How to Cite | Publication History | PlumX Article Matrix
Vinothkanna Annadurai and Sekar Soundarapandian
Department of Industrial Biotechnology, Bharathidasan University, Tiruchirappalli 620024, Tamil Nadu, India.
Corresponding Author E-mail: sekarbiotech@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2678
ABSTRACT: Ayurveda contains a variety of medicines including the polyherbal fermented traditional medicines namely Arishta and Asava. It remains as a rich source of bioactive phytochemicals including antioxidants. There are scanty publications on the antioxidant activity of these medicines. There is a need to explore the natural antioxidants to replace synthetic counterparts which requires an assessment in terms of antioxidant activity by in vitro analysis. This study employs a range of antioxidant assay systems containing various free radicals (DPPH, hydroxyl, hydrogen peroxide, super oxide, nitric oxide and ABTS), Inhibition of free radical generation (metal chelating) and H+ – donating ability (reducing power) were performed. Ashokarishta, Aswagandharishta and Dasamoolarishta exhibited higher degree of antioxidant activities but less than the corresponding standards. Based on IC50 values, the hydrogen peroxide scavenging ability of Ashokarishta is comparable to the standard L-ascorbic acid. In ABTS assay, Aswagandharishta and Dasamoolarishta are comparable to the standard Butylated hydroxytoluene (BHT). These may be due to the phytochemical heterogeneity of the samples assayed. Thus study can lead to the sourcing of Ayuvedic polyherbal fermented medicines as a novel source of natural antioxidant molecules that might be acting as free radical quencher or scavenger and to treat human ailments.
KEYWORDS: Antioxidant activity; Arishta; Ayurveda; Free radicals; Phytochemicals
Download this article as:| Copy the following to cite this article: Annadurai V, Soundarapandian S. Antioxidant Activity of Fermented Traditional Medicines of Indian Ayurveda – Ashokarishta, Aswagandharishta and Dasamoolarishta. Biosci Biotech Res Asia 2018;15(3). |
| Copy the following to cite this URL: Annadurai V, Soundarapandian S. Antioxidant Activity of Fermented Traditional Medicines of Indian Ayurveda – Ashokarishta, Aswagandharishta and Dasamoolarishta. Biosci Biotech Res Asia 2018;15(3). Available from: https://www.biotech-asia.org/?p=31119 |
Introduction
Antioxidants are compounds that quench free radicals generation, terminate the chain reactions, arrest other oxidation reactions and neutralize free radicals which leads to delay or inhibit cellular damage.1 Reactive oxygen species (ROS) is known for the cause of various chronic human diseases. It includes coronary artery blocks, insulin disorders, malignancy, gastric problems, neurodegenerative diseases, cataract, paralysis, aging etc.1 They are caused by the generation of free radicals like O2-., .OH, NO, 1O2 , H2O2, and HOCl.2
Butylated hydroxytoluene (BHT), Butylated hydroxyanisole (BHA), the chelating agent Ethylene Diamine Tetra Acetic acid (EDTA) and propylgallateare synthetic antioxidants. They are scavenging free radicals and chelate transition metals, thus inhibiting progressive autoxidative damage. However, the safety of these synthetic antioxidants remains doubtful.3-5 The natural antioxidants, vitamin C and vitamin E and polyphenols/flavonoids are non-toxic and also effectively scavenge free radicals or interrupt propagation of autoxidation.6
Arishta and Asava are unique and self-generated polyherbal Fermented Traditional Medicines (FTMs) of Indian Ayurveda7,8 and few reports also highlight the importance of FTMs.9-12 Few of the researchers reported the antioxidant activity of a few Arishta/Asava.13-16 Ashokarishta, Aswagandharishta and Dasamoolarishta are widely prescribed by Ayurvedic practioners. Ashokarishta is recommended for the treatment of menstrual disorders, leucorrhoea, menorrhagia, irregular menstruation etc. and excellent tonic for women. Aswagandharishta is used to treat epilepsy, insomnia, fainting, loss of memory and a nervine tonic for all age groups. Dasamoolarishta is used as a tonic in seminal weakness, general debility, emaciation, etc. also used as a heart stimulant, post natal tonic and used in respiratory illness.7,8 Ashokarishta contains Saraca asoca (Roxb.) De wild (bark) as the main herb along with 13 minor ingredients. Similarly, in Aswagandharishta the main herb is Withania somnifera (L.) Dunal. along with 26 minor ingredients. Similarly, Dasamoolarishta contains 10 main herbs and 65 minor ingredients.7,8 Like other Ayurvedic medicines, these FTM may contribute novel phytochemicals to treat various diseases. Among them, search for natural antioxidants is a prerequisite to safeguard the human health from the damages caused by free radicals. This will delay the progression of various chronic life style diseases. Hence it is rational to ascertain the antioxidant properties of Ayurvedic medicines in this search.
