Manuscript accepted on : 28-Mar-19
Published online on: 29-03-2019
Plagiarism Check: Yes
Mansee Kapil Thakur*1, Smital Sameer Kulkarni1, Nimain Mohanty2, Nitin N. Kadam2 and Niharika S. Swain3
1Department of Medical Biotechnology, MGMSBS, MGMIHS, Navi Mumbai, India.
2Department of Pediatrics, MGM Medical College, MGMIHS, India.
3Department of Oral Pathology, MGM Dental College, MGMIHS, India.
Corresponding Author E-mail: mansibiotech79@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2722
ABSTRACT: Many research centres have developed various animal models with Iron Deficiency Anaemia (IDA) by using iron deficient feeds as well as different chemicals. Model for iron deficiency anaemia (IDA) in rats has been created by the use of iron elimination from diet components as much as possible. The present study elaborates and concludes the development of IDA rat model by investigating different parameters like body weight, haematological indices, peripheral smear, immunoassay studies and histopathological studies using commercially available iron deficient diet. 12 Wistar albino female rats weighing 180-200 gm were selected with normal haemoglobin range of 12 - 15 g/dL purchased from Bombay Veterinary College, Parel and divided into two groups – Control (3 no. of rats) and Test (9 no. of rats). The test group was fed with iron deficient diet (VRK Nutritional Solutions) whereas control group was fed with standard diet. The time duration of the study was 5 weeks (35 days) and 6 weeks (42 days). Retro orbital blood for both control and treated was drawn at both time intervals so as to analyse haematological and immunoassay studies. Peripheral smear staining was carried out to observe the gross morphology of RBCs for iron deficient and control rats. The body weights were recorded before and after treatment and statistical significance was calculated. Post exposure rats were dissected and organs like heart, kidney, liver, lungs and spleen were collected for histopathological analysis. Our results showed decreased levels of hemoglobin (Hb), hematocrit (HCT), mean corpuscular hemoglobin (MCH), mean corpuscular volume (MCV), reticulocyte count, serum iron (SI), serum ferritin (SF) and an increase in total iron binding capacity (TIBC). in IDA animals exposed to 42 days of iron deficient diet. Significant difference (p<0.5) was observed in body weights of rats when compared with the data before and after treatment. The peripheral smear has indicated microcytic hypochromic RBCs in test group confirming development of IDA model. The histopathological results revealed the abnormality at cellular level like congestion of blood vessels in heart, congestion and centrilobular hepatocyte with inflammatory cell infiltration in liver, perivasculitis in lungs and decrease in white pulp in spleen whereas kidney were found normal. Our results clearly demonstrate iron deficient rat model when administered with IDA feed. This model can be used for estimation of efficiency of new food products and food supplements enriched with iron.
KEYWORDS: Hematology; Iron Deficiency Anaemia (IDA); Microcytic; Wistar Rats
Download this article as:| Copy the following to cite this article: Thakur M. K, Kulkarni S. S, Mohanty N, Kadam N. N, Swain N. S. Standardization and Development of Rat Model with Iron Deficiency Anaemia Utilising Commercial Available Iron Deficient Food. Biosci Biotech Res Asia 2019;16(1). |
| Copy the following to cite this URL: Thakur M. K, Kulkarni S. S, Mohanty N, Kadam N. N, Swain N. S. Standardization and Development of Rat Model with Iron Deficiency Anaemia Utilising Commercial Available Iron Deficient Food. Biosci Biotech Res Asia 2019;16(1). Available from: https://bit.ly/2I9lhFj |
Introduction
One of the most prevalent nutritional disorders according to WHO is Iron Deficiency Anaemia (IDA) affecting approximately two billion individuals worldwide.1 The highest prevalence is found particularly in young children and women of reproductive age. In children, it can cause various developmental delays and disturbances in behaviour in children. In women of reproductive age, the physical performance is usually affected and also increase in the risk of preterm delivery and babies with low birth weight.2 Iron, one of the essential trace element for living organisms responsible for oxygen transport and storage of molecules and many enzymes catalysing the redox reactions which is required for the generation of energy.3,4 It also acts as a cofactor for many proteins being a modulator of metabolic and physiological functions. Prolonged periods of iron deficiency result in a smaller iron storage pool, the suppression of haemoglobin biosynthesis and the development of anaemia. These results indicate that iron is an essential nutrient associated with a variety of biological activities.5
Even though, many researchers have studied particularly IDA, the main cause of high prevalence of anaemia has not been established yet. Animal models are the key holders, the research on which is always required in order to understand the pathogenesis of any disease.6,7,8 The present study, focuses on development of iron deficient Wistar rat (Rattus nor vegicus) model, which is commonly used model in biomedical research because of its physiologically higher similarities with human body. For the development of model, test groups rats were administered with iron deficient diet. The factors affecting iron deficiency anaemia were recorded and studied by comparing with the control group.
