. Mol. Pharm. 2008;5:505–515.
CrossRef - Budhian A., Siegel S. J., Winey K. I. Production of haloperidol-loaded PLGA nano particles for extended controlled drug release of haloperidol. J. Microencapsul. 2005;22:773–785.
CrossRef - Rizvi S. A. A., Saleh A. M. Applications of nano particle systems in drug delivery technology. Saudi Pharm. J. 2018;26:64–70.
CrossRef - Cheng Q., Feng J., Chen J., Zhu X., Li F. Brain transport of neurotoxin-I with PLA nanoparticles through intranasal administration in rats: a micro dialysis study. Biopharm. Drug Dispos. 2008;29:431–439.
- CrossRef
- Gómez-Gaete C., Tsapis N., Besnard M., Bochot A., Fattal E. Encapsulation of dexamethasone into biodegradable polymeric nanoparticles. Int. J. Pharm. 2007;331: 153–159.
CrossRef - Jong D. W. H., Borm P. J. A. Drug delivery and nano particles: Applications and hazards. Int. J. Nanomedicine. 2008;3:133–149.
CrossRef - Nair L. S., Laurencin C. T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007;32:762–798.
CrossRef - Gunatillake P., Adhikari R. Biodegradable synthetic polymers for tissue engineering. Eur. Cells Mater. 2003;5:1–16.
CrossRef - Azimi B., Nourpanah P., Rabiee M., Arbab S. Poly (ε-caprolactone) Fiber: An Overview. J. Eng. Fiber. Fabr. 2014;9:74–90.
- Ulery B. D., Nair L. S., Laurencin C. T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. B. Polym. Phys. 2011;49:832–864.
CrossRef - Mondal D., Venkatraman S. S. Formulation and characterization of naked DNA and complexed DNA loaded polymer films. Mater. Sci. Eng. C. 2011;31:224–229.
CrossRef - Natu M. V., Gaspar M. N., Ribeiro C. A., Correia I. J., Silva D., de Sousa H. C., Gil M. H. A poly(ε-caprolactone) device for sustained release of an anti-glaucoma drug. Biomed. Mater. 2011;6:25003.
CrossRef - Woodruff M. A., Hutmacher D. W. The return of a forgotten polymer—Polycaprolactone in the 21st century. Prog. Polym. Sci. 2010;35:1217–1256.
CrossRef - Mondal D., Griffith M., Venkatraman S. S. Polycaprolactone-based biomaterials for tissue engineering and drug delivery: Current scenario and challenges. Int. J. Polym. Mater. Polym. Biomater. 2016;65:255–265.
CrossRef - Mahapatro A., Singh D. K. Biodegradable nano particles are excellent vehicle for site directed in-vivo delivery of drugs and vaccines. J. Nano biotechnology. 2011;9:55.
CrossRef - Mohamed R. M., Yusoh K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2016;249–255.
- Labet M., Thielemans W. Synthesis of polycaprolactone a review. Chem. Soc. Rev. 2009;38:3484–3504.
CrossRef - Abedalwafa M., Wang F., Wang L., Li C. Biodegradable poly-epsilon-caprolactone (PCL) for tissue engineering applications a review. Rev. Adv. Mater. Sci. 2013;34:123–140.
- Woodward S. C., Brewer P. S., Moatamed F., Schindler A., Pitt C. G. The intra cellular degradation of poly(ε-caprolactone). J. Biomed. Mater. Res. 1985;19:437–444.
CrossRef - Pathiraja A. G., Adhikari R. Biodegradable Synthetic Polymers for Tissue Engineering. Eur. Cells Mater. 2003;5:1–16.
CrossRef - Wu F., Liu C., O’Neill B., Wei J., Ngothai Y. Fabrication and properties of porous scaffold of magnesium phosphate/polycaprolactone biocomposite for bone tissue engineering. Appl. Surf. Sci. 2012;258:7589–7595.
CrossRef - Sinha V. R., Bansal K., Kaushik R., Kumria R., Trehan A. Poly-ϵ-caprolactone micro spheres and nano spheres an overview. Int. J. Pharm. 2004;278:1–23.
CrossRef - Miladi K., Sfar S., Fessi H., Elaissari A. Nano precipitation Process: From Particle Preparation to In Vivo Applications. In: Polymer Nano particles for Nanomedicines: A Guide for their Design Preparation and Development (Vauthier C., Ponchel G. ed.). Springer International Publishing. 2016;17–53.
