How to Cite | Publication History | PlumX Article Matrix
Sputum Negative Tubercular Adults Spread Infection and Disease to Their Household Children
Diwan Israr Khan1  , Farzana K Beig1
, Farzana K Beig1  , Zuber Ahmad2
, Zuber Ahmad2  , Ghulam Md Ashraf 3,4*
, Ghulam Md Ashraf 3,4*
![]()
1Department of Paediatrics, JNMCH, Aligarh Muslim University, Aligarh, India
2Department of TB and Chest disease, JNMCH, Aligarh Muslim University, Aligarh, India
3King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia
4Department of Medical Laboratory Technology, Faculty of Applied Medical Sciences, King Abdulaziz University, Jeddah, Saudi Arabia
Corresponding Author’s E-mail: ashraf.gm@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2774
ABSTRACT: To examine whether tuberculosis sputum negative adults can spread disease to their household children. A patient based study was conducted over a time period of one year, on children of those adults who were suspected case of “sputum smear positive” as well as “sputum smear negative” diagnosed with pulmonary tuberculosis at JNMCH, India. Various investigations like tuberculin test, chest X-ray, fine needle aspiration cytology (FNAC) of lymph nodes, gastric aspirate, acid-fast bacilli (AFB) sputum smear examination and others were done to attain the goal of this study. Out of 129 children, 69 were in household contact of sputum smear positive and 60 in contact of sputum smear negative adults. Forty children were found to be Mantoux positive; of which the ratio of children that were in contact of sputum positive adult to those in contact of sputum negative adult was 2:1 (27 vs 13). A small percentage (6%) of asymptomatic children was found to be diseased. Sputum positivity, low socio-economic status as well as less than 3 years of age exhibited strong association with infection as well as disease; nonetheless the number of infected and diseased children in sputum smear negative group is not negligible. A considerable number of screened children that were in contact with sputum negative tubercular adult were found to be infected as well as diseased; hence this group should not be ignored. A portion of Mantoux positive children were found to be asymptomatic and one-third of diseased children were Mantoux negative, so Mantoux cannot be taken as a gold standard for diagnosis of tuberculosis.
KEYWORDS: Malnutrition; Mantoux; Sputum Negative; Sputum Positive; Tuberculosis
Download this article as:| Copy the following to cite this article: Khan D. I, Beig F. K, Ahmad Z, Md Ashraf G. Sputum Negative Tubercular Adults Spread Infection and Disease to Their Household Children. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Khan D. I, Beig F. K, Ahmad Z, Md Ashraf G. Sputum Negative Tubercular Adults Spread Infection and Disease to Their Household Children. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2jY4JGq |
Introduction
In developing countries, tuberculosis is said to be one of the most fatal disease and the leading cause of morbidity and mortality amongst all age groups.1 Today, India accounts for nearly 30% of all tuberculosis cases in the world, a figure likely to increase as India’s population grows and the HIV epidemic progresses. Prevalence and incidence of tuberculosis infection has been found in rural as well as urban areas.2 Around 38% of people of all ages are infected with the disease, while infection among males above 40 years of age was as high as 70%. According to the World Health Statistics Report 2015, the number of reported cases in India is 10 times more than leprosy, 38 times more than Pertussis and 30 times more than combined cases of Cholera, Diphtheria, Leishmaniasis, Measles, Rubella and Tetanus.3
WHO global tuberculosis report has showed an estimated 10.4 million new (incident) TB cases worldwide, of which 5.9 million (56%) were among men, 3.5 million (34%) among women and 1.0 million (10%) among children (Global tuberculosis report, 2016).4 In children, the risk of transmission of infection is through air from the patients of pulmonary infection.5 Despite given so much importance to distinguish TB in the adults, children in India are still lagging behind in noticing and detecting the infection caused by Mycobacterium tuberculosis (Mtb). Children come in contact with the adults bearing infection, follow almost same epidemiology of TB as that of adults. The risk of transmission is highest if the index case is ‘sputum smear positive’. Infected children represent large section of society and form a pool from where new cases of TB arise. The distribution of TB infection can be considered as marker for detecting TB in the society as a whole. Delay in the diagnosis and treatment in low age group children may cause increase in the risk factor among adults. Sputum smear negative case in children usually does not cause the spread of infection, but they form pool of infection from where future adult case of infection may arise.
