Manuscript accepted on : 16-Nov-2018
Published online on: 25-12-2018
Plagiarism Check: Yes
Antony V. Jenila and J. Joel Gnanadoss
Microbial and Environmental Biotechnology Research Unit, Department of Plant Biology and Biotechnology, Loyola College, Chennai 600 034, Tamil Nadu, India.
Corresponding Author E-mail: joelgna@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2699
ABSTRACT: Endophytic fungi Fusarium sp. LCJ273 capable of producing L-asparaginase was isolated from the medicinal plant Adhatoda vasica. The aim of the present study was to maximize L-asparaginase production by submerged fermentation through statistical optimization. L-Asparaginase production by Fusarium sp. LCJ273 was studied in five different media. Various nutritional parameters specifically carbon, nitrogen and inducers were optimized for enhancing the production of L-asparaginase. In addition, different statistical based experimental designs were also applied to increase the production of L-asparaginase by Fusarium sp. LCJ273. Dextrose, ammonium sulphate and wheat bran were found to be effective for growth and higher yield of L-asparaginase in Modified Czapek’s Dox Broth. Dextrose at a concentration of 3.0 g/L increased L-asparaginase production up to 9.18±0.9 U/mL, ammonium sulphate at the concentration of 20 g/L showed maximum L-asparaginase production up to 13.69±0.4 U/mL and wheat bran at 2.5 g/L yielded up to 14.24±0.5 U/mL. The maximum L-asparaginase production was observed by Fusarium sp. LCJ273 on 5th day. The study revealed that through optimization, a 2 fold increase in L-asparaginase could be achieved.
KEYWORDS: Endophytic Fungi; Enzyme Production; L-Asparaginase; Submerged Fermentation
Download this article as:| Copy the following to cite this article: Jenila A. V, Gnanadoss J. J. Formulation of A Suitable Medium and its Optimization for Maximizing L-Asparaginase Production from Endophytic Fungi Fusarium Sp. LCJ273. Biosci Biotech Res Asia 2018;15(4). |
| Copy the following to cite this URL: Jenila A. V, Gnanadoss J. J. Formulation of A Suitable Medium and its Optimization for Maximizing L-Asparaginase Production from Endophytic Fungi Fusarium Sp. LCJ273. Biosci Biotech Res Asia 2018;15(4). Available from: https://www.biotech-asia.org/?p=32316 |
Introduction
L-Asparagine is hydrolyzed by L-asparaginase (L-asparagine amido hydrolase E.C.3.5.1.1) to form aspartic acid and ammonia. L-Asparaginase is present in animals, plants and microbes but not in humans. Fungal L-asparaginases are enzymes of great therapeutic significance due to their use in anti-leukemic and antilymphoma treatment.1,2 L-Asparaginase has a growing demand in medical application and food industries.3 L-asparaginase production from microbes has more attention because the process is cost-effective and environmental friendly.4 Bacteria such as Escherichia coli and Erwinia carotova are the best producers of L-asparaginase and are used in pharmaceutical industries. There are only few researches carried out on L-asparaginase production by endophytic fungi.5,6,7 Recently, L-asparaginase obtained from fungi, such as Fusarium, Aspergillus and Penicillium, show additional promise since their L-asparaginases have less adverse effects when compared to bacterial L-asparaginases.8 Various types of cancers including melanosarcoma, reticulosarbom, acute myelocytic leukemia, lymphosarcoma, Hodgkin disease, chronic lymphocytic leukemia, acute myelomonocytic leukemia and acute lymphoblastic leukemia specially in children can be treated using L-asparaginase.9,10 L-Asparaginases are effective because neoplastic cells cannot synthesize L-asparagine and therefore rely on L-asparagine found within blood plasma. Research has demonstrated that plasma L-asparagine levels in the blood can be reduced through intravenous injections of L-asparaginases.11 Submerged fermentation (SmF) is a very effective technique and require low energy and less risk of contamination.12 Screening and estimation of the environmental factors and nutritional parameters are more essential stages of bioprocess.13 Minimum information is available related to the optimization of medium conditions using statistical methods for enzyme production. Response surface methodology (RSM) uses mathematical equations to predict the relationship between response and variables.14,15 The present study was carried out to formulate a suitable medium and to optimize various nutritional factors specifically carbon, nitrogen, natural inducers and culture conditions for maximizing the L-asparaginase production by Fusarium sp. LCJ273 under submerged fermentation. Optimization by RSM was used to observe the correlation between the L-asparaginase production and significant parameters.