CrossRef - Lassalle V., Ferreira M. L. PLA Nano- and Micro particles for Drug Delivery: An Overview of the Methods of Preparation. Macromol. Biosci. 2007;7:767–783.
CrossRef - Badri W., Miladi K., Robin S., Viennet C., Nazari Q. A., Agusti G., Fessi H., Elaissari A. Polycaprolactone Based Nano particles Loaded with Indomethacin for Anti-Inflammatory Therapy: From Preparation to Ex Vivo Study. Pharm. Res. 2017;34:1773–1783.
CrossRef - Bilati U., Allémann E., Doelker E. Development of a nano precipitation method intended for the entrapment of hydrophilic drugs into nano particles. Eur. J. Pharm. Sci. 2005;24: 67–75.
CrossRef - Sawant K., Pandey A., Patel S. Aripiprazole loaded poly(caprolactone) nano particles: Optimization and in vivo pharma cokinetics. Mater. Sci. Eng. C. Mater. Biol. Appl. 2016;66:230-243.
CrossRef - Badri W., El Asbahani A., Miladi K., Baraket A., Agusti G., Nazari Q. A., Errachid A., Fessi H., Elaissari A. Poly (ε-caprolactone) nano particles loaded with indomethacin and Nigella Sativa L. essential oil for the topical treatment of inflammation. J. Drug Deliv. Sci. Technol. 2018;46:234–242.
CrossRef - Çırpanlı Y., Allard E., Passirani C., Bilensoy E., Lemaire L., Çalış S., Benoit J. P. Anti tumoral activity of camp tothecin-loaded nano particles in 9L rat glioma model. Int. J. Pharm. 2011;403:201–206.
CrossRef - Çirpanli Y., Bilensoy E., Doğan L. A., Çaliş S. Comparative evaluation of polymeric and amphiphilic cyclodextrin nanoparticles for effective camp tothecin delivery. Eur. J. Pharm. Biopharm. 2009;73:82–89.
CrossRef - Leroueil-Le V. M., Fluckiger L., Kim Y. I., Hoffman M., Maincent P. Preparation and characterization of nano particles containing an anti hypertensive agent. Eur. J. Pharm. Biopharm. 1998;46:137–143.
CrossRef - Pertuit D., Moulari B., Betz T., Nadaradjane A., Neumann D., Ismaïli L., Refouvelet B., Pellequer Y., Lamprecht A. 5-amino salicylic acid bound nano particles for the therapy of inflammatory bowel disease. J. Control. Release. 2007;123:211–218.
CrossRef - Zili Z., Sfar S., Fessi H. Preparation and characterization of poly-ɛ-caprolactone nano particles containing griseofulvin. Int. J. Pharm. 2005;294:261–267.
CrossRef - Bazylińska U., Lewińska A., Lamch Ł., Wilk K. A. Polymeric nano capsules and nano spheres for encapsulation and long sustained release of hydrophobic cyanine-type photo sensitizer. Colloids Surfaces A Physicochem. Eng. Asp. 2014;442:42–49.
CrossRef - Pohlmann R . A., Weiss V., Mertins O., da Silveira P . N., Guterres S. S. Spray-dried in domethacin-loaded polyester nano capsules and nano spheres development stability evaluation and nano structure models. Eur. J. Pharm. Sci. 2002;16:305–312.
CrossRef - Fessi H., Puisieux F., Devissaguet J. P., Ammoury N., Benita S. Nano capsule formation by interfacial polymer deposition following solvent displacement. Int. J. Pharm. 1989;55:1–4.
CrossRef - Suksiriworapong J., Sripha K., Kreuter J., Junyaprasert V. B. Comparative Study of Ibuprofen and Indomethacin Loaded Poly(caprolactone) Nano particles : Physicochemical Properties. Mahidol Univ. J. Pharm. Sci. 2010;37:17–27.
- Molpeceres J., Guzman M., Aberturas M. R., Chacon M., Berges L. Application of central composite designs to the preparation of polycaprolactone nano particles by solvent displacement. J. Pharm. Sci. 1996;85:206–213.
CrossRef - Mei L., Zhang Y., Zheng Y., Tian G., Song C., Yang D., Chen H., Sun H., Tian Y., Liu K., Li Z., Huang L. A Novel Docetaxel-Loaded Poly (ε-Caprolactone)Pluronic F68 Nano particle Overcoming Multi drug Resistance for Breast Cancer Treatment. Nano scale Res. Lett. 2009;4:1530.