Not much data is available in India on prevalence and incidence of tuberculosis in children. In most surveys carried out in India, children below 5 years, or even 9 years of age were excluded. Little information is available that are conducted among children in the community in India, are interpreted in the light of epidemiological situation on tuberculosis.6-8 Owing to the overall lack of country wide representation, it cannot be said whether the information could be extrapolated to represent the situation in our country. Even then the available information could be helpful in constructing some hypothesis on the tuberculosis situation in the pediatric age group. The objective behind this study was to gain information on the prevalence of disease in children that live in contact with infected or diseased adults. Since children living in contact of sputum negative adults are largely ignored, we decided to include those children and tried to evaluate the whether this can act as risk factor for tuberculosis.
Patients and methods
Study population
This patient based study was conducted over a time period of one year, on children who were suspected case of “sputum smear positive” as well as “sputum smear negative” adults with pulmonary tuberculosis diagnosed at DOTS (Directly Observed Treatment, Short-course) centre of TB and Chest & Pulmonology and Infectious Disease and Out Patient Department (OPD) Clinic of Department of Pediatrics, Jawaharlal Nehru Medical College and Hospital (JNMCH), Aligarh Muslim University (AMU), Aligarh, India. The study was initiated with the approval of the institutional ethics committee and written informed consent of either parent/guardian was obtained prior to enrolment. Children living under the same roof with the suspected case of pulmonary tuberculosis have been regarded as a household contact with those adult patients.
The current study aimed and recognized adults, diagnosed on the basis of suggestive symptoms and signs, confirmed with either presence of Tuberculosis bacilli on Ziehl-Neelsen staining of sputum (hereafter referred to as sputum positive patients) or diagnostic chest radiograph in the absence of Tuberculosis bacilli in sputum specimens (referred to as sputum negative patients). Children under the age of 12 years who were household contacts of newly diagnosed adults with pulmonary tuberculosis constituted the study population.
Inclusion criteria
Under the 12 years of age and in household contacts of adults are registered under DOTS of JNMCH, AMU Aligarh, residing in Aligarh.
Exclusion criteria
Children who had been previously treated for tuberculosis.
Those with concurrent conditions identified as risk factors for tuberculosis like HIV infection, hematological, and / or reticulo- endothelial malignancy.
Those who were previously or currently on immunosuppressive drugs.
All the registered children were subjected to elicitation of detailed history and clinical examination suggestive of tuberculosis like:
Prolonged fever (>15 days) with no apparent cause, persistent cough, failure to thrive, loss of appetite and swellings in the neck.
Nutritional status was decided as per WHO classification (Table S1).9
Socio economic status was decided based on modified Prasad’s classification.10
Class I: Per capita income more than 1200 Rs/month
Class II: Per capita income 600-1199 Rs/month
Class III: Per capita income 280-599 Rs/month
Class IV: Per capita income120-279 Rs/month
Class V: Per capita income 120 Rs and less /month
Class IV and V were considered as lower socio-economic strata.
History suggestive of measles or pertusis was taken.
History of BCG immunization was taken and presence of BCG scar was noted. Children without scar were considered as unvaccinated.
Examination was done to note for lymphadenopathy, hepatosplenomegaly, gibbous of spine, erythema nodosum and phylectenular conjunctivitis.
Chest findings, suggestive of effusion, consolidation, cavitations were looked for.
Tuberculin test
All the children were examined for tuberculin skin testing, performed by the intradermal injection of 5 TU (Tuberculin Unit) of PPD (Purified Protein Derivative)-RT23 (Span laboratories) into the volar surface of the left forearm using a 26 gauge needle and disposable syringe. This was read 72 hours later in good light with the forearm slightly flexed. Induration was measured by the pen method, and transverse induration of greater than 10 mm was considered as a positive tuberculin test.11 The test was performed and read by a single trained technician to obviate interpersonal observational errors. The test was read by pediatrician as well.
Chest X-ray
All children had a postero-anterior chest radiograph in the department of radio diagnosis, which was reported by a consultant radiologist besides pediatrician and a consensus was evolved on radiological findings.