Materials and Methods
Chemicals and Reagents
The chemicals for in vitro antioxidant assays and standards were procured from Himedia, India. Sisco, India and Sigma–Aldrich, USA. Chemicals, reagents and solvents used were of analytical grade.
Sample Collection
The samples of Asokarishta, Aswagandharishta and Dasamoolarishta were collected from the factory premises of M/S. Ashtanga Ayurvedics (P) Ltd, Tiruchirappalli, Tamilnadu.
In vitro Antioxidant Assays
DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay
The scavenging of DPPH by polyherbal FTMs was determined.15 Briefly, 1 ml of each Arishta sample in different ranges (100 to1000 µl/ml) were mixed with distilled water in different test tubes. For each tube, 5 ml DPPH solution (33 mg/litre of methanol) was added, subsequently shaken and the tubes were kept at 27 °C for 20 min in dark. Then, the tubes were centrifuged for 5 min at 3000 g and absorbance of collected supernatant was recorded at 517 nm using UV-Vis Spectrophotometer (JASCO, Model V-650). L-Ascorbic acid was the standard used. DPPH scavenging effects were calculated as follows:
% of DPPH radical scavenging effect = [(A0– A1) / A0 × 100]
Where A0 denotes control; A1 denotes sample.
Hydroxyl Radical Scavenging Assay
The activity of hydroxyl radicals scavenging was assessed by the reaction of Fenton.17 The reaction system constitute ferric chloride (60 µL in 1.0 mM), 1,10-phenanthroline (90 µl in1.0 mM), phosphate buffer (2.4 ml in 0.2 M, pH 7.8), hydrogen peroxide (150 µL in 0.17 M). Various concentration of sample (1.5 ml) at a range of concentrations (10 to 100 µl/ml) were tested. The reaction was started with the addition H2O2. Incubation conditions are 5 min and ambient temperature. It was incubated at room temperature for 5 minutes and the absorbance values were obtained from spectrophotometer at 560 nm (JASCO, Model V-650). L-Ascorbic acid was the reference. Finally, the level of hydroxyl radicals scavenging was calculated.
Hydrogen Peroxide Radical Scavenging Assay
This assay was performed using a solution of hydrogen peroxide (100 mM) prepared in phosphate buffered saline (PBS) pH 7.4.15 Briefly, 0.5 ml of each Arishta at various concentrations (10, 20, 40, 60, 80 and 100 µl/ml) was added to test tube each containing hydrogen peroxide solution (4.5 ml) in PBS. Optical density was measured at 230 nm, after 10 minutes of incubation at ambient temperature. L-ascorbic acid was the standard. Percentage of H2O2 scavenging of Arishta was calculated.
H2O2 scavenged (%) = [(A0– A1) / A0 × 100]
Where A0 denotes control; A1 denotes sample.
