How to Cite | Publication History | PlumX Article Matrix
Rabeeth M.*, Thangarj T., Karaiyan K. and K. Logan Kumar.
Kongunadu Arts and Science College, Coimbatore- 641 029 (India)
ABSTRACT: Phenoloxidase in the cuticle was extracted in different stages of development. Maximum enzyme activity was found at the pharate pupal stage (126 ± 4 hr). Extracted enzyme was partially purified using Sephacryl S-400. Two fractions have been localized in 7-18 and 20-24. Partially purified phenoloxidase was localized in the SDS-PAGE. It is resolved that two molecular species of 204 and 230 Kd has been localized. DDC and PTU inhibited the enzyme maximum when compare to PHZ, SA and SF. The enzyme was thermostable upto 40°C and activity showed a broad spectrum of pH. In situ treatment of newly ecdysed puparial cuticle with phenylthiourea inhibited brown colour pigment formation.
KEYWORDS: Cuticle; Phenoloxidase; H. armigera
Download this article as:| Copy the following to cite this article: Rabeeth M, Thangarj T, Karaiyan K, Kumar K. L. Purification And Inhibition Of Cuticular Phenoloxidase In The Cotton Bollworm, Helicoverpa Armigera (H.) Lepidoptera. Biosci Biotechnol Res Asia 2003;1(2) |
| Copy the following to cite this URL: Rabeeth M, Thangarj T, Karaiyan K, Kumar K. L. Purification And Inhibition Of Cuticular Phenoloxidase In The Cotton Bollworm, Helicoverpa Armigera (H.) Lepidoptera. Biosci Biotechnol Res Asia 2003;1(2). Available from: https://www.biotech-asia.org/?p=3397 |
Introduction
In insects and invertebrates, the mechanism of nonself recognition may be controlled by the phenoloxidase (Po) pathway. The enzyme is involved in the encapsulation and melanization of infecting bacterial and fungi and sclerotization of the cuticle (Soderhall and Smith, 1986). It is known that during moulting, the old cuticle is cast off and new cuticular layer is formed. The epicuticle of the newly formed layer is composed of sclerotin. The sclerotin in the epicuticle is synthesized by the action of the key enzyme, namely phenoloxidase. Po (E.E. 1.14.18.1) is a bifunctional copper containing enzyme, which catalyzed both the o-hydroxylation of monophenols and the oxidation of diphenols to quinines (Lerch, 1988). Thus, the enzyme is able to convert tyrosine to DOPA, as well as DOPA to DOPA- quinone. The ultimate product of the non-enzymatic reactions that spontaneously follow in melanin, a brown or yellowish pigment. In arthropods, this pigment is produced in the cuticle after wounding or as a result of parasite attack (Ratcliffe et al, 1985). Phenoloxidase, in both the inactive prophenoloxidase (proPo) or active Po form can be demonstrated in the vertebrates species (Ratcliffe et al, 1984; Saul et al, 1987; Soderhall and Smith, 1983).
The characterization of Po has been determined for various insects and invertebrates. The molecular weight of Po varies greatly with the insect from which the enzyme is isolated (Ashida, 1971; Gillespie et al, 1991; Morgan et al, 1990). Difficulty in ascertaining the molecular weight of Po pertains to whether it is found in an aggregated form that is resistant to chemical separation or if the enzyme is found in a native form composed of one sub unit (Anderson et al, 1989; Gillepsie et al, 1991). There is a difference in the physical and chemical characteristics of the cuticle and haemolymph forms of Po ever with in the same insect as found in Manduca sexta (Aso et al, 1985; Morgan et al, 1990; Thomas et al, 1989).
The characterization of Po was determined for H. armigera using cuticle. The molecular weight of Po was determined by SDS-PAGE. The optimal pH, optimal temperature, at which Po is active, were determined. Along with the physical characteristics, chemical characteristics such as substrate specificity, and inhibitors of Po were also studied. In addition, in situ localization of Po in the cuticle also examined. Determining the characteristics of Po will provide a better understanding of the optimal conditions needed by Po for maximum activity of H. armigera.