Fine needle aspiration cytology (FNAC) of lymph nodes
All children with significant lymphadenopathy were subjected to FNAC, in Department of pathology.12 Cytomorphological classification of tubercular lymph node was followed as described by Bailey et al (1985).13
Epitheloid granulomas without necrosis.
In which epitheloid cells i.e. modified macrophages with lymphoid cells at periphery were seen.
Epitheloid granuloma with necrosis.
Epitheloid cells and caseation necrosis seen as structure less eosinphilic material containing granular debris.
Only necrosis.
Seen as structure less eosinphilic material containing granular debris.
Abscess.
seen as marked neutrophilic infiltration with few plasma cells.
Chronic non-specific inflammation.
Seen as mixture of histocytes, lymphocytes and polymorphonuclear cells were classified as reactive lymph node.
Gastric aspirate
Under the age of five, children with a chest radiograph consistent with tuberculosis were admitted and underwent gastric lavage after an overnight fast of eight hours. This was done by aspirating the stomach contents, instilling 50 ml distilled water through a nasogatric tube, and aspirating again. Both aspirates were mixed, subjected to concentration and homogenization as for sputum, and stained by the Ziehl-Nielsen technique and inoculated for culture on Lowenstein Johnson medium in Department of Microbiology, JNMCH, AMU, Aligarh.
Acid-Fast Bacilli (AFB) sputum smear examination
Children of six years and above who could produce sputum with a chest radiograph consistent with tuberculosis were subjected to three smear examination for AFB and culture in Department of Microbiology, JNMCH, AMU, Aligarh.
Other investigations
CT scan head was done when tubercular meningitis was suspected. The diagnosis was based on modified criteria.14 This includes as (i) exudates in the basal cisterns or in the sylvian fissures, (ii) hydrocephalus (iii) infarcts and (iv) gyral enhancement. Presence of two or more of the above criteria was taken as evidence of tubercular meningitis. Ultrasound (USG) skull was done as an alternative to CT in younger children as the cost was unaffordable and anterior fontanel was open. In cases suspected of abdominal/disseminated tuberculosis USG abdomen was done and looked for the following suggestive findings.15, 16 Parameters are (i) mesenteric thickness of 15 mm or more, (ii) increased mesenteric chogenicity, (iii) mesenteric lymphadenopathy, (iv) dilated small bowel loops and (v) ascites. Children who presented as index cases of tuberculosis with a positive family history of contact, parents were subjected to investigations as per RNTCP (Revised National TB Control Programme) algorithm and classified into sputum smear positive and sputum smear negative for the purpose of the study. Based on clinical findings and investigations the children were classified as per criteria given by Indian Academy of Pediatrics.17
Group 1: Preventive therapy
Asymptomatic Mantoux positive <3 years.
Asymptomatic Mantoux positive < 5 years with severe malnutrition.
Mantoux positive – recent converter / no signs.
Children < 3 years with H/O contact.
Children < 5 years with H/O contact with severe malnutrition.
Group 2: Primary complex (lungs)
Symptomatic Mantoux positive <3 years without localization.
Symptomatic Mantoux positive <5 years with severe malnutrition without localization.
Isolated lymphadenitis.
Pleural effusion.
Group 3
Progressive pulmonary disease.
Multiple tubercular lymphadenitis.
Group 4
Miliary/disseminated disease.
Cavitatory disease/bronchopneumonia, osteoarticular disease.
Abdominal, pericardial, genitourinary disease.
Group 5
Neurotuberculosis.
All registered patients diagnosed as having tuberculosis were treated in Pulmonology and Infections Disease OPD under Department of Pediatrics, JNMCH AMU Aligarh.
Statistical analyses
Data entry, analysis, and tabulation were carried out using SPSS 18 software (SPSS Inc., Chicago, Illinois, USA). Analysis of outcome parameters in terms of infection (latent tuberculosis) and disease between contacts of sputum positive and sputum negative patients was done using Student’s t test & chi square test. P value, 95% confidence interval was calculated for various risk factors between affected and unaffected household contacts.