Superoxide Anion Scavenging Assay
It was determined on the basis of superoxide radicals produced in a phenazine methosulphate (PMS) – NADH system coupled with NADH oxidation and through reduction of Nitroblue Tetrazolium chloride (NBT).17 Briefly, The superoxide radicals were generated in sodium phosphate buffer (3 ml in 100 mM, pH 7.4) containing NBT (1 ml in 150 µM), NADH (1 ml in 468 µM,) and various dilutions of samples (10, 20, 40, 60 and 100 µl/ml). Then 1 ml phenazine methosulphate solution (60 µM in phosphate buffer) was added to onset the reaction and incubated at 25 ºC for 5 min. Employing UV-Vis spectrophotometer, A560nm was measured. The standard used was L-Ascorbic acid. Decrease in A560nm values indicates increase in scavenging activity. Inhibition (%) was calculated by the formula.
Super oxide scavenged (%) = [(A0– A1) / A0 × 100]
Where A0 denotes control; A1 denotes sample.
Nitric oxide Scavenging Assay
This activity of Ayurvedic polyherbal FTM samples were measured.18 Briefly, sodium nitroprusside (5 mM, 0.5 ml) prepared in PBS (pH 7.4) and aliquots of sample (v/v) (10, 20, 40, 60 and 100 µl/ml) of the test samples were mixed. This reaction mixture was incubated at 25 ºC for 150 min. After incubation, the quantity of nitrite produced was measured by the addition of Griess reagent (1% sulphanilamide, 5% phosphoric acid and 0.1% 1-naphthalene diamine dihydrochloride in water). Finally, the A570nm was measured. The inhibition (%) of nitric oxide generation was determined with reference to control standard (L-ascorbic acid).
Nitric oxide scavenged (%) = [(A0– A1) / A0 × 100]
Where A0 denotes control; A1 denotes sample.
ABTS (2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) radical scavenging assay
This activity of Ayurvedic polyherbal formulations were determined by the decolorization assay of ABTS radical cation.19 This cation radical was generated by mixing equal ratio of ABTS (7 mM) and potassium persulfate (2.45 mM) prepared 12-16 h in advance and stored in dark at ambient temperature. This solution was diluted accordingly to set an absorbance value of 0.700 (734 nm) using double distilled water. Briefly, freshly prepared ABTS solution (1 ml) was mixed with equal volume of the various concentrations (v/v) (10, 20, 40, 60, 80 and 100 µl/ml) of Ayurvedic polyherbal formulations, vortexed for few seconds and incubated for 6 min. A734nm values of samples prepared in triplicates were measured. The percentage of scavenging activity was calculated with reference to BHT (standard) and calculations were performed as per DPPH assay.
Metal chelating assay
It was performed based on the chelation of ferrous ions (Fe2+).17 Briefly, 0.5 ml of each Arishta sample in different concentrations (v/v) (10, 20, 40, 60 and 100 µl/ml) of Ayurvedic polyherbal formulations was added with ferric chloride (50 µl in 2 mM) and mixed vigorously for 30 sec. To start the reaction, ferrozine (100 µl in 5 mM) solution was added. It was followed by vortexing and 10 min incubation at ambient temperature. The A562nm values of Fe2+– Ferrozine complex formed was measured. The inhibition (%) of formation of Fe2+– Ferrozine complex was calculated with reference to citric acid standard.
Chelating activity (%) = [(A0– A1) / A0 × 100]
Where A0 denotes control; A1 denotes sample.
Reducing Power Assay
This property of the polyherbal formulations was assessed as per Manmode et al. (2012).14 Different dilutions (v/v) (10, 20, 40, 60 and 100 µl/ml) of formulations were used. The reaction consists of 1ml sample, phosphate buffer (2.5 ml in 0.2 M, pH 6.6), potassium ferricyanide (2.5 ml in 1%) and subjected to water bath incubation for 20 min at 50 °C. To inhibit the reaction TCA was used (2.5 ml in 10%). Finally, the tubes were centrifuged (3000 g) for 10 min. From this supernatant, 2.5 ml was mixed with equal volume of distilled water and ferric chloride (0.1 ml in 0.1% w/v) followed by 10 min incubation. A700nm was measured with reference to L-ascorbic acid standard. Higher absorbance values denote higher reducing power.