Material and Methods
Microorganism and Growth Medium
The fungal strain Fusarium sp. LCJ273 was isolated from Adhatoda vasica collected from Chennai. It was grown in Potato Dextrose Agar (PDA) medium containing Potato (200 g/L), Dextrose (20 g/L) and agar (15g/L). The fungal strain were periodically sub-cultured once in two weeks and stored at 4ºC.16
Culture Conditions for L-Asparaginase Production
Fusarium sp. LCJ273 was grown in 5 different basal media specifically Modified Czapek’s Dox Broth (MDCB) (Medium 1), Agar based modified broth (Medium 2), Glucose Asparagine Broth (GAB) (Medium 3), Asparagine Dextrose Salts Broth (ADSB) (Medium 4) and Inorganic Salts Starch Asparagine liquid medium (ISA) (Medium 5)17,18,19 for maximizing the L-asparaginase production. Endophytic fungi Fusarium sp. LCJ273 showed higher activity when Modified Czapek’s Dox medium was used. Composition of Modified Czapek’s Dox medium (g/L): Glucose 2.0, L-asparagine 10.0, KH2PO4 1.52, MgSO4.7H2O 0.52, KCL 0.52, traces of Cu(NO3).3H2O, ZnSO4.7H2O, FeSO4.7H2O.
Submerged fermentation was done using 100 mL liquid medium in 250 mL conical flasks. The medium was sterilized and Streptomycin (Hi-media) was added to liquid medium to avoid bacterial contamination. After sterilization, the mycelium of Fusarium sp. LCJ273 was inoculated into the medium and incubated in rotatory shaker at 120 rpm for 5 – 8 days. After every 24h of fermentation, 0.5 mL of crude culture filtrate was taken and centrifuged at 10,000 rpm for 10 mins and the clear supernatant was collected. The activity of L-asparaginase was measured at every 24 h interval using the supernatant as a enzyme source.
Assay for L-Asparaginase
The activity of L-asparaginase was evaluated quantitatively by estimating the amount of ammonia liberated from L-asparagine using Nessler’s reagent by Imada et al.,20 in short: 0.5 mL of crude culture filtrate, 0.5 mL of 0.5 M tris HCL buffer (7.2), 0.5 mL of 0.04 M asparagine and 0.5 mL of distilled water were combined and incubated at 37ºC for 30 min. The reaction was terminated by adding 0.5 mL of 1.5 M tricholoro acetic acid (TCA). After termination of the reaction, the mixture was centrifuged at 10,000 rpm for 10 min and 0.1 mL of clear supernatant was added to 3.7 mL of distilled water. 0.2 mL of Nessler’s reagent was then added and incubated for 20 min. Absorbance was measured at 450 nm using spectrophotometer. One international unit (IU) of L-asparaginase needed to release 1 μM of ammonia per minute under specified conditions.
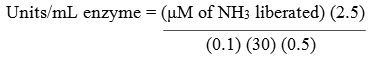
2.5 = Initial volume of reaction mixture (mL)
0.1 = Volume of supernatant used (mL)
30 = Time of incubation (min)
0.5 = Volume of crude enzyme sample used (mL).
Estimation of Protein
Protein estimation was done by the method of Lowry et al.,21 using Bovine serum albumin as the standard.
Optimization Studies for L-Asparaginase Production
Optimization of medium was done by changing any one carbon, nitrogen and natural inducers variable while keeping all the other variables at a fixed level. Optimization of the fermentation medium is to study the process conditions to maximize the enzyme production. Carbon, nitrogen and inducers are considered as the key parameters for medium optimization to increase the L-asparaginase production. They also play a major role in the synthesis of fundamental nutrients needed for the organism growth in the liquid medium. Optimization of nutritional factors by “one-factor-at-a-time” technique was done. Carbon sources such as dextrose, maltose, lactose, glycerol, sucrose and mannitol were studied at different concentrations from 0.5 to 6.0 g/L to determine the effect of carbon source in L-asparaginase production. Nitrogen sources such as ammonium sulphate, yeast extract, sodium nitrate and peptone were also studied at different concentrations from 5 to 25 g/L for the production of L-asparaginase by Fusarium sp. LCJ273.
Effect of Carbon Sources and Their Concentration on L-Asparaginase in Modified Czapek Dox Medium
The effect of carbon source on the production of L-asparaginase by Fusarium sp. LCJ273 was studied using dextrose, lactose, maltose, sucrose, glycerol and mannitol at a concentration of 10 g/L. Other components in the medium were kept unchanged while original medium was used as a control.
The best carbon source was further optimized by using different concentrations from 0.5 to 6.0 g/L. L-Asparaginase and protein activity were determined and best carbon source was chosen for further studies.
Effect of Nitrogen Sources and Their Concentration on L-Asparaginase in Modified Czapek Dox Medium
L-Asparaginase production by Fusarium sp. LCJ273, was studied using four different nitrogen sources. Modified Czapex Dox broth was amended with different nitrogen sources using ammonium sulphate and ammonium nitrate (inorganic sources), peptone, yeast extract (organic sources) at a concentration of 10 g/L and other variables in the medium were kept constant. The original medium was used as a control.