Results
This hospital based, observational study was conducted over a period of 12 months. Total 129 children under the age of 12 years were enrolled as cases. Sixty nine (53.49%) children were in household contact of 25 “sputum smear positive” and 60 (46.51%) children were in household contact of 23 “sputum smear negative” adults with pulmonary tuberculosis (Fig. 1). Maximum number of patients belonged to age group 6-12 years followed by 3-6 and 0-3 years in that order (Table S2). Mean age was 5.9 ±3.1 years and male to female ratio was 1.15:1.
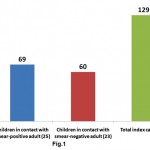 |
Figure 1: Distribution of children viz a viz sputum smear status of adult: Total number of children that are in contact with sputrum positive and negative adults are shown. ( index casesa re given in parenthesis) |
Table 1: Nutritional status of children as suggested by WHO in 1995 (9)
| Variables | Moderate under nutrition | Severe under nutrition |
| Symmetrical edema | No | Yes edematous malnutrition |
| Weight for height (measure of washing) | SD score -2 to -3 (70-79% of expected) wasting | SD score < -3 (<70% of expected) Severe wasting |
| Height for age (measure of stunting) | SD score -2 to -3 (85-89% of expected) Stunting | SD score < -3 (<85% of expected) Severe stunting |
Table 1 shows demographic characteristic as a whole. Out of 129 children, 78 were under six and remaining were over six years of age. Male female ratio was almost equal in both the groups. Of total, 72.86% cases were undernourished, almost 40% were unvaccinated and nearly half of total children belong to low socio-economic strata of the society. There was no statistically significant difference between demographic characteristics of household contacts of two groups of adults except that significantly more number of children from low socioeconomic strata were in contact of sputum smear positive adult (P<0.05). Forty children were Mantoux positive (31%), of which 2/3rd was in contact with sputum positive and 1/3rd in contact with sputum smear negative adults (Fig. 2). In individual age group, maximum numbers of Mantoux positive children were seen in age groups of 6-12 years followed by 3-6 and 0-3 years in that order (Table 2). Male children (40.37%) that were in contact sputum positive adult were found to be infected with Mtb. Surprisingly, 22.58% children were found to be infected in spite of being in contact with sputum negative adults (P<0.05).
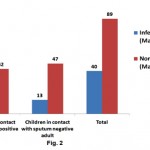 |
Figure 2: Distribution of infected children against sputum smears status of adult: children that are in contact with sputrum positive and negative adults were screened for Mtx Click here to view figure |
Table 2: Different age groups of children (male and female)
| AGE | MALE | FEMALE | TOTAL |
| 0-3 YRS | 16 (53.33%) | 14 (46.67%) | 30 (23.26%) |
| 3-6 YRS | 24 (50.00%) | 24 (50.00%) | 48 (37.21%) |
| 6-12 YRS | 29 (56.86%) | 22 (43.14%) | 51 (39.53%) |
| TOTAL | 69 (53.49%) | 60 (46.51%) | 129 (100%) |
A total of 55% of Mantoux positive children were vaccinated; 38.64% of these children were in contact with sputum positive adults whereas only 14.29% of vaccinated (P<0.05) were in contact with sputum negative adults. Similar behaviour was not noted in unvaccinated children. In normal and moderately undernourished children, the Mantoux positivity was more than twice in sputum smear positive group than sputum smear negative group (19.05 vs. 7.14 for normal nourished children & 50.00 vs. 22.22 for moderately undernourished children) (Table 2), whereas in severely undernourished children there was no difference. There was a preponderance of lower socio-economic strata in sputum smear negative group but there was no reflection on Mantoux status on either group. 63.89% of symptomatic children living in contact of sputum positive adult, were found to be Mantoux positive as compared to 31.03% in contact of sputum smear negative adult (P<0.05). Manifestation of disease in the children of adult contacts was performed by clinical examination and investigation was done as per guidelines.17 Mantoux positive children were further screened for the presence of tuberculosis. X-ray chest showed hilar adenopathy (Fig. S1A), consolidation (Fig. S1B), miliary shadow (Fig. S1C) and progressive pulmonary disease (Fig. S1D). FNAC of children showing lymphadenopathy exhibited granulomatous lesion (Fig. S2A). AFB sputum was also done wherever feasible, to demonstrate the presence of acid fast bacilli as shown in Fig. S2B.
