Manuscript accepted on : 28-Mar-2019
Published online on: 29-03-2019
Plagiarism Check: Yes
Layin Muthoharoh1, Hanik Faizah2, Popy Hartatie Hardjo3, Alfinda Novi Kristanti4 and Yosephine Sri Wulan Manuhara*1
1Department of Biology, Airlangga University, Surabaya 60115, Indonesia.
2Department of Biology, UIN Sunan Ampel, Surabaya, Indonesia.
3Faculty of Biotechnology, Universitas Surabaya, Indonesia.
4Department of Chemistry, Airlangga University, Surabaya 60115, Indonesia.
Corresponding Author E-mail: wulanmanuhara@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2729
ABSTRACT: Gynura procumbens (Lour) Merr is a medicinal plant that has been widely used as an ingredient of herbal medicine. G. procumbens is known to contains secondary metabolite compounds namely flavonoids. The aim of this study was to investigate the effect of carbon sources on biomass and flavonoid content of G. procumbens adventitious root culture grown in agitated liquid medium. Adventitious roots were induced from leaf explants on Murashige and Skoog (MS) medium supplemented with 3% sucrose and 8 g/Lagar. G. procumbens adventitious root cultures were performed in MS liquid medium containing different carbon source of 3% sucrose, 5% sucrose, 3% glucose and 4% fructose, and supplemented with5 mg/L IBA. Two grams of adventitious roots as the initial inoculum were cultured in1000 mL Erlenmeyer flask containing 250 mL MS medium and agitated at 70 rpm in dark conditions for 28 days.The results showed that medium with the addition of 5% sucrose produced the highest fresh weight (10.23 ± 0.86 g) followed by 3% sucrose (8.39 ± 0.60 g), while the addition of 3% glucose (3.35 ± 0.11 g) and 4% fructose (3.68 ± 1.13 g) produced adventitious root biomass lower compared to the addition of sucrose.The production of G. procumbens adventitious roots biomass increased 5-fold of the initial inoculum. The highest flavonoid content was obtained in MS medium supplemented with 3% glucose with the content of kaempferol and quercetin reached 25.44g/L per 1gdry weight and 7.08 g/L per gdry weight, respectively.
KEYWORDS: Adventitious Root; Biomass; Carbon Source; Gynura Procumbens; Metabolite; Secondary Flavonoid
Download this article as:| Copy the following to cite this article: Muthoharoh L, Faizah H, Hardjo P. H, Kristanti A. N, Manuhara Y. S. W. Effect of Carbon Source on Biomass and Flavonoid Content of Gynuraprocumbens (Lour.) Merr Adventitious Root in Liquid Culture. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Muthoharoh L, Faizah H, Hardjo P. H, Kristanti A. N, Manuhara Y. S. W. Effect of Carbon Source on Biomass and Flavonoid Content of Gynuraprocumbens (Lour.) Merr Adventitious Root in Liquid Culture. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2uLguSh |
Introduction
Gynura procumbens (Lour.)Merr is a medicinal plant that has been widely used as an ingredient of herbal medicine in Southeast Asia such as Indonesia, Malaysia and Thailand.1 G. procumbens leaves have a sweet and savorless in taste. G. procumbens has been known contain secondary metabolite compounds flavonoids.2 Flavonoids in plants haverolesas protection of UV-B radiation and against pathogen attack3; for human health, this compound acts as an anti-allergy, antihypertensive, antiviral, antitumor, antioxidant, anti-inflammatory effects,4 anticancer,5 antibacterial6 and anticarcinogenic properties.7
The current demand secondary metabolites for pharmaceutical, healthy food and cosmetic products is increasing.8 Many plant secondary metabolites accumulate in the roots. However, conventional root harvesting for secondary metabolite compounds will destroy the whole plant, so it is necessary to develop tissue culture method to produce secondary metabolite compounds.9
Tissue culture technique as an alternative method has a better prospect than conventional propagation methods. Therefore, in this study used tissue culture method using adventitious roots. Liquid medium has been widely used for cell cultivation,10 somatic embryos11 and plant organs.12,13 Tissue culture technique uses a liquid medium to enhance biomass and secondary metabolite content in adventitious roots, such as in Astragalus membranaceus,14 Hypericum perforatum8 and Oplopana xelatus.15
Cultures in the liquid medium are better than in the solid medium because explants continuously submerged provide an opportunity for hormones and nutrients to be efficiently absorbed and utilized for the growth of explant. Moreover, the liquid medium provides better homogeneity compared to the solid medium.16 G. procumbens adventitious root culture has been developed in liquid culture in an agitated Erlenmeyer flask. The biomass of adventitious roots increased at50 g/Lsucrose concentration reached 7.8 g and the content of quercetin and kaempferol were 165mg/L per g dry weight and 733 mg/L per g dry weight, respectively.17 However, the addition of carbon sources in the form of glucose and fructose in G. procumbens adventitious roots culture has never been studied.