Statistical Analysis
Experimental samples were run in triplicates. The values are given as mean ± standard deviation. The tool used was Graphpad software ( Instat and Prism 5.0 Demo version). The assay values were subjected to Two-way ANOVA comprising Dunnet’s multiple comparison tests. The mean difference is considered as significant at the levels of *p<0.05, **P<0.01 and ***P<0.001. The IC50 values were obtained by linear-regression probit analysis using SPSS software 16.0.
Results and Discussion
DPPH Radical Scavenging Assay
The Ayurvedic polyherbal formulations, Ashokarishta, Aswagandharishta and Dasamoolarishta samples upon treatment with DPPH, react with methanol and yield purple DPPH radicals. DPPH is a stable nitrogen centered free radical and organic molecule. The antioxidant molecule scavenge DPPH radicals (donating hydrogen atom or electron transfer) and convert them to a colorless/yellow substrate (2,2-diphenyl-1-hydrazine).20-22 Among the Ayurvedic polyherbal FTMs tested, Aswagandharishta had highest radical scavenging ability (73.75%) followed by Ashokarishta (71.63%) and Dasamoolarishta (70.46%). The antioxidant standard L-ascorbic acid showed highest activity (80.58%) than others (Fig. 1). The result of DPPH radical scavenging activity clearly indicated that Ayurvedic polyherbal formulations contain wide range of phytochemicals which act as good antioxidants. Aswagandharishta required lowest amount to scavenge 50% of DPPH radicals, followed by Ashokarishta and Dasamoolarishta while the IC50 value for L-ascorbic acid was the lowest when compared to others (Table 1). The present study suggested that the polyherbal formulations may produce good amount of hydrogen donor atoms which may interrupt or inhibit the propagation of free radicals.
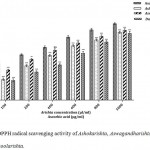 |
Figure 1: DPPH radical scavenging activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Hydroxyl Radical Scavenging Assay
Hydroxyl radicals are hyper reactive oxygen species which damage biological macromolecules including carbohydrates, proteins, polyunsaturated fatty acids and DNA.23 The Ayurvedic FTMs were examined for their ability to scavenge hydroxyl radical. The hydroxyl radical scavenging ability of the Ayurvedic FTMs were in the following sequence: Dasamoolarishta (76.02%) > Ashokarishta (77.56%) >Aswagandharishta (79.44%) > L-ascorbic acid (84.81%) (Fig. 2). Our findings suggested that, the Ayurvedic FTM shows quenching effect on hydroxyl radicals that may help to protect biological system from free radical generation. Similarly based on IC50 values, Ayurvedic formulation Ashokarishta required lowest amount to scavenge 50% of hydroxyl radicals followed by Aswagandharishta and Dasamoolarishta. However, the positive control L-ascorbic acid required the least (30.22 µg/ml) among all to scavenge 50% of hydroxyl radicals (Table 1). Our results revealed that the Ayurvedic formulations may generate a wide range of phytochemical compounds which may stimulate hydrogen donating ability or scavenging of free radicals.