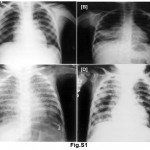 |
Figure S1: (A) Shows chest Radiograph reveals bilateral hilar adenopathy (PPC), (B) consolidation in right lower lobe (PPD), (C) bilateral, diffuse, tiny, discrete, pinpoint opacities (miliary tuberculosis) and in (D) bilateral extensive bronchopneumonic tuberculosis, more marked on the right side has clearly distinguished. Click here to view figure |
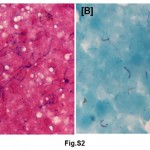 |
Figure S2: (A) H & E stain shows caseous necrotic material, epithelioid cell granuloma and (B) (D) rod shaped well defined tubercle bacilli stained with AFB stain. Click here to view figure |
Out of total 129 children studied, 30 (23%) were diseased and out of them 3/4th were in contact with sputum smear positive and 1/4th in contact with sputum smear negative cases (Fig. 3A). As shown in Table 3, almost 40% diseased children under the age of six (16 children out of 40) were in contact of sputum smear positive adult as compared to 5.26% children that were in contact of sputum negative adult (P<0.05). The ratio of diseased female children that were in contact with sputum positive adult as compared to those in contact of sputum negative adult was 3:1 (32.26% vs 10.34%) (P<0.05). About 50% of vaccinated children developed disease, of which 29.55% were in contact of sputum positive adult, whereas only 5.71% of vaccinated contacts of sputum negative were diseased (P<0.05). The same is not noted in unvaccinated children. About 23.81% of children with normal nutritional status and in contact of sputum positive adult were diseased, but no one was diseased in sputum smear negative adult; whereas in severely undernourished children the ratio was 3:2 in sputum positive and negative group respectively. Almost equal percentage of children from low socio-economic strata was affected in both type of contact. Children not from low socio-economic strata were significantly diseased in contact of sputum smear positive contacts (P<0.001). Of symptomatic children, 55.56% diseased children were in contact of sputum positive adult as compared to 20.69% in contact of sputum smear negative adult (P<0.05). It was observed that age < 3 years, low socio-economic status, severe under nutrition, symptomatic children and contact with sputum smear positive adults significantly increase the disease rate in children (Table 4). Children are likely to be diseased in presence of one or more of these factors.
Table 3: Demographic characteristics of children in household contact with adult patients
| Variables | contacts of sputum positive patients n=69 | contacts of sputum negative patients n=60 | Total N=129 |
Z score | p Value | |
| Age | <6yrs | 40 (51.28%) | 38 (48.72%) | 78 (60.47%) | 0.58 | NS |
| >6yrs | 29 (56.86%) | 22 (43.14%) | 51 (39.53%) | 0.58 | NS | |
| Nutritional status | Normal | 21 (60.00%) | 14 (40.00%) | 35 (27.13%) | 0.37 | NS |
| Mod under nutrition | 36 (50.00%) | 36 (50.00%) | 72 (55.81%) | 0.91 | NS | |
| Severe under nutrition | 12 (54.55%) | 10 (45.45%) | 22 (17.05%) | 0.00 | NS | |
| Gender | Male | 38 (55.07%) | 31 (44.93%) | 69 (53.49%) | 0.34 | NS |
| Female | 31 (51.67%) | 29 (48.33%) | 60 (46.51%) | 0.34 | NS | |
| BCG Status | Vaccinated | 44 (55.70%) | 35 (44.30%) | 79 (61.24%) | 0.68 | NS |
| Unvaccinated | 25 (50.00%) | 25 (50.00%) | 50 (38.76%) | 0.68 | NS | |
| S-E Back Ground | Low Socio Economic | 38 (64.41%) | 21 (35.59%) | 59 (45.74%) | 2.30 | <0.05 |
| Above Low Socio Economic | 31 (44.29%) | 39 (55.71%) | 70 (54.26%) | 2.30 | NS | |
Table 4: Demographic characteristics of Mtx +ve children viz a viz sputum smear status of source
|
Variables |
Sputum +ve Adult | Sputum –ve Adult | Total N=40 | p Value | |||
| total | Mtx +ve | total | Mtx +ve | ||||
| Age | 0-3 YRS N=30 |
17 | 6 (35.29%) | 13 | 2(15.38%) | 8 (20.00%) | NS |