L-Asparaginase production by Fusarium sp. LCJ273 was studied at different concentrations of selected nitrogen source ranging from 5 to 25 g/L. L-Asparaginase and protein activity were determined and best nitrogen source which showed maximum L-asparaginase production was selected for further study.
Effect of Natural Inducers and Their Concentration on L-Asparaginase in Modified Czapek Dox Medium
The effects of cheap natural inducers on the L-asparaginase production by Fusarium sp. LCJ273 was studied using groundnut oil cake, coconut oil cake, wheat bran, paddy straw and bagasse at the concentration of 5 g/L and other variables were kept constant, while original medium was used as a control.
The best inducer was further optimized by using different concentrations from 0.5 to 3.0 g/L. L-Asparaginase and protein activity were determined and best natural inducer which showed maximum L-asparaginase production was selected for further study.
Optimization of L-Asparaginase Production Using Response Surface Methodology
The optimized medium components for enzyme production was studied using Central Composite Design (CCD), a method commonly used in Response Surface Methodology (RSM). Central composite design methodology which is applied to explain the suitable conditions for the production of L-asparaginase. The level of major parameters namely dextrose, ammonium sulphate and wheat bran were selected for studies using Face Centre Central Composite Design (FCCCD). The results of the experiments were estimated using statistical software Design Expert (version 10, Stat-Ease, Inc., Minneapolis, MN). The three independent parameters were studied at three different levels (-1, 0, +1) shown in Table. 2.
Thirty experiments were generated and all the parameters were taken at the central coded points to estimate the pure error. The minimum and maximum ranges of parameters were studied and the proper response to the independent parameters. The response of
L-asparaginase production is presented in the following polynomial equation employed to fit the experimental data.
Y= b0 + b1X1 + b2X2 + b3X3 + b11X12 + b22X22 + b33X32 + b12X1X2 + b23X2X3 + b13X1X3
In this equation, Y is the predicted response (L-asparaginase production); b1, b2, b3 linear coefficients; b11, b22, b33 squared coefficients; b12, b13, b23 interaction coefficients.
To validate the statistical model, the coefficients were designed by regression analysis and their significance level. L-Asparaginase production was subjected to Analysis of Variance (ANOVA) with statistic software program (version 10, Stat-Ease, Inc., Minneapolis, MN) suitable to the design of the experiments. The response surface curves were acquired for determining the finest levels of the parameters for high L-asparaginase production.
Original and Optimized Medium for L-Asparaginase Production in Modified Czapek Dox Medium
Comparison was done between original and optimized medium to prove the efficiency of the optimized medium in the L-asparaginase production by Fusarium sp. LCJ273. The mycelium of Fusarium sp. LCJ273 was inoculated in 1000 mL of original and optimized medium in separate set of experiments and incubated for 3-8 days. L-Asparaginase activity was determined by measuring the absorbance at 450 nm.
Results
Screening and Identification of L-Asparaginase Producing Endophytic Fungi
L-Asparaginase activity was done using plate assay on Czapek’s Dox media. Among the isolates while were positive, Fusarium sp. LCJ273 was considered as a highly potential strain (Fig 1). 18S rRNA gene sequence of the fungi has been deposited in GenBank under accession number KY238311.
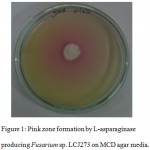 |
Figure 1: Pink zone formation by L-asparaginase producing Fusarium sp. LCJ273 on MCD agar media.
|
Effect of Carbon Sources and Their Concentration on L-Asparaginase in Modified Czapek Dox Medium
The results showed that, an addition of dextrose increased the L-asparaginase production when compared to the other carbon sources. L-Asparaginase activity of 7.06 U/mL was observed in dextrose (Table. 1). The effect of different concentration of dextrose from 0.5 to 6.0 g/L in MCDB was studied further.
Table 1: Effect of different carbon sources, nitrogen sources and natural inducers in the culture medium for L-asparaginase production by Fusarium sp. LCJ273.
| Sources | L-Asparaginase activity U/mL |
| Carbon sources (10 g/L) | |
| Fructose | 6.81±1.1 |
| Glycerol | 6.38±0.5 |
| Maltose | 6.84±0.4 |
| Starch | 6.51±1.2 |
| Lactose | 6.38±0.4 |
| Sucrose | 7.03±1.0 |
| Mannitol | 6.70±1.1 |
| Dextrose | 7.06±0.9 |
| Nitrogen sources (10 g/L) | |
| Peptone | 2.46±0.9 |
| Yeast extract | 2.97±1.0 |
| Ammonium sulphate | 10.21±1.1 |
| Ammonium nitrate | 8.45±0.8 |
| Natural inducers (5 g/L) | |
| Groundnut cake | 12.45±0.7 |
| Coconut cake | 12.63±0.6 |
| Wheat bran | 13.29±0.4 |
| Paddy straw | 12.43±0.4 |
| Bagasse | 11.78±0.5 |
Highest L-asparaginase activity of 9.18 U/mL was observed at 3.0 g/L of dextrose on the 5th day of incubation Fig 2. Dextrose concentration above 3 g/L decreased L-asparaginase activity. Minimum L-asparaginase production was observed in glycerol and lactose. The maximum specific activity of L-asparaginase in Fusarium sp. LCJ273 was found to be 52.77 U/mg in dextrose.