Material and Methods
Test insects
Helicoverpa armigera eggs were obtained from the Bio-control laboratory, Tamil Nadu Agricultural University, Coimbatore. The larvae were reared on artificial diet media (Singh and Rembold, 1992) in individual sterile glass bottles. Each bottles contained 10 ml of diet media. The bottles were held at 26-28°C in a 12:12 (L:D) hour photoperiod.
Cuticular sample preparation
The pharate pupa was dissected and the cuticle with attached trachea and muscles was separated in physiological saline in an ice-cold condition. Total of 100 cuticles were cut into small pieces and incubated in known volume of borate buffer for the exaction of the enzyme. The extracted enzyme was subjected to ammonium sulphate (80%) precipitation. After 24 hr of incubation at 4°C, the mixture was centrifuged at 1000 rpm for 2 min. The precipitate obtained was dissolved in a known volume of phosphate buffer (0.01 M pH 7.0) and dialyzed against the same buffer for 24 hr at 4°C. The dialysate was taken for further purification.
Purification
The dialysate of 2 ml was subjected to get filtration on Sephacryl S-400 (Sigma) 1.2 cm x 60 cm column (Bio-rad). The enzyme was eluted with phosphate buffer (0.01 M, pH 7.0) at the flow rate of 20 ml / hr. Protein content in the fractions were localized at 280 nm using UV visible spectrophotometer – 118 (Systronics). The phenoloxidase activity was localized in the fractions as detailed by Andersen (1980) using the substrates, tyramine, dopamine and 4-methyl hydroquinone. Fractions that showed enzyme activity were freeze dried (Savant, USA) and stored until further use.
Assay of phenoloxidase activity
The assay mixture consisted of 100 µl of the enzyme and 100 µl of the substrate, dopamine (10 mM). The reaction mixture was incubated for 10 min. with 1.8 ml of phosphate buffer (0.01 M, pH 7.0). The enzyme activity was measured at 470 nm. For the control 100 µl of the buffer used instead of the enzyme.
Activity towards para-diphenol was determined with 4-methyl hydroquinone as the substrate. 0.3 ml of 4-methyl hydroquinone (10 mM) was added to 1.6 ml assay buffer at room temperature (30°C).
The reaction was started by the addition of 100 µl of the enzyme. The increase in the absorbency at 250 nm was registered for 20 min.
Characterization
Substrate specificity
The enzyme activity towards various phenolic substrates was observed. The substrates employed in the present study were tyrosine, tyramine, L-tyrosine ethylester, L-tyrosine methyl ester, N-acetyl L-tyrosine, dihydroxy phenyl alanine (DOPA), dopamine, N-acetyl dopamine, L-B. 3-4-dihydroxy phenylalanine methyl ester, syringaldazine, resorcinol, 4-methyl catechol, pyrogallol, hydroquinone and 4-methyl hydroquinone. The activity of the enzyme was assayed as detailed in the previous section, and appropriate controls were kept along with the experimental tubes.
Effect of temperature, pH and inhibitors on the activity of the enzyme phenolozidase
The stability of the enzyme was determined by incubating 100µl of enzyme at different temperature ranging from 20 to 80°C for 10 min. After the incubation period, the enzyme was brought to room temperature and 100µl of the substrate and 1.8ml of buffer was added. The activity was measured at 470nm.
The pH ranges used for PO enzymatic activity were from pH 3.00 to pH 7.5 100µl of enzyme was incubated with 100µl of phosphate buffer of different pH for 30 min. and the activity of the enzyme was determined. Appropriate controls were maintained along with experimental tubes.