| 3-6 YRS N=48 |
23 | 8 (34.78%) | 25 | 3 (12.00%) | 11 (27.50%) | NS | |
| 6-12 YR N=51 |
29 | 13 (44.83%) | 22 | 8 (36.36%) | 21 (52.50%) | NS | |
| Gender | Male N=69 |
38 | 18 (47.37%) | 31 | 7 (22.58%) | 25 (62.50%) | 0.05 |
| Female N=60 |
31 | 9 (29.03%) | 29 | 6 (20.69%) | 15 (37.50%) | NS | |
| BCG status | Vaccinated N=79 |
44 | 17 (38.64%) | 35 | 5 (14.29%) | 22 (55.00%) | 0.05 |
| Unvaccinated N=50 |
25 | 11 (44.00%) | 25 | 7 (28.00%) | 18 (45.00%) | NS | |
| Nutritional status | Normal N=35 |
21 | 4 (19.05%) | 14 | 1 (7.14%) | 5 (12.50%) | NS |
| Mod Under nutrition N=72 |
36 | 18 (50.00%) | 36 | 8 (22.22%) | 26 (65.00%) | 0.05 | |
| Severe Under nutrition N=22 |
12 | 5 (41.67%) | 10 | 4 (40.00%) | 9 (22.50%) | NS | |
| Socio-economic back ground | Low Socio Economic Status N=59 |
38 | 18 (47.37%) | 21 | 8 (38.10%) | 26 (65.00%) | NS |
| Above Low Socio Economic Status N=70 |
31 | 9 (29.03%) | 39 | 5 (12.82%) | 14 (35.00%) | NS | |
| Clinical features | Symptomatic N=65 |
36 | 23 (63.89%) | 29 | 9 (31.03%) | 32 (80.00%) | 0.05 |
| Asymptomatic N=64 |
32 | 4 (12.90%) | 32 | 4 (12.90%) | 8 (20.00%) | NS | |
A large proportion of Mantoux positive as well as Mantoux negative children were not diagnosed with TB. In total, 99 non-tubercular cases were found, of which 78 cases were found in Mantoux negative children and 21 in Mantoux positive children. A total of 40 children were diagnosed with various form of TB, in which 19 were Mantoux positive and 11 were Mantoux negative (Fig. 3B). The types of tuberculosis seen at the time of study were progressive pulmonary disease (n=8), tubercular lymphadenitis (n=8) asymptomatic Mantoux positive (n=6), pulmonary primary complex (n=3), disseminated disease (n=2), tubercular meningitis (n=2) and miliary tuberculosis (n=1) (Table 5). Statistically, insignificant differences were found in Mantoux status of the children that was irrespective of sputum smear status of adult. However, significant number of children that were in contact of sputum smear positive adult developed disease as compared to those that remain in contact with sputum smear negative adult (P<0.05) (Fig. 3A).
 |
Figure 3: (A) Sputum status of adult against infection and diseased status in children living in contacts of those adults. (B) showing presence or absence of tuberculosis in children with relation to their Mantoux status. Click here to view figure |
Discussion
Tuberculosis is a progressive disease that spreads by air to air transmission. Individuals that are repeatedly in close contact with active TB afflicted person are at great risk of developing the disease. Thus, crowded places such as jam-packed quarters, market, theatres, closed work space, and urban living increase the chances of acquisition of Mtb.18, 19 Clinical features of the individual with pulmonary TB that augment the risk of contacts becoming infected include cavitary disease, sputum smear grade, and extent of ailment based on X-ray.20-23
Earlier studies showed that household contacts of individual with TB pose greater risk of infection than the general population.24-26 This was confirmed by several recent studies conducted among children in New York City27 Botswana28, and Brazil29, in which contact with a person with active pulmonary TB came out as one of the strongest risk factor for TB infection. Several risk factors such as lower socio-economic status, demography and age of the children, have also been recognized that contribute to the transmission of tuberculosis infection among household contacts. Some of these have been previously described and confirmed by our study.30 Our study has shown that sputum positivity, low socio economic status and less than 3 years of age exhibited strong association with infection as well as disease.