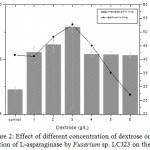 |
Figure 2: Effect of different concentration of dextrose on the production of L-asparaginase by Fusarium sp. LCJ23 on the 5th day.
|
Effect of Nitrogen Sources and Their Concentration on L-Asparaginase in Modified Czapek Dox Medium
Ammonium sulphate was found to be best nitrogen source when compared to other nitrogen sources. Maximum L-asparaginase yield of 10.21 U/mL was observed in Fusarium sp. LCJ273 (Table 1). The effect of different concentration of ammonium sulphate (5 to 25 g/L) was further studied for maximizing the production of enzyme.
Maximum L-asparaginase yield of 13.69 U/mL was observed at 20 g/L of ammonium sulphate on 5th day of incubation (Fig 3). L-Asparaginase production decreased by the addition of yeast extract and peptone (organic nitrogen sources). The maximum specific L-asparaginase activity by Fusarium sp. LCJ273 was 67.02 U/mg when in ammonium sulphate was used. L-Asparaginase activity decreased above 20 g/L ammonium sulphate.
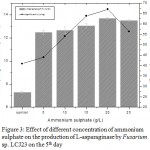 |
Figure 3: Effect of different concentration of ammonium sulphate on the production of L-asparaginase by Fusarium sp. LCJ23 on the 5th day.
|
Effect of Natural Inducers and Their Concentration on L-Asparaginase in Modified Czapek Dox Medium
The results showed that addition of wheat bran favoured maximum L-asparaginase production compared to other tested inducers. Higher L-asparaginase yield of 12.45 U/mL was observed in wheat bran (Table 1). The influence of different concentration of wheat bran from 0.5 to 3.0 g/L by Fusarium sp. LCJ273 in MCDB was further evaluated.
Table 2: Experimental range and levels of the three independent variables used for production of L-asparaginase by Fusarium sp. LCJ273 using RSM in terms of actual and coded factors.
| Varibles | Actual | Coded | Actual | Coded | Actual | Coded |
| Dextrose (X1) | 2 | -1 | 3 | 0 | 4 | +1 |
| Ammonium sulphate (X2) | 15 | -1 | 20 | 0 | 25 | +1 |
| Wheat bran (X3) | 2 | -1 | 2.5 | 0 | 3 | +1 |
Maximum activity of 14.24 U/mL was observed at 2.5 g/L of wheat bran on 5th day of incubation (Fig. 4). L-Asparaginase activity decreased when the concentration of wheat bran was increased above 2.5 g/L. Minimum activity was observed in bagasse and paddy straw. The maximum specific activity of L-asparaginase in Fusarium sp. LCJ273 was found to be 67.02 U/mg in wheat bran. However, the medium contained 5 g/L of L-asparagine as a substrate.
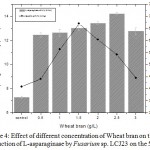 |
Figure 4: Effect of different concentration of Wheat bran on the production of L-asparaginase by Fusarium sp. LCJ23 on the 5th day.
|
Optimization of L-Asparaginase Production Using Response Surface Methodology
The influence of three parameters namely dextrose, ammonium sulphate and wheat bran was evaluated using response surface methodology. Both dextrose and ammonium sulphate were found to be highly significant (P-value = 0. 001) and also increased the L-asparaginase production. The interaction between wheat bran and dextrose, wheat bran and ammonium sulphate were significant as shown by the P-value (P<0.05). The results indicated that optimal concentration of A, B and C (dextrose, ammonium sulphate and wheat bran) were between 2 to 2.5 g/L.
The face centered central composite design used for studying the effects of three independent parameters namely dextrose, ammonium sulphate and wheat bran for L-asparaginase production are shown in Table 3 along with observed and predicted value. The regression equation coefficients were analysed by following equation:
Y= 102.53 – 2.18 X1 – 4.10 X2 – 0.5400 X3 – 23.81 X1X2
+ 33.21 X2X3 + 39.85 X1X3 – 0.0650 X12 – 2.54 X22 – 0.1089 X32
Table 3: Face Centered Central Composite Design for L-asparaginase production with observed and predicted values.