To observe the behaviour of the enzyme in the presence of various inhibitors, such as phenylthiourea (PTU), sodium diethyl dithiocarbamate (DDC), phenyl hydrazine (PHZ), sodium azide (SA) and sodium fluoride (SF), the enzyme was incubated with different concentrations of inhibitors for 15 min. The remaining activity was measured using the substrate, dopamine as detailed is the previous section.
In situ localization and inhibition of cuticular phenoloxidase
Pharate pupal cuticles were isolated along with the newly formed cuticle at 4 hr before the larval to pupal moult without disturbing the newly formed layer. Cuticles were washed in physiological saline and incubated in the solution containing the substrate, dopamine (10 mM) and MBTH (3-Methyl 2-benzothiazolinone hydrazone hydrochloride, 0.3%) dissolved in 90% ethanol. The enzyme was localized in the cuticle after 10 min. of incubation.
Table 1 : Purification scheme of cuticular phenoloxidase of H. armigera
| Source | Volume | Protein | Total activity | Activity / mg protein | |||
| mg/ml | Total | Dopamine 4-methyl | Dopamine | 4-methyl | |||
| hydroquinone | hydroquinone | ||||||
| Crude | 2 | 8.319 | 16.640 | 4.360 | 3.800 | 0.264 | 0.230 |
| Sephacryl | |||||||
| S-400 | 3 | 0.100 | 0.300 | 4.680 | 0.000 | 15.600 | 0.000 |
| Fraction | |||||||
| A(No.12) | |||||||
| Fraction-C | 3 | 0.263 | 0.789 | 0.450 | 1.830 | 0.570 | 2.320 |
Table 2 : Activity of phenoloxidase in the of cuticular H. armigera (OD/mg protein/min.)
| Substrates | Activity | |
| Enzyme A | Enzyme B | |
| Monophenols | ||
| Tyramine | 0.372 ± 0.033 | – |
| Tyrosine | 0.192 ± 0.021 | – |
| L-Tyrosine methyl ester | 0.266 ± 0.058 | – |
| L-Tyrosine ethyl ester | 0.158 ± 0.033 | – |
| N-Acetyl L-Tyrosine | 0.094 ± 0.040 | – |
| Ortho di-phenols | ||
| Dopamine | 1.532 ± 0.059 | 0.049 ± 0.005 |
| L-B-3,4 Dihydroxy | ||
| phenylalanine methyl ester | 0.552 ± 0.072 | 0.023 ± 0.005 |
| DOPA | 1.397 ± 0.024 | 0.037 ± 0.004 |
| 4-Methyl catecol | 1.039 ± 0.056 | 0.018 ± 0.001 |
| N-acetyl dopamine | 0.944 ± 0.031 | 0.027 ± 0.004 |
| Resorcinol | 0.488 ± 0.042 | 0.023 ± 0.004 |
| Para phenols | ||
| 4-Methyl hydroquinone | 0.152 ± 0.047 | 0.149 ± 0.006 |
| Hydroquinone | 0.084 ± 0.010 | 0.121 ± 0.008 |
| Syringaldazine | 0.050 ± 0.019 | 0.037 ± 0.007 |
| Polyphenols | ||
| Pyrogallol | 0.078 ± 0.039 | 0.024 ± 0.004 |
Monophenolic substrates and dopamine, L-B-3,4 dihydroxy phenylamine methyl ester was read at 430 nm; DOPA and 4-methyl catecol at 470 nm; N-acetyl dopamine at 390 nm; resorcinol, syringaldazine and pyrogallol at 420 nm; 4-methyl hydroquinone at 250 nm. Each value represents the mean of 5 determinations.
The enzyme activity was inhibited in the cuticle by incubating the cuticle with inhibitor for 15 min. After washing the cuticle with distilled water, they were transferred to dopamine – MBTH mixture (0.3%) and allowed for 15 min. In another set of experiment, cuticle were incubated in the solution containing only MBTH without dopamine, which served as a control.