The first and foremost factor is malnutrition that seems to play a dual role in the spread of tuberculosis (Tables 3 & 4). Malnourished children are at greater risk of developing the disease due to their weakened immune state. Infection itself can result in failure to thrive bringing the child to a malnourished state;31 this further establishes the infection leading to a fully developed diseased state. Moreover, malnourishment suppresses the hypersensitivity to tuberculin testing, interfering with accurate elucidation of the result; ultimately resulting in false negative outcomes. Thus, these individuals may in fact be diseased while not showing up on the tuberculin test radar. Our observations are in accordance with reports that have not found a significant difference in the prevalence of positive tuberculin test among malnourished compared to normally nourished children;32 suggesting that a large number of malnourished children are able to respond to the tuberculin test. Our findings also suggest that the cut-off of 10 mm induration is applicable even in severely malnourished children. Most of Mantoux positive children in our study were having symptoms (63.89%) at the time of screening in contrast to other studies,33 possibly because most of the adults may be diagnosed late during which children go from asymptomatic Mantoux positive to symptomatic Mantoux positive state.
Children that live in close contact of Mtb infected individual follow the same epidemiology as that in adults. Infected children can act as reservoir of latent TB infection from where new cases can arise. Thus, Mtb distribution in children can be taken as an indicator of the TB transmission pattern in a community as a whole. This applies to both sputum positive and sputum negative cases, as we have shown here that sputum negative cases can act as carrier (Fig. 3A). Our study has shown that about one third (31%) of children of household contact with adult TB patients are infected with the organism and about 23.25% are suffering from the deadly disease. This is a significant assessment, particularly for a country where about one-third of the population below the age of 12 years illustrates a positive tuberculin test.34 In a previous study, prevalence of tuberculosis was shown to be 31%.33 This could be due to the inclusion of only contacts of sputum smear positive cases and their high prevalence. From our study, it can be inferred that the proportion of infected individuals acquiring the disease is far less. Among other factors, high BCG vaccination coverage could be a possible reason. However, almost half of Mantoux positive children were unvaccinated; so one can rule out this possibility. Probably some other factors such as socio-economic status, demography and age could explain the above observed result. Although there has been a decline in tuberculosis mortality rates, most global tuberculosis incidence cases and deaths in individuals are in men and boys.39
It has been shown that the children in contact with sputum positive patients have a higher prevalence of tuberculosis. They form a major pool of susceptible carriers that can in turn lead to the spread of the disease. However, there is no published information on transmission studied in a prospective fashion. In this study, we found that sputum positive index cases (adults) transmit infection as expected (Fig. 2). However, it is likely that sputum negative cases harbor the pathogen and can transmit it to healthy persons; although the spreading frequency is low. In our case, we observed that the number of infected and diseased children in contact with sputum smear negative adults is not negligible; hence this group should not be ignored (Fig. 2 and Fig. 3A). This is in accordance with works from previous studies30, 35, 36 and puts an end to the older notion that only “open cases” (sputum positive) can transmit infection. Among children with positive tuberculin test, the ratio of those that were in contact with sputum positive individuals to those in contact with sputum negative cases was 1.5:1. Likewise, the incidence of disease is almost three times higher in the former. This differs from study that has shown the ratio to be 7:1.30 The reason may be that we had more number of undernourished children among sputum smear negative contacts.
It is generally acceptable that tuberculin test positivity reflects infection in children. However, in our study a large portion of Mantoux positive children were found to be asymptomatic. This can be explained on the basis of the fact that a small proportion of mantoux positive children were diagnosed to be non-tubercular (Fig. 3B). In recent years, there has been an increase in the number of children admitted with both pulmonary and extra-pulmonary tuberculosis during the past decade. Studies have reported a much higher rate of TB (65%) in patients with extra-pulmonary disease, consistent with our results.40 A study conducted by Jain et al41 has showed that a large proportion (46%) had extra-pulmonary disease, which was found to be predominantly meningeal. In our case, tubercular lymphadenitis, progressive pulmonary disease and latent tuberculosis were the main culprits of non-tubercular disease (Table 5).