| Run | Dextrose | Ammonium sulphate | Wheat bran | L-Asparaginase activity (U/mL) Observed Predicted | |
| 1 | 0 | 0 | 0 | 10.95 | 10.37 |
| 2 | 0 | 0 | 0 | 6.73 | 7.51 |
| 3 | 1 | 1 | -1 | 12.05 | 12.20 |
| 4 | 0 | -2 | 0 | 11.69 | 11.00 |
| 5 | 0 | 0 | 2 | 11.82 | 12.28 |
| 6 | -1 | -1 | 1 | 10.77 | 10.89 |
| 7 | -1 | 1 | 1 | 11.53 | 11.36 |
| 8 | -1 | -1 | -1 | 10.25 | 11.79 |
| 9 | 1 | 1 | -1 | 11.46 | 12.01 |
| 10 | 0 | 0 | 0 | 10.88 | 11.24 |
| 11 | 1 | -1 | 1 | 10.02 | 11.06 |
| 12 | -1 | 1 | -1 | 9.68 | 10.69 |
| 13 | 2 | 0 | 0 | 11.73 | 10.88 |
| 14 | 0 | 0 | 0 | 6.82 | 7.51 |
| 15 | 0 | 0 | 0 | 7.01 | 7.51 |
| 16 | 1 | -1 | 1 | 9.9 | 9.56 |
| 17 | 0 | 0 | 0 | 10.55 | 7.51 |
| 18 | 1 | -1 | -1 | 10.77 | 10.50 |
| 19 | 1 | -1 | -1 | 11.21 | 12.31 |
| 20 | 0 | 0 | -2 | 13.24 | 12.55 |
| 21 | -1 | -1 | 1 | 12.54 | 11.95 |
| 22 | -1 | -1 | -1 | 12.04 | 11.05 |
| 23 | 0 | 2 | 0 | 12.53 | 12.99 |
| 24 | 0 | 0 | 0 | 6.96 | 7.51 |
| 25 | -2 | 0 | 0 | 11.14 | 11.76 |
| 26 | 1 | 1 | 1 | 11.36 | 11.91 |
| 27 | 1 | 1 | 1 | 12.64 | 11.77 |
| 28 | -1 | 1 | 1 | 14.48 | 14.05 |
| 29 | -1 | 1 | -1 | 14.54 | 13.06 |
| 30 | 0 | 0 | 0 | 7 | 7.51 |
Where Y, L-asparaginase production; X1, X2 and X3 the coded levels of dextrose, ammonium sulphate and wheat bran. The results analysed using Analysis of Variance (ANOVA) are shown in Table 4. In this analysis the R2 value was 0.81, which indicated 95% of the variability of the response for L-asparaginase production explained by the model. The coefficient of variance was 11.60. The adequate precision value was 7.6052. The Lack of Fit F-value of 4.72 suggests that the Lack of Fit is not significant. It identifies the model can be used to direct the design space for L-asparaginase production, ratio greater than 4.0 is desirable. The predicted sum of squares was 86.04 and this is used to measure the particular model.
Table 4: Analysis of variance (ANOVA) for the experiment.
| ANOVA | |
| Std. Dev | 1.25 |
| Mean | 10.74 |
| Coefficient of variance | 11.60 |
| R2 | 0.8149 |
| Adjusted R2 | 0.6421 |
| Predicted R2 | 0.3161 |
| Adeq Precision | 7.6052 |
| PRESS | 86.04 |
| F-Value | 4.72 |
Three-dimentional response surface graphs shown in Figures 5, 6, and 7 represents the response of L-asparaginase production, main effect, interaction effect and squared effect of three parameters such as dextrose, ammonium sulphate and wheat bran, which shows the best combination for production of L-asparaginase. Finally the highest yield of L-asparaginase was determined using dextrose (2 g/L), ammonium sulphate (25 g/L) and wheat bran (2 g/L). Maximum L-asparaginase activity 14.05 U/mL was predicted using this combination. The maximum enzyme activity reached in this experiment was 14.54 U/mL and also revealed that L-asparaginase activity decreased at the highest levels of these three factors. After statistical optimization, L-asparaginase yield increased to two times higher compare to original medium. Within the designed space, the model was validated for three parameters, predicted values were very close to observed value and the model was successfully verified.
 |
Figure 5: Three-dimensional response surface plots showing the interaction between dextrose and ammonium sulphate on the production of L-asparaginase by Fusarium sp. LCJ273. |
 |
Figure 6: Three-dimensional response surface plots showing the interaction between dextrose and wheat bran on the production of L-asparaginase by Fusarium sp. LCJ273. |
 |
Figure 7: Three-dimensional response surface plots showing the interaction between ammonium sulphate and wheat bran on the production of L-asparaginase by Fusarium sp. LCJ273. |
Original and Optimized Medium for L-Asparaginase Production in Modified Czapek Dox Medium
The L-asparaginase production by Fusarium sp. LCJ273 in original medium was 6.91 U/mL. After optimization of medium components, the L-asparaginase yield was 14.24 U/mL. Thus a 2 fold increase was possible through optimization.