Sugar is a carbon source that supply energy for tissue growth, organ induction and differentiation. Kumar et al18 reported the effect of carbon source (sugar types) on the control of phenolic secretion on Gossypium hirsutum L. callus culture and it was known that 3% maltose and 3% glucose were the most effective carbon sources. The study of Gauchan et al19 showed that the maximum growth of root and shoot length were obtained on the medium supplemented with 1% dextrose. This study was conducted to determine the effect of carbon sources (sucrose, glucose, and fructose) on biomass and flavonoid content of G. procumbens adventitious roots in liquid culture.
Materials and Methods
Material
Gynura procumbens (Lour.) Merr was obtained from Purwodadi Botanical Gardens, Pasuruan, East Java, Indonesia.
Adventitious Roots Induction
G. procumbens leaves were washed using detergent and rinsed with tap water, then sterilized with 10% clorox (v/v) (Bayclin, Johnson, Indonesia) for 5 minutes and rinsed 3 times with sterile distilled water. The leaves were drained on filter paper and cut to an area of 1 cm2. Explants were cultured on MS medium20 supplemented with 3% sucrose and 8 g/L agar.Cultures were maintained for 28 days at 25°C ± 3°C in dark conditions. After 28 days, the adventitious roots were harvested and separated from leaves explants then washed with sterile distilled water to remove residual agar.
Cultivation of Adventitious Roots in Liquid Medium
The MS medium was supplemented with the plant growth regulator of 5 mg/L Indol Butyric Acid (IBA) and different carbon sources 3% sucrose, 3% glucose and 4%fructose. The pHmedium was adjusted to 5.8. Medium was sterilized by autoclave for 15 minutes at 1 atm and 121°C. Adventitious roots were inoculated in 1000 mL erlenmeyer flask containing 250 mL medium and incubated in a rotary shaker (Lab Tech) at 70 rpm with temperature 25 ± 2°C in the dark for 28 days.
Fresh and Dry Weight Measurements
The adventitious roots of each treatment were weighed using an analytical balance to determine the fresh weight. Dry weight was obtained by dryingthe roots using an oven (Memo INB200) at 50°C for 72 hours and weighed using an analytical balance.
Extraction and Flavonoids Analysis
Dried adventitious roots were crushed with mortar into powder. A 0.1 g of adventitious roots powder was soaked with 10 mL of methanol at room temperature for 24 hours.This procedure was done twice. The extract was filtered and then concentrated at room temperature. The methanol extract was partitioned using 1: 1 n-hexane to remove non-polar compounds, then 0.25 mL of methanol extract was added with 1.25 mL of distilled water and 75 μL of of 5% sodium nitrate solution, and dissolved for 6 min. Then, 0.5 mL of 1 M NaOH and distilled water were added to the solution until it reached a volume of 2.5 mL. The extract was measured the absorbance values using a UV spectrophotometer at a wavelength of 510 nm (BOECO S-22, Germany). Flavonoid content was obtained by calculating absorbance values using a linear regression equation based on the standard of quercetin and kaempferol. Result and Discussion
Effect of Carbon Sources on Adventitious Roots Biomass
Among the types of sugars that used as carbon sources, 5% sucrose showed the highest biomass followed by 3% sucrose reached 5 and 4 times of the initial weight of the inoculums, respectively, while 3% glucose and 4% fructose produced lower biomass (Figure 1). Maximum fresh weight (10.23 ± 0.86 g) and maximum dryweight (0.55 ± 0.02 g) were obtained at 5% sucrose treatment. Adventitious root culture with the addition of sucrose and glucose produced brownish yellow roots, while the addition of fructose produced brown roots (Figure 2).