Newly emerged white pupae were collected to test the effects of inhibitors on the activity of cuticular phenoloxidase. The white cuticular layer was topically applied with phenylthiourea (1mg/ ml) for three times with an interval of 30 min. for 1.30 hr duration. Time taken for the melanization of cuticle was noticed.
Electrophoretic localization of phenoloxidase Phenoloxidase were localized in the SDS-PAGE (7%) as followed by Nellaiappan and Vinayagam (1986). After electrophoresis the gel was washed with phosphate buffer several times and incubated with the substrate-MBTH mixture for 5-10 min. The substrate dopamine (10 mM) dissolved in phosphate buffer (0.01 M, pH 7.0) and MBTH (0.3%) dissolved in 90% ethanol was mixed in the ratio of 4:1. The quinone-MBTH condensation product was localized in the gel as an intense band.
Table 3 : Effect of inhibitors on the activity of the cuticular phenoloxidase (enzyme A) of H. armigera
| Inhibitor (% of inhibition) | |||||
| Concentration | PTU | DDC | PHZ | SA | SF |
| (mM) | |||||
| 4 | – | – | – | 38.97 | 27.94 |
| 3 | – | – | – | 25.73 | 22.79 |
| 2 | 98.53 | 98.53 | 44.85 | 15.44 | 8.82 |
| 1 | 98.53 | 98.53 | 39.71 | 7.35 | 0.74 |
| 0.5 | 97.79 | 95.59 | 30.88 | 1.47 | – |
| 0.25 | 95.59 | 88.97 | 25.00 | – | – |
| 0.12 | 95.59 | 86.76 | 10.29 | – | – |
| 0.06 | 93.38 | 83.09 | 4.41 | – | – |
| 0.03 | 87.50 | 67.65 | – | – | – |
| 0.015 | 80.15 | 61.03 | – | – | – |
| 0.007 | 72.79 | 41.91 | – | – | – |
| 0.003 | 56.62 | 27.21 | – | – | – |
| 0.0015 | 44.12 | 16.91 | – | – | – |
| 0.0008 | 34.59 | 11.76 | – | – | – |
Each value is represents the average of 5 determinations. The assays were performed at pH 7.0. Dopamine was used as substrate; PTU – Phenylthiourea, DDC- Sodium diethyl dithiocarbomate, PHZ- Phenylhydrazine, SA- Sodium azide, SF- Sodium fluoride.
 |
Figure 1 : Purification of pharate cuticular phenoloxidase in Helicoverpa armigera |
The column was eluted with phosphate buffer (0.01M, pH 7.0). 3.0ml fractions were collected at the rate of 20ml/hr. Protein content in the fractions were localized at 280nm. And enzyme A at 470 nm. And enzyme B at 250 nm. All the fractions were assayed for phenoloxidase activity using the substrate dopamine, tyramine and 4-methyl hydroquinone.
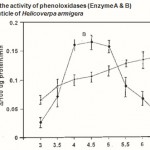 |
Figure 2 : Effect of pH on the activity of phenoloxidases (Enzyme A & B)in the pharate pupal cuticle of Helicoverpa armigera |
The pH ranges used were from pH 3.0 to pH 7.5 Phosphate buffer 0.01M used. Absorbance was read at 470 nm after 30 min. incubation. Error bars represent ±SD of five replications.
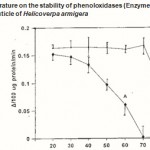 |
Figure 3 : Effect of temperature on the stability of phenoloxidases (Enzyme A & B) in the pharate pupal cuticle of Helicoverpa armigera |
Temperature (°C)
The optimal temperature of phenoloxidase activity was expressed as the phenoloxidase activity found after a preincubation period at the indicated temperature. Error bars represent ±SD of 5 replications.The gels were stored at 7% acetic acid and photographed.