CrossRef - Kho K., Cheow W. S., Lie R. H., Hadinoto K. Aqueous re-dispersibility of spray-dried antibiotic-loaded polycaprolactone nano particle aggregates for inhaled anti-biofilm therapy. Powder Technol. 2010;203:432–439.
CrossRef - Byun Y., Hwang J. B., Bang S. H., Darby D., Cooksey K., Dawson P. L., Park H. J., White side S. Formulation and characterization of α-tocopherol loaded poly ɛ-caprolactone (PCL) nano particles. LWT – Food Sci. Technol. 2011;44:24–28.
- Snehalatha M., Venugopal K., Saha R. N. Etoposide-Loaded PLGA and PCL Nano particles I: Preparation and Effect of Formulation Variables. Drug Deliv. 2008;15:267–275.
CrossRef - Sonaje K., Italia J. L., Sharma G., Bhardwaj V., Tikoo K., Kumar M. N. V. R. Development of Biodegradable Nano particles for Oral Delivery of Ellagic Acid and Evaluation of Their Antioxidant Efficacy Against Cyclosporine A-Induced Nephro toxicity in Rats. Pharm. Res. 2007;24:899–908.
CrossRef - Arias J. L., López-Viota M., Sáez-Fernández E., Ruiz M. A. Formulation and physicochemical characterization of poly(ɛ-caprolactone) nanoparticles loaded with ftorafur and diclofenac sodium. Colloids Surfaces B Biointerfaces. 2010;75:204–208.
CrossRef - Aminu N., Baboota S., Pramod K., Singh M., Dang S., Ansari S. H., Sahni J. K., Ali J. Development and evaluation of triclosan loaded poly-ε-caprolactone nano particulate system for the treatment of periodontal infections. J. Nano particle Res. 2013;15:2075.
CrossRef - Kumar D. V., Ranjan P. P. V. Development of a poly (ε Caprolactone) based nano particles for oral delivery of Quercetin. Res. J. Pharm. Technol. 2015;8:836–840.
CrossRef - Joseph E., Reddi S., Rinwa V., Balwani G., Saha R. DoE based Olanzapine loaded poly-caprolactone nano particles decreases extra pyramidal effects in rodent model. Int. J. Pharm. 2018;541:198–205.
CrossRef - Khanum R., Qureshi M. J., Mohandas K. Antibiofilm Potential of Meropenem-Loaded Poly(Ɛ-Caprolactone) Nano particles Against Klebsiella pneumoniae. Int. J. Pharm. Clin. Res. 2016;8:1343–1350.
- Merkli A., Tabatabay C., Gurny R., Heller J. Biodegradable polymers for the controlled release of ocular drugs. Prog. Polym. Sci. 1998;23:563–580.
CrossRef - Malikmammadov E., Tanir T. E., Kiziltay A., Hasirci V., Hasirci N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018;29:863–893.
CrossRef - Kasinathan N., Amirthalingam M., Reddy N. D., Jagani H. V, Volety S. M., Rao J. V. In-situ implant containing PCL-curcumin nano particles developed using design of experiments. Drug Deliv. 2016;23:1007–1015.
- Varan C., Bilensoy E. Cationic PEGylated polycaprolactone nano particles carrying post-operation docetaxel for glioma treatment. Beilstein J. Nanotechnol. 2017;8:1446–1456.
CrossRef - Shenoy D. B., Amiji M. M. Poly(ethylene oxide)-modified poly(ɛ-caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. Int. J. Pharm. 2005;293:261–270.
CrossRef - Damgé C., Maincent P., Ubrich N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J. Control. Release. 2007; 117: 163–170.
CrossRef - Choi C., Chae S. Y., Nah J. -W. Thermo sensitive poly(N-isopropylacrylamide)-b-poly(ε-caprolactone) nano particles for efficient drug delivery system. Polymer (Guildf). 2006; 47:4571–4580.
CrossRef - Bakre L. G., Sarvaiya J. I., Agrawal Y. K. Synthesis Characterization and Study of Drug Release Properties of Curcumin from Polycaprolactone /Organomodified Mon tmorillonite Nano composite. J. Pharm. Innov. 2016;11:300–307.
CrossRef - Prabu P., Chaudhari A. A., Dharmaraj N., Khil M. S., Park S. Y., Kim H. Y. Preparation, characterization, in-vitro drug release and cellular uptake of poly(caprolactone) grafted dextran copolymeric nano particles loaded with anticancer drug. J. Biomed. Mater. Res. Part A. 2008;90:1128–1136.

This work is licensed under a Creative Commons Attribution 4.0 International License.