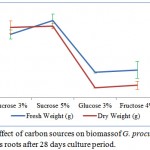 |
Figure 1: Effect of carbon sources on biomassof G. procumbens adventitious roots after 28 days culture period.
|
 |
Figure 2: Adventitious root culture of G. procumbens in liquid medium for 28 days, a) G. procumbens, (b) adventitious root induction in MS solid medium, (c) adventitious root culture in MS + 5 mgL-1IBA supplemented with 3% sucrose, (d) 5% sucrose, (e) 3% glucose, (f) fructose 4%, (g-j) morphology of adventitious roots in each treatment.
|
Carbohydrates are an important source of carbon and energy in plant cell and organ cultures. In vitro planlets require carbon sources from culture medium for biological processes such as for survival, growth, development, and bioactive compounds accumulation. The growth rate of biomass is directly correlated with the consumption of sugar. Sucrose is the most common source of carbon in the tissue culture medium, since it is the main sugar trans located in the phloem of many plants. However, the other carbohydrates such as glucose, fructose and monosaccharide mixtures are also used to improve embryogenic or organogenic processes.21
The percentage of fresh weight and dry weight increased with increasing concentration of sucrose,it had similar result with the study of Manuhara et al22 which also produced the highest biomass in the treatment of 5% sucrose in agitated liquid culture. Sucrose is an essential substrate for carbon source and metabolism energy and polymer biosynthesis at in vitro culture technology.23 Murthy and Praveen24 also reported that the use of the carbon source of sucrose in Withania somnifera (L.) Dunal showed an increase in the accumulation of adventitious roots fresh and dry weights followed by increasing production of secondary metabolite of withanolide that had the highest production compared to other carbon sources such as glucose, fructose, and maltose. Sucrose is a type of disaccharide sugar. In tissue culture technique, explants excrete invertase enzyme that can convert sucrose to glucose and fructose which are the reducing sugar.
Effect of Carbon Sources on Flavonoid Content of Adventitious Roots
The highest production of flavonoid compounds was achieved in the addition of 3% glucose with the content of kaempferol and quercetin reached 25.44 g/L per g dry weight and 7.08g/L per gdry weight, respectively, followed by the production of flavonoid compounds in the addition of 5% sucrosewith the content of kaempferol and quercetin reached 23.33g/L per g dry weight and 6.45g/L per g dry weight, respectively.Production of flavonoid compounds in the addition of 4% fructose resulted in kaempferol of 18.66 g/L perg dry weight and quercetin of 5.05 g/L per g dry weight, whereas the lowest flavonoid content found in the adventitious roots in medium with the addition of 3% sucrose that produced kaempferol of 15.44 g/L per g dry weight and quercetin 4.08g/L per g dry weight (Fig. 3).
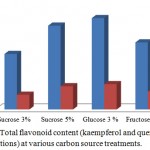 |
Figure 3: Total flavonoid content (kaempferol and quercetin concentrations) at various carbon source treatments.
|
In this study, the highest flavonoid content was not obtained from the treatment of carbon sources with the highest fresh weight and dry weight, but the highest flavonoid content was shown in the sample with the lowest fresh and dry weights found in the treatment of 3% glucose.The result was contrary with the study of Jung et al.25 that the addition of fructose produced catharanthine compounds of Catharanthusroseus2-fold than the treatment of glucose.A strategy to increase biomass production using two culture stages for the optimization of culture yield can be applied to G.procumbens according to Jung et al. (1992) strategy, in the first stage, the adventitious roots are cultured in MS medium with the addition of carbon source that produces maximum biomass (5% sucrose) then in the next stage, the adventitious roots are sub-cultured into medium with 3% glucose (for optimization the production of flavonoid). In this study, the second highest flavonoid content was obtained in the treatment of 5% sucrose followed by acquiring high fresh and dry weights. Sivakumar et al.26 reported that there was an increase in reducing sugar content, total sugar, and starch in hairy roots of Panax ginseng along with increasing the concentration of sucrose in the culture medium. Sucrose and glucose can affect directly(such as signalling molecules) or indirectly on various metabolic pathways.27 The study conducted by Morkunas et al.28 indicated that sucrose induces the production of phenylalanine ammonia-lyase (PAL), chalcone synthase (CHS), chalcone isomerise (CHI) and isoflavone synthase (IFS) that catalyze flavonoid biosynthesis on the shikimat pathway.
The pH value of the medium at each treatment was measured before sterilization, after sterilization, and at the end of cultivation. The pH value of MS liquid medium before sterilization was adjusted to 5.8 in all treatments. After sterilization, the pH of medium in all treatments decreased, the most drastic decrease was found in the treatment of 4% fructose (4.2), whereas in the other treatment, the pH value of the medium after sterilization was between 5.0-5.3. At the end of cultivation (28 days culture period) the pH value of the medium decreased slightly, except for the 4% fructose treatment in which the pH value increased from 4.2 to 5.0. This is perhaps due to the absorption of nitrate in certain amount which will change the pH of the medium becomes more alkaline at the end of culture.