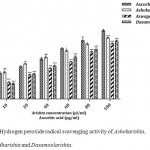 |
Figure 2: Hydroxyl radical scavenging activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Hydrogen Peroxide Scavenging Assay
Hydrogen peroxide (H2O2) has ability to inactivate a few enzymes, generally due to the oxidation of thiol (-SH) groups. It has the ability to penetrate cross cell membranes, react with metal ions (iron and copper) to generate hydroxyl radical leading to toxic effects.14 In this study, the hydrogen peroxide scavenging abilities of the Ayurvedic FTMs were in the following sequence: Aswagandharishta (67.49%) > Dasamoolarishta (70.54%) > Ashokarishta (74.18%) > L-ascorbic acid (83.12%) (Fig. 3). Our results indicate that all formulations showed considerable level H2O2 radical scavenging activity. Minimum IC50 value for scavenging H2O2 was 30.79 µl/ml for Ashokarishta followed by 50.24 µl/ml for Dasamoolarishta and 58.08 µl/ml for Aswagandharishta. The Positive control L-ascorbic acid showed better IC50 value of 29.98 µl/ml (Table 1) which is comparable to Ashokarishta. The hydrogen peroxide scavenging activity of Ayurvedic formulations can inhibit free radical generation and decomposition of peroxides by the phytochemical compounds present within.
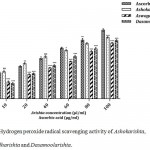 |
Figure 3: Hydrogen peroxide radical scavenging activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Superoxide Anion Scavenging Assay
Superoxide anion radicals are most harmful radical because they are the precursor for other major Reactive Oxygen species like hydrogen peroxide and hydroxyl radical. They cause oxidative damage to biomolecules.17,19 In the present study, all the polyherbal formulations showed super oxide anion scavenging ability and results are depicted (Fig. 4). The formulations Ashokarishta (71.89%), Aswagandharishta (76.32%) and Dasamoolarishta (78.38%) possessed low level activity upon comparison to the standard (84.63%). The superoxide radical scavenging abilities of the polyherbal formulations were in the following sequence: Ashokarishta > Aswagandharishta > Dasamoolarishta. Dasamoolarishta (37.53 µl/ml) exhibited effective IC50 value followed by Ashokarishta (53.79 µl/ml) and Aswagandharishta (53.98 µl/ml). The standard L-ascorbic acid required 25.40 µl/ml of compound to quench 50% of super oxide anion radicals (Table 1). Similar work on flavonoids indicate effective scavenging of superoxide anion by the inhibition of free radical chain reactions, mostly hydroxyl radical scavenging.24 Our results also demonstrated that all the polyherbal formulations were able to quench or neutralize free radicals by the stabilization of reactive species.
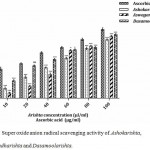 |
Figure 4: Super oxide anion radical scavenging activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Nitric Oxide Scavenging Assay
This radical is generated in L-arginine metabolism by nitric oxide synthases that are toxic to biological tissues. The toxicity is increased, when nitric oxide radicals form a highly reactive peroxynitrite anion (ONOO–) upon reaction with superoxide radical. This is responsible for many physiologic and pathologic activities. In our study, Ayurvedic polyherbal formulations had considerable scavenging ability of nitric oxide radical.22,25 Ashokarishta exhibited highest (77.97%) nitric oxide radical scavenging activity followed by Aswagandharishta (75.38%) and Dasamoolarishta (73.5%). Nitric oxide scavenging activity of polyherbals formulations are presented (Fig. 5). Positive control L-ascorbic acid (85.14%) possesses higher activity when compared to others. The IC50 values of Ashokarishta, Aswagandharishta and Dasamoolarishta were 50.21, 43.73 and 43.75 µl/ml respectively (Table 1). These formulations were found to be effective to scavenge nitric oxide in the order Ashokarishta > Dasamoolarishta > Aswagandharishta. The results showed that Aswagandharishta and Dasamoolarishta have comparable IC50 values. The phytochemicals such as flavonoids, alkaloids and saponins were shown to have better nitric oxide free radical scavenging ability.18 Therefore, the results indicated that all the formulations were able to scavenge nitric oxide free radicals by the presence of such compounds.