Results
Partial purification of cuticular phenoloxidase Two peaks of activity were identified. Peak 1 (enzyme A) was eluted in the early fractions 9 to 18 and peak 2 (enzyme B) was eluted in the fractions 19 to 24. The peak 1 oxidized both mono-phenols and o-diphenols whereas, peak 2 exhibited more activity in 4-methyl hydroquinone and dopamine (Fig. 1). Based on the performance of the activity on the substrate, the peak 1 is designated as the tyrosinase, which is 60 times purified, whereas, the peak 2 designated as the laccase, purified only 10 times (Table 1).
Activity of the enzyme on various phenolic substrates
The behaviour of cuticular phenoloxidase on various phenolic substrates was tested. The enzyme A showed activity towards monophenolic and o-diphenolic, and exhibited higher activity to dopamine, DOPA, 4-methyl catechol and N-acetyl dopamine and lesser activity on the substrates 3,4 di hydroxy benzoic acid, pyrogallol and resorcinol.
The enzyme B exhibited activity towards o-diphenolic and p-phenolic substrates only, but not to monophenolic substrates (Table 2).
 |
Figure 4 |
Effect of pH and thermostability
The optimum pH for the enzyme activity was determined using the phosphate buffer. The enzyme A showed a broad range of optimum pH 5-7 using the substrate, dopamine and the enzyme B showed maximum activity in between pH 4.0 and 5.0 when methyl hydroquinone used as substrated (Fig. 2).
Thermostability of the enzyme A and B types were tested under various temperatures ranging from 20 to 100°C. The enzyme A retains its activity maximum upto 40°C and thereafter, the activity was completely lost at 70°C. The enzyme B was found more thermostable and over 93% of the activity remains after the treatment of the enzyme at 80 °C for 5 min. thereafter the enzyme loss its activity completely (Fig. 3).
Effect of inhibitors
Partially purified enzyme was subjected to the treatment of various chemicals. It was noticed that the enzymes were completely inhibited by sodium di-ethyldithio carbamate and phenylthiourea. It showed 99% of inhibition at the concentration of 1mM and 45% at 2 mM phenylhydrazine. Sodium azide and sodium fluoride required higher concentration of 4mM to inhibit 39% and 28% of the enzyme activity, respectively (Table 3).
Electrophoretic localization of the cuticular phenoloxidases
Phenoloxidases were localized by means of simple and efficient MBTH quinone coupling method. Two bands of phenoloxidase were identified in the cuticle of H. armigera. The band 1 behaved as a high molecular weight protein retained in the 4% stacking gel having the molecular weight of more than 230 Kd. The second broad band showed molecular weight of 204 Kd.
In situ inhibition of pharate pupal and newly ecdysed puparial cuticular phenoloxidase
The behaviour of phenoloxidase (in situ) in the presence of various chemicals, such as phenyl thiourea (PTU) sodium diethyl dithio carbamate (DDC), phenyl hydrazine (PHZ), sodium azide (SA) and sodium fluoride (SF) was noticed. Cuticles incubated with PTU and DDC were inhibited maximum when mono phenol, tyramine and o-diphenol, dopamine were used. However, the cuticles in the presence of phenyl hydrazine, sodium azide and sodium fluoride resulted with moderate inhibition (Plate 2a).
Cuticle of the newly emerged pupa (control) become reddish brown in colour within half an hour, whereas, newly emerged pupa treated with phenyl thiourea did not show reddish brown colour within half and hour as like the normal pupae indicating that the phenyl thiourea sensitive phenoloxidase responsible for colour development might be inhibited. Newly emerged pupae treated with phenyl thiourea were allowed for 90 min. for melanization. The results revealed that intense melaniztion was observed in the untreated pupae whereas, phenylthiourea treated pupae after 90 min. did not show equal melanization as compared to the untreated.
The results suggested that although the inhibitors inhibited the enzyme to some extend it could not prevent complete melanization. The reason might be the penetration of inhibitor was very slow to have contact with the enzyme entangled in the cuticular matrix (Plate 2b).