Discussion
The anti-leukaemic activities of L-asparaginase have high medicinal value and also used for treating many diseases.22,23 In the present study, Fusarium sp. LCJ273 was isolated from Adhatoda vasica. It was screened for L-asparaginase activity and optimization studies were done for maximum L-asparaginase production. Medium optimization was done under submerged fermentation using nutritional parameters specifically dextrose, ammonium sulphate and wheat bran. Dextrose and ammonium sulphate play a major role in synthesis of essential nutrients and promote growth of the organism. For maximizing L-asparaginase production nitrogen source play a vital role. In both submerged and solid state fermentation L-asparaginase production was done.24,25,26 The formulation and optimization of medium is done for enhancement of microbial growth and L-asparaginase production in a large scale microbial fermentation processes.27 Fungi are the most potent producers for L-asparaginase, which has the significant value.28
In the present study, the dextrose and ammonium sulphate in the Czapex Dox medium were optimized for the L-asparaginase production by Fusarium sp. LCJ273. When dextrose is used as a carbon source, the production of L-asparaginase was 7.06 U/mL and the concentration of dextrose was further optimized in the range of 1 to 6 g/L. Dextrose at 3.0 g/L showed 9.18 U/mL of L-asparaginase activity using Czapek’s Dox broth. Similarly in earlier studies glucose has resulted in high production of L-asparaginase at the concentration of 3.0 g/L.29,30,31 Glycerol and lactose showed minimum production of L-asparaginase. Dextrose has been indicated as the best carbon source when compared to other sources and also it is economical.32
When ammonium sulphate is used as a nitrogen source, the production of L-asparaginase was maximum of 10.21 U/mL at the concentration of 20 g/L. The minimum activity of L-asparaginase was 2.46 U/mL observed when peptone was used as a nitrogen source. The various natural inducers such as paddy straw, coconut oil cake, bagasse, groundnut oil cake and wheat bran were selected and screened for L-asparaginase from Fusarium sp. LCJ273. The maximum yield of 13.29 U/mL was observed in the presence of wheat bran. L-Asparaginase production by Fusarium sp. LCJ273 has been studied using different nitrogen sources such as ammonium sulphate, ammonium nitrate, yeast extract and peptone.33,34 Previous work also strongly suggested that wheat bran acts as a best substrate for the production of L-asparaginase.35 L-asparaginase production was further optimized by using different concentration of wheat bran 0.5 to 3 g/L, 14.24 U/mL was observed at 2.5 g/L of wheat bran. Paddy straw and bagasse showed minimum activity of L-asparaginase.
Highest yield of L-asparaginase was observed by optimum combination of dextrose (3.0 g/L), ammonium sulphate (20 g/L) and wheat bran (2.5 g/L). L-Asparaginase production increases 2 fold after optimization process compared to un-optimized condition. The three variables such as dextrose, ammonium sulphate and wheat bran were selected and optimal level were identified using Response Surface Methodology (RSM).36,37 RSM is used to investigate the importance of independent variables at different level. Surface plots used to observe the significant interaction between three variables such as dextrose, ammonium sulphate and natural inducers, among them dextrose and ammonium sulphate acts as a best substrate.
In statistical optimization using Response Surface Methodology the yield of L-asparaginase was 14.54 U/mL, the medium composition was as follows dextrose 2 g/L, ammonium sulphate 25 g/L and wheat bran 2 g/L. There have been a number of studies conducted on statistical optimization using different carbon and nitrogen sources for the production of L-asparaginase.38,39,40 In the present study, Fusarium sp. LCJ273 produces maximum L-asparaginase production using dextrose, ammonium sulphate, wheat bran and L-asparagine as substrate.
In conclusion, Fusarium sp. LCJ273 was isolated from the medicinal plant Adhatoda vasica showed maximum production of L-asparaginase using an optimized medium containing dextrose, ammonium sulphate along with natural inducer wheat bran. In this study, L-asparaginase production was two times higher compared to the original medium. L-Asparaginase production from microbes is much cheaper compared to others and also enzyme has more importance in pharmaceutical industries. The study concentrates on the selection of different parameters such as carbon, nitrogen and natural inducer, would influence cost-effective production of L-asparaginase. However, further studies on purification of Fusarium sp. LCJ273 will help to extent its application in various industries.
Conflict of Interest
There is no conflict of interest.
Acknowledgements
The authors are thankful to the management, Loyola College, Chennai for providing necessary facilities and encouragement.
References
- Moses J. R., Leelavathy R., Vanapalli V. S. V., Thirumurugan G., Rajaram M. S. Effect of inducers and physical parameters on the production of L-asparaginase using Aspergillus terreus. Journal of Bioprocessing and Biotechniques. 2011;1:1-6.
- Jenila V. A and Gnanadoss J. J. Characterization of extracellular enzyme producing endophytic fungi from medicinal plants with reference to L-asparaginase. International Journal of Scientific Research and Review. 2018;7(8):425-446.