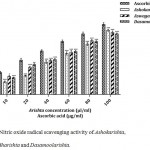 |
Figure 5: Nitric oxide radical scavenging activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
ABTS Scavenging Assay
ABTS is a cation free radicals solution which is soluble in water and organic solvents. The determination of this activity is based on the transfer of hydrogen atoms and electrons to ABTs radical.19 Aswagandharishta (80.40%) exhibited better ABTS radical scavenging activity than Dasamoolarishta (76.44%) and Ashokarishta (73.82%). The standard BHT (84.26%) showed highest activity compared to the tested drugs (Fig. 6). The IC50 values of Ashokarishta Aswagandharishta and Dasamoolarishta were 58.72, 32.72, and 32.81 µl/ml respectively. BHT required only 28.50 µg/ml to scavenge 50% of ABTS free radicals (Table 1). Among them, Aswagandharishta and Dasamoolarishta IC50 values were comparable with each other. Our results explained that the all the polyherbal formulations may have large amount of hydrogen donor molecules which may neutralize or reduce the generation of free radicals and results in decolorization.
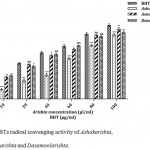 |
Figure 6: ABTs radical scavenging activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Metal Chelating Assay
Iron is vital for carrying oxygen, respiration and for enzymatic activities. It can initiate lipid peroxidation by Haber–Weiss and Fenton reactions promoting the generation of hydroxyl and superoxide anion radicals.23 Ferrous ion chelating agents inhibit lipid peroxidation (by stabilization of transition metal), free radical generation and resultant oxidative damage.26 It can form complexes with ferrozine producing violet color. The chelating ability (interruption of complex formation) was determined by the measurement reduction of the color of solution. The metal chelating activity of various Ayurvedic polyherbal formulations are depicted in Fig. 7. Among them, Dasamoolarishta (74.22%) showed better chelating activity followed by Ashokarishta (70.30%) and Aswagandharishta (68.13%). The standard citric acid (81.44%) had highest chelating ability compared to others. The IC50 values of the Ayurvedic FTMs were in the higher level than positive control. The IC50 values of the chelating ability were in the following sequence: Aswagandharishta (64.10 µl/ml) > Ashokarishta (56.37 µl/ml) > Dasamoolarishta (40.03 µl/ml) and > Citric acid (22.75 µg/ml) (Table 1). This study suggested that Ayurvedic polyherbal formulations have the vital iron chelating capacity by the presence of phytochemicals that enhance the natural antioxidant defense mechanism.
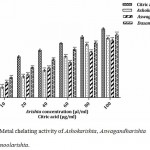 |
Figure 7: Metal chelating activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Determination of Reducing Power
The antioxidants act as reductones and reduce Fe3+ to Fe2+ which reflects scavenging of free radical by the donation of hydrogen atom.17,26 Increase in absorbance (at 700 nm) reflects higher level of reducing power. The Dasamoolarishta (75.55%) showed better reducing power followed by Ashokarishta (71.02 %) and Aswagandharishta (68.64%). The standard L-ascorbic acid (86.10%) exhibited highest activity than the tested formulations (Fig. 8). The IC50 values of the Ayurvedic formulations were comparable with each other. The standard L-ascorbic acid (25.73 µg/ml) required minimum amount to scavenge free radicals than other formulations (Table 1). The hydrogen donating ability of Ayurvedic formulation determines their reducing power. The antioxidant activity could be because of phytochemicals present therein that may react and stabilize free radicals which in turn arrest chain reactions.