Discussion
Phenoloxidase is an enzyme that may be involve in nonself recognition by insects. Phenoloxidase is found in arthropods, insects and plants (Ashida and Dohke, 1980). Even though Po is found in many organisms, it may still have different molecular weight, optimum pH, temperature sensitivity and different Po activators and inhibitors depending on the organism (Thomas et al, 1989). The molecular weight of the Po enzyme of H. armigera was 204 and 235 Kd. The molecular weight of heamolymph Po of H. virescens was 250 Kd (Lockey and Ourth, 1992). The molecular weight of grasshopper Po was 212 Kd (Gillespie et al, 1991) and tyrosinase from Xenopus skin was 229 Kd (Wittenberg and Triplett, 1985) are similar to that of H. armigera. In H. armigera the optimal pH for Po is 7.0 for enzyme A and 4.5 for enzyme B (Fig. 2). This is a different pH value of Manduca sexta and H. virescens which has an optimal pH of 9.0 (Lockey and Ourth, 1992; Tsukamoto et al, 1986). The thermostability of enzyme A was 20-30°C and labile at 70°C and enzyme B was stable upto 80°C and labile at 100°C. In Manduca sexta cuticular Po activity was stable at a temperature of 45°C and labile at 75°C (Aso et al, 1984) . Different inhibitors of Po were analyzed for their effects on Po activity (Table 3). In the case of H. armigera Po activity was significantly inhibited by PTU and DDC when compared to that of PHZ, SA and SF. But H. virescens, SDS caused a significant decrease in Po activity when the enzyme was assayed in the presence of SDS (Lockey and Ourth, 1992). However in the gypsy moth and greater wax moth, EDTA caused a reduction in Po activity (Dunphy, 1991). Recently Muller et al (1999) have shown that presence of six pro-phenol oxidase genes which expresses towards the synthesis of pro-phenol oxidase in cell culture system using 4G 3B cell lines. Three of the genes identified are noval. The Pro Po genes show expression profile from the embryo to adult in an overlapping manner.
In this study, the physical and chemical characteristics of cuticular Po from H. armigera were determined. Morgen (1990) has found that the Po enzyme can be different even within the same species as seen in M. sexta. Although there are similar characteritics to other insenct soluble haemolymph and cuticular phenoloxidase, no other insect Po enzyme was identical to, or closely related to, the Po of H. armiger based on our data. All of the insect phenoloxidass seem to have the same enzymatic activity including the oxidation of monophenols and diphenols. Our conclusion, then, is that the Po enzymes isolated from the different insect species, including H. armigera, are not identical in their physical and chemical properties. Enzymatic characterization on Po is important in that it provides a better understanding of this enzyme which is needed for melanization and sclerotization reactions. In addition, melanization may that it provides a better understanding of this enzyme which is needed for melanization and sclerotization reactions. In addition, melanization may be important in the insect defense response. Phenoloxidase, and its involvement in these important enzymatic reactions, is therefore necessary for insect survival.