Material and Methods
Development of Iron Deficient Rat Model
Two forms of diets were procured from VRK Nutritional Solutions, Pune. A balanced diet that included the daily iron requirements and an iron-deficient diet. Both types of diets were formulated to provide all the recommended nutrients as follows: protein, fat, fibre, calcium, phosphorous, carbohydrates, mineral mixture and corn oil. In case of iron deficient diet, Fe deficient corn oil and mineral mix was utilized.
12 wistar albino female rats were acclimatized at MGMIHS animal house in stainless steel cages with 12 x 15 x 25 cm dimensions for 7 days before initiating the study. Optimum temperature of 21°C ± 3°C and 12/12hr light/dark cycle was maintained at the animal house. The inclusion criteria for selecting the rats was 180-200 gms of body weight and haemoglobin between 12 to 15 g/dL. All selected rats were randomly divided into 2 groups: Control (n = 3) and Test (n = 9). Test rats were administered with iron deficient diet and timepoints of the study were 5 weeks (35 days) and 6 weeks (42 days). The research protocol of this study was approved by MGM’s Institutional Animal Ethics Committee (IAEC) with approval no. 2016/7/12, MGMIHS. Rats were allowed free access to their diet and to deionised water during the experiment.
Sampling Procedure
Body weight and food consumption were periodically measured throughout the experimental period. After 35 days on Iron deficient diet, 4 animals were anesthetized using isofluorane and a volume of 0.1–0.3 ml of blood was extracted from the retro-orbital sinus and collected in micro tubes containing EDTA as anticoagulant as well as plain tube. The total haemoglobin content of blood was measured using automated Sysmex KX-21, haematology machine in order to verify the haemoglobin content. All rats had haemoglobin content < 9 g/dL, which demonstrated depletion of iron. Also immunoassay studies were carried out to record the levels of serum iron, TIBC, serum ferritin and transferrin saturation using semi-auto analyzer Beckman Coulter AU480. After an additional 7 days, all the 12 animals (n = 3 control & n = 9 test) were anesthetized using isofluorane and dissected for further analysis. The organs were collected for histopathological analysis and blood samples were collected in both EDTA and plain tubes for haematology and immunoassay studies. The reticulocyte counts were also recorded after 5th and 6th week to determine the percentage of immature RBCs which are usually found to be low in case of iron deficiency anaemia. Statistically, the data was analysed using paired t-tests to distinguish the parameters between control and treated groups. P< 0.05 were considered to be the level of significance.
Results and Discussion
Effect of Iron Deficient Diet on Body Weight
Animals exhibited similar weight (203.3 ± 1.7), and no significant differences were noted among groups at the beginning of the study. Body weight was increased in control group (233.9 ± 1.7), whereas body weight gain was markedly lower in the group fed with iron deficient diet (211.9 ± 1.6) after exposure of 42 days (Table 1).The statistical analysis showed significant differences in groups, after treatment with p < 0.05. Decreased rat body weight in our study was in line with Tanaka’s study.9 Similar results were also found (Susanti et al, 2017) showing loss of body weight and decrease in the levels of haemoglobin after administration of IDA diet in 15 days. Rats were fed with 15.6g/day standard diet in all control and Iron deficient diet for treated groups till the end of the study but Tanaka and co-worker fed their experimental rats with a standard diet depending on weekly rat consumption. Therefore, growth retardation in our study may result from iron-deficient diet instead of malnutrition.