- Monica T., Lincoln L., Niyonzima F. N., Sunil S. M. Isolation, Purification and Characterization of Fungal Extracellular L-Asparaginase from Mucor hiemalis. Journal of Biocatalysis and Biotransformation. 2013;9:12-14.
- Mangamuri U., Vijayalakshmi M., Ganduri V. S. R. K., Rajulapati S. B., Poda S. Extracellular L-Asparaginase from Streptomyces labedae VSM-6: Isolation, Production and Optimization of Culture Conditions Using RSM. Pharmacognosy Journal. 2017;9(6).
- Kamble V. P., Rao R. S., Borkar P. S. Purification of L-asparaginase from a bacteria Erwinia carotovora and effect of a dihydropyrimidine derivative on some of its kinetic parameters. Indian Journal of Biochemistry and Biophysics. 2006;43:391-394.
- Zalewska-Szewczyk B., Gach A., Wyka K., Bodalski J., Młynarski W. The cross-reactivity of anti-asparaginase antibodies against different L-asparaginase preparations. Clinical and Experimental Medicine. 2009;9(2):113-6.
CrossRef - Pieters R., Hunger S. P., Boos J., Rizzari C., Silverman L., Baruchel A., Goekbuget N., Schrappe M., Pui C. H. L‐asparaginase treatment in acute lymphoblastic leukemia: a focus on. Erwinia. Cancer. 2011;117(2):238-249.
CrossRef - Stecher A. L., De Deus P. M., Polikarpov I., Abrahao-Neto J. Stability of L-asparaginase an enzyme used in leukemia treatment. Pharmaceutica Acta Helvetiae. 1999;74(1):1-9.
CrossRef - Aparna C., Raju J. Optimization of process parameters for L-asparaginase production by Aspergillus terreus MTCC1782 under solid state fermentation using mixed substrate. International Journal of Research in Engineering and Technology. 2015;4(5):354-360.
CrossRef - Ravindranath Y., Abella E., Krischer J. P., Wiley J., Inoue S., Harris M., Chauvenet A., Alvarado C. S., Dubowy R., Ritchey A. K. Acute myeloid leukemia (AML) in Down’s syndrome is highly responsive to chemotherapy: experience on Pediatric Oncology Group AML Study 8498. Blood. 1992;80(9):2210-2214.
CrossRef - Elshafei A. M., Hassan M. M., Abd M., Abouzeid E., Mahmoud D. A., Elghonemy D. H. Purification, characterization and antitumor activity of L-asparaginase from Penicillium brevicompactum 2 NRC 829 3. Methodology. 2011.
- Sarquis M. I. D. M., Oliveira E. M. M., Santos A. S., Costa G. L. D. Production of
L-asparaginase by filamentous fungi. Memorias do Instituto Oswaldo Cruz. 2004;99(5):489-492.
CrossRef - Venil C. K., Nanthakumar K., Karthikeyan K., Lakshmanaperumalsamy P. Production of l-asparaginase by Serratia marcescens SB08: optimization by response surface methodology. Iranian Journal of Biotechnology. 2009;7(1):10-18.
- Kenari S. L. D., Alemzadeh I., Maghsodi V. Production of l-asparaginase from Escherichia coli ATCC 11303: Optimization by response surface methodology. Food and Bioproducts Processing. 2011;89(4):315-321.
CrossRef - Thenmozhi C., Sankar R., Karuppiah V., Sampathkumar P. L-asparaginase production by mangrove derived Bacillus cereus MAB5: optimization by response surface methodology. Asian Pacific Journal of Tropical Medicine. 2011;4(6):486-491.
CrossRef - Fouda A. H., Hassan S. E. D., Eid A. M., Ewais E. E. D. Biotechnological applications of fungal endophytes associated with medicinal plant Asclepias sinaica (Bioss.). Annals of Agricultural Sciences. 2015;60(1):95-104.
CrossRef - Moorthy V., Ramalingam A., Sumantha A., Shankaranaya R. T. Production, purification and characterisation of extracellular L-asparaginase from a soil isolate of Bacillus. African Journal of Microbiology Research. 2010;4(18):1862-1867.
- Waksman S. A. The Actinomycetes: Classification, identification and descriptions of genera and species. The Williams and Wilkins Company. 1961;2:61-292.
- El-Naggar N. E. A., Moawad H., El-Shweihy N. M., El-Ewasy S. M. Optimization of culture conditions for production of the anti-leukemic glutaminase free L-asparaginase by newly isolated Streptomyces olivaceus NEAE-119 using response surface methodology. Bio.Med Research International. 2015;2015.
- Imada A., Igarasi S., Nakahama K., Isono M. Asparaginase and glutaminase activities of micro-organisms. Microbiology. 1973;76(1):85-99.
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193(1):265-275.