References
- Anderson S O, Cuticular sclerotization. In Cuticle techniques in Arthropods by Miller T A, 185-215. Springer, New York (1980)
- Andersson, K, Sun S C, Boman H G, Steiner H, Purification of the prophenoloxidase from Hyalophora cecropia and four proteins involved in its activation. Insect Biochem, 19, 629-637 (1989)
- Ashida M, Purification and characterization of prophenoloxidase from hemolymph of the silk worm Bombyx mori. Archs Biochem. Biophys. 144, 749-762 (1971)
- Ashida M, Dohke K, Activation of prophenoloxidase by the activating enzyme of the silk work, Bombyx mori. Insect Biochem, 10, 37-47 (1971)
- Aso Y, Kramer K J, Hopkins T L, Whetzel S Z, Properties of tyrosinase and DOPA quinone immune conversion factor from pharate pupal cuticle of Manduca sexta (L) Insect Biochem, 14, 463-472 (1984)
- Aso Y, Kramer K J, Hopkins T L, Lookhart G L, Characterization of haemolymph protyrosinase and a cuticular activator from Manduca sexta(L) Insect Biochem, 15, 9-17 (1985)
- Dunphy G B, Phenoloxidase activity in the serum of two species of insects, the gypsy moth, Lymantria dispar (Lymantriidae) and the greater wax moth, Galleria mellonela (Pyralidae). Biochem Physiol, 98B, 535-538 (1991)
- Gillespie J P, Bidochka M J, Khachatourians G G, Separation and characterization of grasshopper hemolymph phenoloxidase by sodium dodecyl sulfate polycrylamide gel electrophoresis Comp Biochem Physiol, 98C, 351-358 (1991)
- Lerch K, Protein and active site structure of tyrosinase. In advances in pigment cell research, Ed. by Bagnara J T 85-98, Liss, New York (1988)
- Lockey T D, Ourth D D, Isolation and characterization of Hemolymph phenoloxidase from Heliothis virescens Comp Biochem Physiol, 102B, 891-896 (1992)
- Morgan T D, Thomas B R, Yonekura M, Czapla T H, Kramer K J, Hopkins T L, Soluble tyrosinases from pharate pupal integument of the tobacco hornworm, Manduca sexta (L) Insect Biochem, 20, 251-260 (1990)
- Muller H M, Dimopoulos G, Blas C, Kafatos F C, A haemocyte like cell line established from the malaria vector Anopheles gambie expresses sex phenoloxidase gene J Biol Chem, 274, 11727-11735 (1999)
- Nelliappan K, Vinayakam A, A rapid method for detection of tyrosinase activity in electro-phoresis. Stain Technol. 61, 269-272 (1986)
- Ratcliffe N A, Leonard C, Rawley A F, Prophenoloxidase activation: Nonself recognition and cell co operation in insect immunity. Science, 226, 557-559 (1984)
- Ratcliffe N A, Rowley A F, Fitzgerald S W, Rhodes C P, Invertibrate immunity: Basic concepts and recent advances Int Rev Cytol, 97, 183-350 (1985)
- Saul S J, Bin L, Sugumaran M, The majority of prophenoloxidse in the haemolymph of Manduca sexta in present in the plasma and not in the hemocytes, Comp. Immunol, 11, 479-485 (1987)
- Singh A K, Rembold H, Maintenance of the cotton bollworm, Heliothis armigera (Lepidoptera: Noctuidae) on laboratory culture 1. Rearing on semi-synthetic diet. Insect Sci Appl, 333-338, (1992)
- Soderhall K, Smith V J, Separation of the haemocyte populations of Carcinus maenas and other marine decapods, and prophenoloxidase distribution, Devl Comp Immunol, 7, 229-239 (1983)
- Soderhall K, Smith V J, Prophenoloxidase activating cascade as a recognition and defence system in arthropods. In Hemocytic and Humoral Immunity in Arthropods. Ed. by Gupta A P, 251-285, John Wiley and Sons, New York (1986)
- Thomas B R, Yonekura M, Morgan T D, Czapla T H, Hopkins T L, Kramer K J, A trypsin solubilized laccase from pharate pupal integument of the tobacco hornworm, Manduca sexta, Insect Biochem, 19, 611-622 (1989)
- Tsukamoto T, Ishuguro M, Funatsu M, Isolation of latent phenoloxidase from prepupal of the housefly, Musca domestica, Insect Biochem, 16, 573-581 (1986)
- Wittenberg C, Triplett E L, A detergent activated tyrosinase from Xenopus laevis Purification and partial characterization, J Biol Chem, 260, 12535-12541 (1985)

This work is licensed under a Creative Commons Attribution 4.0 International License.