Table 1: Weight loss and food consumption of Wistar albino female rats (n = 3 for control and n = 9 for test group) after 6th week (42 days) of inducing IDA showed significant differences (p<0.05) in comparison with control.
| Negative Control | Test | |
| Initial weight (g) | 203.3 ± 1.7 | 203.2 ± 1.7 |
| Final weight (g) | 233.9 ± 1.7 | 211.9 ± 1.6* |
| Food consumption (g animal-1 day-1) (After 42 days) | 15.6 ± 0.5 | 9.8 ± 0.4* |
RBC and WBC counts, Hb levels, HCT, MCV, MCH and MCHC levels
Hb levels before and after induction of IDA experiments are shown in Table 2. Hb is the red blood cells protein responsible for the oxygen delivery to the tissues, in which iron has a central position in the Hb structure. IDA could cause Hb decrease, due to the iron-deficient diet, the Hb content in the IDA group was significantly lower compared with the normal control group (P < 0.05). The differences in the Hb levels in the normal control group were not significant after the induction of IDA experiment (P > 0.05). When the intake of iron is inadequate for daily needs, body iron stores drop to a too low amount to support normal RBC production, thus developing IDA, followed by a low reticulocyte Count, HCT, MCV, MCH or MCHC.10 Control group showed normocytic normochromic RBCs whereas test group has revealed changes in the RBCs which showed microcytic hypochromic morphology (Figure 1). At the end of this experiment, RBC counts and HCT, MCV, MCH, and MCHC levels in the IDA group significantly decreased (P < 0.05) compared with the normal control group (Table 2). Similar pattern was observed by Lazaro et al (2017) and Zhu. et al (2016) for alterations in the levels of haemoglobin, reticulocytes, MCV, MCHC and body weight of animals, similar results were obtained in our studies.11,12 In a similar study Xiao et al (2016) developed anaemic model where the experimental rats were given a standard diet with 8 mg Fe/kg for 21 days and mean Hb levels < 10 g/dl.10 Other study conducted by Susanti et al (2017) established a model in 15 days by modification in their standard diet but they lack information on some important parameters of iron deficiency anaemia like morphological changes on RBCs, overall haematological indices with reticulocyte count, iron studies etc studied.6 Few studies had used the mixture of low iron based feed, trace elements and vitamin. Depending on this, they changed the casein instead of egg albumin, changed cellulose into microcrystalline cellulose.13
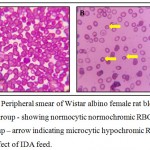 |
Figure 1: Peripheral smear of Wistar albino female rat blood – 1A Control group – showing normocytic normochromic RBCs and 1B Test group – arrow indicating microcytic hypochromic RBCs due to effect of IDA feed.
|
Table 2: Haematological indices (WBC, RBC, HGB, HCT, MCV, MCH, MCHC, PLT) of Wistar albino female rats after 5th week (35 days) and 6th week (42 days) of inducing IDA showed significant differences (p<0.05) in comparison with control.
| Haematological Indices of Wistar Albino Rats | |||
| Control | Test | ||
| After 5 weeks | After 6 weeks | ||
| HGB (g/dL) | 14.5 ± 0.9 | 7.7 ± 0.5* | 7.5 ± 0.3* |
| WBC (/ul) | 15.8 ± 0.7 | 14.1 ± 0.4* | 13.9 ± 0.6* |
| RBC (/ul) | 6.6 ± 0.1 | 5 ± 0.1* | 4.8 ± 0.2* |
| Retic Count (%) | 1.7 ± 0.4 | 0.5 ± 0.2* | 0.4 ± 0.2* |
| HCT (%) | 41.4 ± 0.8 | 18.4 ± 0.2* | 18.2 ± 0.3* |
| MCV (fL) | 64.6 ± 1.4 | 46.8 ± 1.9* | 46.5 ± 2.4* |
| MCH (pg) | 19.3 ± 0.4 | 16.1 ± 0.3* | 15.8 ± 0.2* |
| MCHC (g/dL) | 30 ± 0.2 | 21.7 ± 0.1* | 21.4 ± 0.3* |
| PLT (ul) | 1000 ± 21.5 | 1374.7 ± 22.4* | 1377 ± 23.3* |
Immunoassay Studies
The content of SI, TIBC, is shown in Table 3. The iron content of the serum SI, SF and TS were significantly lower in IDA rats than the control group (P < 0.05). Serum TIBC in the IDA rats was significantly higher than the control group and iron due to progressive Fe depletion from the body stores. All these findings were expected and consistent with induced severe IDA in rats. The degree of iron deficiency produced by our iron-restricted diet was severe enough to impair weight gain and to reduce final body weight, as found previously.14,15,16 The data showed significant differences by paired t-test with p < 0.05. Wang et al. (2014) in their study fed low iron diet for 4 weeks to induce IDA in rats. After iron deprivation, lower levels of haemoglobin concentration, serum iron and serum ferritin were observed whereas increased levels of TIBC were found, these results are in line of our study.17
Table 3: Immunoassay studies (SI, TIBC, SF and TS) of Wistar albino female rats after 5th week (35 days) and 6th week (42 days) of inducing IDA showed significant differences (p<0.05) in comparison with control.