In addition to the types and concentrations of carbon sources, the pH value of the medium may also affect the production of fresh weight, dry weight, and secondary metabolite accumulation of adventitious root culture. In this study, the pH value of liquid medium before sterilization was adjusted at 5.8 in all treatments. It was an attempt to provide the same conditions for explants to grow; good medium pH for root growth ranged from 5.0 to 6.3, since the range of pH values is available for nutrients plant.29 A similar result was also reported by Saiman et al.30 that the best production of fresh weight of G. procumbens adventitious roots was obtained in the initial pH of medium of 5.5.
After the sterilization process, there was a decrease in pH value in all medium. This condition may occur due to the presence of sucrose in MS medium. The pH of MS medium supplemented with sucrose will decrease after sterilization process.In addition, it can occur due to the lack of solidifying material (agar) in the medium, since solidifying material can serve to stabilize and raise the pH of the medium.29 In this study, the drastically decreased of pH value after sterilisation was found in the treatment of 4 % fructose with medium pH value of 4.2 which resulted in the lowest production of fresh and dry weightabout 3.68 ± 1.13 g and 0.095 ± 0.03 g,respectively. The minimum production of fresh and dry weights in medium with low pH value was also obtained in Withania somnifera (L.), that the lower the pH of the medium, the lower production of fresh and dry weights. The optimum fresh weight and dry weight was at pH of 5.8 and will be decrease when pH value was too alkaline (above 6).24
Conclusion
Production of Gynura procumbens (Lour) Merr adventitious roots biomass in agitated Erlenmeyer flask increased 5-fold of the initial inoculum inthe addition of 5% sucrose as carbon source. While the highest secondary metabolite production obtained from the addition of 3% glucose.
Acknowledgments
This study was supported by Mandatory Research of Airlangga University (grant No. 1922/UN3.1.8/LT/2018).
References
- Kaewseejan N., Puanpronpitag D and Nakornriab M. Evaluation of phytochemical composition and antibacterial. property of Gynura procumbens Asian Journal of Plant Sciences. 2012;11(2):77-82.
CrossRef - Akowuah G. A., Sadikun A and Mariam A. Flavonoid identification and hypoglycaemic studies of butanol fraction form Gynuraprocumbens. Pharmaceutical Biology. 2002;40(6):405-410.
CrossRef - Par J. M and Bolwell G. P. Phenols in plant and in man, the potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. Journal of the Science and Agriculture. 2000;80(7):985-1012.
CrossRef - Jin J. H., Lim H., Kwon S. Y., Son K. H and Kim H. P. Anti-inflamatory activity of the total flavonoid fraction from Broussonetiapapyriferain combination with Lonicera japonica. Biomolecules and Therapeutics. 2010;18(2):197-204.
CrossRef - Cibin T. R., Devi D. G and Abraham A. Chemoprevention of skin cancer by the flavonoid fraction of Sacaranasoka. Phytotherapy Research. 2010;24(5):666-672.
- Naeem I., Saddiqie Z., Pateland Hello C. Analysis of flavonoid and antimicrobial activity of extracts of Hypericum perforatum. Asian Journal of Chemistry. 2010;55(8):443-446.
- Merken H. M., Casandra D. MandGary R. B. Kinetics method for the quantitation of anthocyanidins, flavonols, and flavons in foods.Journal of Agricultural and Food Chemistry. 2001;49:2727-2732.
CrossRef - Cui X. H., Chakrabarty D., Lee E. J and Paek K. Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresource Technology. 2010;101:4708–4716.
CrossRef - Kusuma D. Y., Kristanti A. N and Manuhara Y. S. W. Effect of sucrose and immersion frequency on production of adventitious roots and secondary metabolites of Gynuraprocumbens (Lour.) Merr in temporary immersion bioreactors. Asian Journal of Plant Sciences. 2017;16(1):24-36.
CrossRef - Ahmadian M., Babaci A., Shokri S and Hessami S. Micropropagation of carnation (Dianthus caryophyllus L.) in liquid medium by temporary immersion bioreactor in comparison with solid culture. Journal of Genetic Engineering and Biotechnology. 2017;15:309–315.
CrossRef - Mujib A., Ali M., Isah T and Dipti. Somatic embryo mediated mass production of Catharanthusroseus in culture vessel (bioreactor) – a comparative study. Saudi Journal of Biological Sciences. 2014;21:442-449.
CrossRef - Zhang Z., Yu Z., Jin Z., Liu J and Li Y. Liquid Culture of Adeventitious Roots is a potential alternative to field cultivation for Psammosilenet unicoides, a rare and endangered endemic medicinal plant. Advance Journal of Food Science and Technology. 2013;5(2):127-131.