Table 1: Antioxidant activity of Ashokarishta, Dasamoolarishta and Aswagandharishta.
| IC50 value | |||||
| Assays | Standard (µg/ml) | Ashokarishta (µl/ml) | Dasamoolarishta (µl/ml) | Aswagandharishta (µl/ml) | |
| Scavenging of free radicals | DPPH | 216.97 (Ascorbic acid) | 408.38 | 515.62 | 301.69 |
| Hydroxyl radical | 30.22 (Ascorbic acid) | 40.69 | 48.83 | 47.70 | |
| Hydrogen peroxide | 29.98 (Ascorbic acid) | 30.79 | 50.24 | 58.08 | |
| Super oxide anion | 25.40 (Ascorbic acid) | 53.79 | 37.53 | 53.98 | |
| Nitric oxide | 29.00 (Ascorbic acid) | 50.21 | 43.75 | 43.73 | |
| ABTS | 28.50 (BHT) | 58.72 | 32.81 | 32.72 | |
| Inhibition of free radical generation | Metal chelating | 22.75 (Citric acid) | 56.37 | 40.03 | 64.10 |
| H+ – donating ability | Reducing power | 25.73 Ascorbic acid) | 51.08 | 51.76 | 54.33 |
IC50 value denotes effective concentration at which the particular free radical was scavenged by 50% and obtained by linear-regression probit analysis.
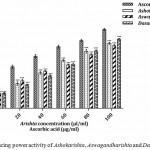 |
Figure 8: Reducing power activity of Ashokarishta, Aswagandharishta and Dasamoolarishta.
|
The values indicate mean ± SD (n=3). The mean difference is significant at the levels of *p<0.05, **P<0.01 and ***P<0.001 Vs standard. ns – non significant.
Conclusion
The study indicated that the three preparations, Ashokarishta Aswagandharishta and Dasamoolarishta had the ability to scavenge many free radicals. Generally, earlier studies were performed only with one or two antioxidant assay systems. But in the current study, many antioxidant systems were employed delivering a wide of range of free radicals. Moreover, these samples can offer a wide variety of natural antioxidants to cure diseases as they contain a variety of herbal ingredients. The antioxidants present in herbal ingredients shall promote various quenching reactions to stabilize free radicals.
Acknowledgements
The authors thank Bharathidasan University, Tiruchirappalli, India for the facilities and award of UGC for RGNF fellowship to AV. Authors are indebted to TN. Narayanan Varier and TR. Sasi Varier of Ashtanga Ayurvedics (P) Ltd, Tiruchirappalli, India.
Conflict of Interest
The authors declare that they have no conflict of interest
References
- Mishra V., Shah C., Mokashe N., Chavan R., Yadav H., Prajapati J. Probiotics as potential antioxidants: a systematic review. J. Agric. Food. Chem. 2015;63(14):3615-26.
CrossRef - Lü J. M., Lin P. H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: experimental approaches and model systems. J. Cell. Mol. Med. 2010;14(4):840-60.
CrossRef - Whysner J., Williams G. M. Butylated hydroxyanisole mechanistic data and risk assessment conditional species-specific cytotoxicity, enhanced cell proliferation and tumor promotion. Pharmacol. Ther. 1996;71(1-2):137-51.
CrossRef - Williams G. M., Iatropoulos M. J., Whysner J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food. Chem. Toxicol. 1999;37(9-10):1027-38.
CrossRef - Patel V. R., Patel P. R., Kajal S. S. Antioxidant activity of some selected medicinal plants in western region of India. Adv. Biol. Res. 2010;4(1):23-6.
- Brewer M. S. Natural antioxidants: sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food. Sci. Food. Saf. 2011;10(4):221-47.
CrossRef - Sekar S., Mariappan S. Fermented medicines of Ayurveda: a treatise. Germany:LAP LAMBERT Academic Publishing. 2010.
- Sekar S., Mariappan S. Traditionally fermented biomedicines, Arishtas and Asavas from Ayurveda. Indian. J. Tradit. Know. 2008;7:548-56.
- Chaudhary A., Singh N., Dalvi M., Wele A. A progressive review of sandhana kalpana (biomedical fermentation): An advanced innovative dosage form of Ayurveda. AYU. 2011;32(3):408-17.
CrossRef - Lee J. H., Lee J. H., Jin J. S. Fermentation of traditional medicine: present and future. Orient. Pharm. Exp. Med. 2012;12(3):163-5.