| Control | Test | ||
| After 5 weeks | After 6 weeks | ||
| Serum iron (µg/dl) | 114.8 ± 5.4 | 77 ± 2.5* | 76.8 ± 1.4* |
| TIBC (µg/dl) | 281 ± 8.1 | 383.2 ± 2.7* | 384.5 ± 2.9* |
| Serum ferritin (ng/ml) | 85.1 ± 0.5 | 65.1 ± 1.4* | 64.8 ± 1.5* |
| Transferrin saturation (%) | 41.2 ± 1.4 | 20.3 ± 0.1* | 19.9 ± 0.3* |
Histopathological Studies
The histological findings involved in the study allowed for assessing the abnormality in rat tissues due to consumption of IDA through iron deficient diet. Figure 2A showed normal architecture of heart muscle tissue with normal blood vessel whereas Figure 2B (arrow) showed congestion of small blood vessels with normal heart muscle tissue. The normal architecture of kidney showing glomerulus, proximal convoluted tissue and distal convoluted tissue were observed in control as well as test rat tissues (figure 2C & 2D). The specimen of liver and lungs has shown congestion and centrilobular hepatocyte with inflammatory cell infiltration (figure 2F) and inflammation of perivascular sheath and surrounding tissue i.e. perivasculitis (figure 2H) respectively. Normal architecture of hepatocyte radiating from central vein with central vesicular nuclei (figure 2E)was observed. Similar studies by Rothenbacher et al (1980) has revealed accumulation of fat, cellular swelling and nuclear degeneration in hepatocytes. Spleen showed decrease in white pulp (figure 2J) when compared with control rat tissue observations (figure 2I). The study also revealed hemopoiesis and lymphopoiesis which were found to be depressed in spleen of iron deficient rats.18
Our results are in line with the histopathological results of Ibrahim et al (2011) studies which concluded that induced iron deficiency anaemia by tannic acid in male albino rats leads to dilatation and congestion of sinusoids with lipid droplets, tyknotic nuclei, congestion of central vein associated with hyalinization area around it in iron deficient rats.19,20 Heart revealed fatty hydropic and hyallin granular degeneration, pyknosis and karyolysis with dilatation and congestion of blood vessels were observed whereas it was found that kidneys were normal in our study in contrast with Ibrahim et al (2011) studies where they found that the iron deficient kidney revealed pyknosis and karyolysis, haemorrhage and lymphocyte infiltration were observed in interstitial tissue, renal cortex exhibited focal degeneration in glomeruli and congestion of their capillaries.
On the basis of the presented results, it is expected that absorption of iron strongly depends on the iron status of the organism being fed. Thus, iron deficient feed leads IDA model.
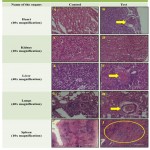 |
Figure 2: Micrographs of Rat Tissues after 42 exposure of Iron deficient feed:
|
Sections of rat tissues showing normal architecture in Control (A, C, E, G, I). Histological specimen of Heart (B) – arrow showing congestion of small blood vessels, Kidney (D) showing normal histological structure of glomerulus, proximal convoluted tubules and distal convoluted tubules. Liver (F) congestion and centrilobular hepatocyte with inflammatory cell infiltration. Lungs (H) showed perivasculitis i.e. inflammation of perivascular sheath and surrounding tissue. Spleen (J) showed decrease in white pulp.
Conclusion
The IDA rat model has been successfully developed in 42 days by modification of standard diet i.e. corn oil without iron and Fe deficient mineral mix. The model has been confirmed by assessing the levels of haematological indices, reticulocyte count serum iron, TIBC, serum ferritin and transferrin saturation. The morphology of RBCs has also confirmed the development of IDA rat model.
Acknowledgements
We would like to thank all staff in the Animal House & Central Research laboratory, MGMIHS, Navi Mumbai for maintaining rats during this study and testing various parameters. We also acknowledge MGMIHS for providing funds to support this study.
Conflict of Interest
There is no conflict of interest.
Funding Source
The infrastructure and animal house facility were provided by MGMIHS.
References
- Who U. UNU. Iron deficiency anaemia: assessment, prevention and control, a guide for programme managers. Geneva: World Health Organization. 2001.