CrossRef - Jose B., Silja P. K., Dhanya B and kumar K. S. In vitro cultivation of hairy roots of Plumbagorosea L. in a customized reaction kettle for the production of plumbagin – An anticancer compound. Industrial corps and products. 2016;87:89-85.
CrossRef - Wu S. Q., Lian M. L., Gao R., Park S. Y., Piao X. C. Bioreactor application on adventitious root culture of Astragalus membranaceus. In Vitro Cell Development Biology Plant. 2011;47:719–724.
CrossRef - Jiang Y. J., Piao X. C., Liu J. S., Jiang J., Lian X., Kim M. J and Lian M. I. Bioactive compound production by adventitious root culture of Oplopana xelatus in balloon-type airlift bioreactor systems and bioactivity property.Plant Cell Tissue Organ Culture. 2015;123(2):423-425.
CrossRef - Mariateresa C., Suarez C., Maria C and Giuseppe C. Influence of ozone treatments on in vitro propagation of Aloe barbandensis in continueus immersion bioreactor. Industrial Crops and Products. 2014;55(3):194-201.
CrossRef - Noviyanti R., Sari L. K. S., Kristanti A. N., Yachya R and Manuhara Y. S. W. Biomass and flavonoid production of Gynura procumbens adventitious roots induced by sucrose, phenylalanine and tyrosine. Bioscience Research. 2017;14(4):934-941.
- Kumar G. P., Sivakumar S., Siva G., Vinoth S., Vigneswaran M., Kanakachari M., Senthil K. T and Jayabalan N. Evaluation of different carbon sources for high frequency callus culture with reduced phenolic secretion in cotton (Gossypium hirsutum L.) cv. SVPR-2. Biotechnology Reports. 2015;7:72–80.
CrossRef - Gauchan D. P. Effect of different sugars on shoot regeneration of maize (Zea mays L.).Kathmandu University Journal of Science, Engineering and Technology. 2012;8(1):119-124.
- Murashige T and Skoog F. Revised medium for rapid growth and bioassays in tobacco tissue culture. Physiology Plant. 1962;15:473-493.
CrossRef - Last D. I and Brettell R. I. S. Embryo yield in wheat anther culture is influence by the choice of sugar in the culture medium. Plant Cell Reproduction. 1990;9:14-16.
CrossRef - Manuhara Y. S. W., Kusuma D. Y., Sari R. L and Kristanti A. N. K. Biomass Production of Gynura procumbens Adventitious Roots in Different Type of Liquid Culture. Biosaintifika: Journal of Biology & Biology Education. 2017;9(3):523-529.
CrossRef - Paek K. Y., Murthy H. N and Zhong J. J. Production of Biomass and Bioactive Compounds Using Bioreactor Technology. 2014. Springer.
CrossRef - Murthy H. N and Praveen N. Carbon sources and medium pH affects the growth of Withania somnifera (L.) Dunal adventitious roots and withanolide A production. Natural Product Research: Formerly Natural Product Letters. 2013;27(2):185–189.
CrossRef - Jung K. H., Kwak S. S., Kim S. W., Lee H., Choi C. Y and Liu J. R. Improvement of the catharanthine productivity in hairy root cultures of Catharanthus rosesus by using monosaccharides as a carbon source. Biotechnology Letters. 1992;14:695-700.
CrossRef - Sivakumar G., Yu K. W., Hahn E. J and Paek K. Y. Optimization of organic nutrients for ginseng hairy roots production in largescale bioreactors. Current Science India. 2005;89:641–649.
- Krapp A., Hofmann B., Schafer C and Stitt M. Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the ‘sink regulation’ of photosynthesis. Plant Journal. 1993;3:817–828.
CrossRef - Morkunas I., Formela M., Floryszak-Wieczorek J., Marczak L., Narozma D., Nowak W and Bednarski W. Cross-talk interactions of exogenous nitric oxide and sucrose modulates phenylpropanoid metabolism in yellow lupine embryo axes infected with Fusarium oxysporum. Plant Science. 2013;211:102-121.
CrossRef - Thorpe T., Stasolla C., Yeung E. C., de Klerk G. J., Roberts A and George E. F. The Components of plant tissue culture media II: Organik additions, and pH effects and Support systems. In George E. F., Hall M. A and de J. ed. Plant propagation by tissue cultue. 2008;1:115-173. 3rd Edition, Netherlands.
- Saiman M. Z., Mustafa N. R., Schulte A. E., Verpoorte R and Choi Y. H. Induction, characterization, and NMR-based metabolic profiling of adventitious root cultures from leaf explant of Gynuraprocumbens. Plant Cell Tissue Organ Culture. 2012;109(3):465-475.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