CrossRef - Sabu A., Haridas M. Fermentation in ancient Ayurveda: Its present implications. Front. Life. Sci. 2015;8(4):324-31.
CrossRef - Egea T., Signorini M. A., Bruschi P., Rivera D., Obón C., Alcaraz F.,Palazón J. A. Spirits and liqueurs in European traditional medicine: Their history and ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharmacol. 2015;175:241-55.
CrossRef - Yogesh D. A., Shankar L. L. Antioxidant assessment of Ashokarishta–A fermented polyherbal Ayurvedic formulation. J. Pharm. Res. 2012;5(6):3165-8.
- Manmode R., Manwar J., Vohra M., Padgilwar S., Bhajipale N. Effect of preparation method on antioxidant activity of Ayurvedic formulation J. Homeop. Ayurv. Med. 2012;1:2-5.
- Manwar J., Mahadik K., Sathiyanarayanan L., Paradkar A., Patil S. Comparative antioxidant potential of Withania somnifera based herbal formulation prepared by traditional and non-traditional fermentation processes. Integr. Med. Res. 2013;2(2):56-61.
CrossRef - Swapna E., Mohan G. K., Nagaveni P., Shanker K. Evaluation of Antioxidant activity of Marketed Ayurvedic Formulations-Balarishta. Res. J. Pharmacogn. Phytochem. 2015;7(2):107-10.
CrossRef - Sharma S. K., Singh A. P. In vitro antioxidant and free radical scavenging activity of. Nardostachys jatamansi J. Acupunct. Meridian. Stud. 2012;5(3):112-8.
CrossRef - Boora F., Chirisa E., Mukanganyama S. Evaluation of nitrite radical scavenging properties of selected Zimbabwean plant extracts and their phytoconstituents. J. Food Processing. 2014. http://dx.doi.org/10.1155/2014/918018.
CrossRef - Rameshkumar A., Sivasudha T., Jeyadevi R., Sangeetha B., Ananth D. A., Aseervatham G. S., et al. In vitro antioxidant and antimicrobial activities of Merremia emarginata using thio glycolic acid-capped cadmium telluride quantum dots. Colloids. Surf. B. Biointerfaces. 2013;101:74-82.
CrossRef - Brand-Williams W., Cuvelier M. E., Berset C. L. Use of a free radical method to evaluate antioxidant activity. LWT-Food. Sci. Technol. 1995;28(1):25-30.
- Hseu Y. C., Chang W. H., Chen C. S., Liao J. W., Huang C. J., Lu F. J., et al. Antioxidant activities of Toona Sinensis leaves extracts using different antioxidant models. Food. Chem. Toxicol. 2008;46(1):105-14.
CrossRef - Alam M. N., Bristi N. J., Rafiquzzaman M. Review on in vivo and in vitro methods evaluation of antioxidant activity. Saudi. Pharm. J. 2013;21(2):143-52.
CrossRef - Abirami A., Nagarani G., Siddhuraju P. In vitro antioxidant, anti-diabetic cholinesterase and tyrosinase inhibitory potential of fresh juice from Citrus hystrix and C. maxima fruits. Food. Sci. Hum. Welln. 2014;3(1):16-25.
CrossRef - Robak J., Gryglewski I. R. Flavonoids are scavengers of superoxide anions. Biochem. Pharmacol. 1988;37(5):837–41.
CrossRef - Balavoine G. G., Geletti Y. V. Peroxynitrite scavenging by different antioxidants. Part 1 convenient study. Nitric Oxide. 1999;3(1):40–54.
CrossRef - Pavithra K., Vadivukkarasi S. Evaluation of free radical scavenging activity of various extracts of leaves from Kedrostis foetidissima (Jacq.) Cogn. Food. Sci. Hum. Welln. 2015;4(1):42-6.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