- McLean E., Cogswell M., Egli I., Wojdyla D., De Benoist B. Worldwide prevalence of anaemia, WHO vitamin and mineral nutrition information system, 1993–2005. Public health nutrition. 2009;12(4):444-54.
CrossRef - Lucca P., Hurrell R., Potrykus I. Fighting iron deficiency anemia with iron-rich rice. Journal of the American College of Nutrition. 2002;21(3):184S-90S.
- Chua A. C., Graham R. M., Trinder D., Olynyk J. K. The regulation of cellular iron metabolism. Critical reviews in clinical laboratory sciences. 2007;44(5-6):413-59.
CrossRef - Zhang X. G., Wei G. X., Wang W. N., Ma G. D., Tang P., Chen X. Q. Effects of Fe-YM1504 on iron deficiency anemia in rats. Food & function. 2016;7(7):3184-92.
CrossRef - Susanti T., Dirgahayu P., Indarto D. Development of Rat Model with Iron Deficiency Anemia by Modification of Its Standard Food. In2nd Public Health International Conference (PHICo 2017) Atlantis Press. 2017.
- Fernandes M. I., Galvao L. C., Bortolozzi M. F., Oliveira W. P., Zucoloto S., Bianchi M. L. Disaccharidase levels in normal epithelium of the small intestine of rats with iron-deficiency anemia. Brazilian journal of medical and biological research. 1997;30:849-54.
CrossRef - Nicolas G., Bennoun M., Porteu A., Mativet S., Beaumont C., Grandchamp B., Sirito M., Sawadogo M., Kahn A., Vaulont S. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proceedings of the National Academy of Sciences. 2002;99(7):4596-601.
CrossRef - Tanaka M., Kusuda M. T., Abe K., Nagasaka M. Effects of Iron Deficiency Anemia on Growth Rate of Rats.
- Xiao C., Lei X., Wang Q., Du Z., Jiang L., Chen S., Zhang M., Zhang H., Ren F. Effects of a tripeptide iron on iron-deficiency anemia in rats. Biological trace element research. 2016;169(2):211-7.
CrossRef - Lázaro E., Santas J., Rafecas M. Recovery from dietary iron deficiency anaemia in rats by the intake of microencapsulated ferric saccharate. Journal of food science and technology. 2017;54(9):2913-8.
CrossRef - Zhu Q., Qian Y., Yang Y., Wu W., Xie J., Wei D. Effects of carbonyl iron powder on iron deficiency anemia and its subchronic toxicity. journal of food and drug analysis. 2016;24(4):746-53.
CrossRef - Rat Model for Iron Deficiency Anemia (IDA) (http://www.cloud-clone.com/products/DSI595Ra02.html)
- Borel M. J., Smith S. H., Brigham D. E., Beard J. L. The impact of varying degrees of iron nutriture on several functional consequences of iron deficiency in rats. The Journal of nutrition. 1991;121(5):729-36.
CrossRef - Strube Y. N., Beard J. L., Ross A. C. Iron deficiency and marginal vitamin A deficiency affect growth, hematological indices and the regulation of iron metabolism genes in rats. The Journal of nutrition. 2002;132(12):3607-15.
CrossRef - Murphy A. T., Witcher D. R., Luan P., Wroblewski V. J. Quantitation of hepcidin from human and mouse serum using liquid chromatography tandem mass spectrometry. Blood. 2007;110(3):1048-54.
CrossRef - Wang F. R., Xie Z. G., Ye X. Q., Deng S. G., Hu Y. Q., Guo X,. Chen S. G. Effectiveness of treatment of iron deficiency anemia in rats with squid ink melanin–Fe. Food & function. 2014;5(1):123-8.
CrossRef - Rothenbacher H., Sherman A. R. Target organ pathology in iron-deficient suckling rats. The Journal of nutrition. 1980;110(8):1648-54.
CrossRef - Ibrahim E. R., Yehia M. N. Biochemical and histological studies on the Ameliorative role of fenugreek products in tannic acid-induced iron deficiency anemia in Albino rats. The Egyptian Journal Of Experimental Biology (Zoology). 2011;7(2):185-96.
- Markandeywar N. R. Evaluation of Efficacy and Safety of an Ayurvedic Hematinic Formulation in an Experimental Model of Iron Deficiency Anaemia. [MD Pharmacology Thesis] University of Mumbai. 2011.

This work is licensed under a Creative Commons Attribution 4.0 International License.