- Ronghe M., Burke G. A. A., Lowis S. P., Estlin E. J. Remission induction therapy for childhood acute lymphoblastic leukaemia: clinical and cellular pharmacology of vincristine, corticosteroids, L-asparaginase and anthracyclines. Cancer Treatment Reviews. 2001;27(6):327-337.
CrossRef - Basha N. S., Rekha R., Komala M., Ruby S. Production of extracellular anti-leukaemic enzyme lasparaginase from marine actinomycetes by solidstate and submerged fermentation: Purification and characterisation. Tropical Journal of Pharmaceutical Research. 2009;8(4):353-360.
CrossRef - Pandey A., Soccol C. R., Mitchell D. New developments in solid state fermentation: I-bioprocesses and products. Process Biochemistry.2000;35(10):1153-1169.
CrossRef - Mahajan R.V., Saran S., Kameswaran K., Kumar V., Saxena R.K. Efficient production of L-asparaginase from Bacillus licheniformis with low-glutaminase activity: optimization, scale up and acrylamide degradation studies. Bioresource Technology. 2012;125:11-16.
CrossRef - Prakasham R. S., Rao C. S., Rao R. S., Lakshmi G. S., Sarma P. N. l‐asparaginase production by isolated Staphylococcus–6A: design of experiment considering interaction effect for process parameter optimization. Journal of Applied Microbiology. 2007;102(5):1382-1391.
CrossRef - Murali T. S. L-asparaginase from marine derived fungal endophytes of seaweeds. Mycosphere. 2011:147-155.
- Lapmak K., Lumyong S., Thongkuntha S., Wongputtisin P., Sardsud U. L-asparaginase production by Bipolaris BR438 isolated from brown rice in Thailand. Chiang Mai Journal of Science. 2010;37:160-164.
- Pallem C., Nagarjun V., Srikanth M. Production of a tumour inhibitory enzyme, L-asparaginase through solid state fermentation using Fusarium oxysporum. International Journal of Pharmaceutical Sciences Review and Research. 2011;7(2):189-192.
- Hosamani R., Kaliwal B. B. L-asparaginase an anti-tumor agent production by Fusarium equiseti using solid state fermentation. International Journal of Drug Discovery. 2011;3(2):88-99.
CrossRef - Kumar S., Dasu V. V., Pakshirajan K. Localization and production of novel L-asparaginase from Pectobacterium carotovorum MTCC 1428. Process Biochemistry. 2010;45(2):223-229.
CrossRef - Sreenivasulu V., Jayaveera K. N., Rao P. M. Optimization of process parameters for the production of L-asparaginase from an isolated fungus. Research Journal of Pharmacognosy and Phytochemistry. 2009;1(1):30-34.
- Ahmed M. A., Dahab N. A., Taha T., Hassan S. M. J. Production, purification and characterization of L-Asparaginase from marine endophytic Aspergillus ALAA-2000 under submerged and solid state fermentation. Journal of Microbial and Biochemical Technology. 2016;7(3):165-172.
- Kalyanasundaram I., Nagamuthu J., Srinivasan B., Pachayappan A., Muthukumarasamy S. Production, purification and characterisation of extracellular L-asparaginase from salt marsh fungal endophytes. World Journal of Pharmacy and Pharmaceutical Sciences. 2015;4(3):663-677.
- Kumar N. S. M., Ramasamy R., Manonmani H. K. Production and optimization of l-asparaginase from Cladosporium using agricultural residues in solid state fermentation. Industrial Crops and Products. 2013;43:150-158.
CrossRef - Sunitha M., Ellaiah P., Devi R. B. Screening and optimization of nutrients for L-asparaginase production by Bacillus cereus MNTG-7 in SmF by plackett-burmann design. African Journal of Microbiology Research. 2010;4(4):297-303.
- Farag A. M., Hassan S. W., Beltagy E. A., El-Shenawy M. A. Optimization of production of anti-tumor l-asparaginase by free and immobilized marine Aspergillus terreus. The Egyptian Journal of Aquatic Research. 2015;41(4):295-302.
CrossRef - Badoei-Dalfard A. Purification and characterization of l-asparaginase from Pseudomonas aeruginosa strain SN004: Production optimization by statistical methods. Biocatalysis and Agricultural Biotechnology. 2015;4(3):388-397.
CrossRef - Hymavathi M., Sathish T., Rao C. S., Prakasham R. S. Enhancement of L-asparaginase production by isolated Bacillus circulans (MTCC 8574) using response surface methodology. Applied Biochemistry and Biotechnology. 2009;159(1):191-198.
CrossRef - Erva R. R., Goswami A. N., Suman P., Vedanabhatla R., Rajulapati S. B. Optimization of L-asparaginase production from novel Enterobacter sp. by submerged fermentation using response surface methodology. Preparative Biochemistry and Biotechnology. 2017;47(3):219-228.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