Table 5: Possible risk factors affecting prevalence of tuberculosis among children in house hold contact with adult patients
| Variables | Tubercular | Non Tubercular | P Value | Odd ratio | Chi square | 95% CI |
| Age < 3 Years (N =30) |
11 | 19 | <0.05 | 2.438 | 3.94 | 0.951-5.965 |
| Severe Under nutrition (N=22) | 11 | 11 | <0.001 | 4.632 | 10.6 | 1.752-12.243 |
| Contact with Sputum positive adult(N=69) | 23 | 46 | <0.01 | 3.786 | 8.4 | 1.573-10.166 |
| Low Socio Economic Status (N=59) | 19 | 40 | <0.05 | 2.548 | 4.9 | 1.094-5.929 |
| Symptomatic children (N=65) | 26 | 39 | <0.001 | 10 | 10 | 3.241-30.877 |
Table 6: Disease pattern versus nature of contact
| Type of tuberculosis | Smear Sputum +VE | Smear Sputum -VE | Total
% |
| Asymptomatic Mantoux positive | 5 | 1 | 6(20.00) |
| Pulmonary primary complex | 2 | 1 | 3(10.00) |
| Progressive pulmonary disease | 6 | 2 | 8(26.67) |
| Miliary tuberculosis | 1 | 0 | 1(3.32) |
| Tubercular lymphadenitis | 6 | 2 | 8(26.67) |
| Disseminated disease | 1 | 1 | 2(6.67) |
| Tubercular meningitis | 2 | 0 | 2(6.67) |
Table 7: Demographic characteristics of diseased children viz a viz sputum smear status of source
|
Variables |
Sputum +ve Adult | Sputum –ve Adult | Total N=30 | p Value | |||
| total | Diseased | total | Diseased | ||||
| Age | 0-3 Years N=30 |
17 | 9 (52.94%) | 13 | 2 (15.38%) | 11 (36.67%) | 0.05 |
| 3-6 Years N=48 |
23 | 7 (30.43%) | 25 | 0 (0.00%) | 7 (23.33%) | 0.05 | |
| 6-12 Years N=51 |
29 | 7 (24.14%) | 22 | 5 (22.73%) | 12 (40.00%) | NS | |
| Gender | Male N=69 |
38 | 13 (34.21%) | 31 | 4 (12.90%) | 17 (56.67%) | NS |
| Female N=60 |
31 | 10 (32.26%) | 29 | 3 (10.34%) | 13 (43.33%) | 0.05 | |
| BCG status | Vaccinated N=79 |
44 | 13 (29.55%) | 35 | 2 (5.71%) | 15 (50.00%) | 0.05 |
| Unvaccinated N= 50 |
25 | 10 (40.00%) | 25 | 5 (20.00%) | 15 (50.00%) | NS | |
| Nutritional status | Normal N=35 |
21 | 5 (23.81%) | 14 | 0 (0.00%) | 5 (16.67%) | NS |
| Mod Under nutrition N=72 |
36 | 11 (30.56%) | 36 | 3 (8.33%) | 14 (46.67%) | 0.05 | |
| Severe Under nutrition N=22 |
12 | 7 (58.33%) | 10 | 4 (40.00%) | 11 (36.67%) | NS | |
| Socio-economic back ground | Low Socio Economic Status N=59 |
38 | 13 (34.21%) | 21 | 6 (28.57%) | 19 (63.33%) | NS |
| Above Low Socio Economic Status N=70 |
31 | 10 (32.26%) | 39 | 1 (2.56%) | 11 (36.67%) | 0.001 | |
| Clinical features | Symptomatic N=65 |
36 | 20 (55.56%) | 29 | 6 (20.69%) | 26 (86.67%) | 0.01 |
| Asymptomatic N=64 |
33 | 3 (9.09%) | 31 | 1 (3.23%) | 4 (13.33%) | NS | |
It is noteworthy that a significant portion (36%) of diseased children was Mantoux negative. Two implications can be made based on this observation. Firstly, children that failed to react to tuberculin can also develop tuberculosis; therefore this group should also be screened for the presence of tuberculosis by other investigations. A previous report identified a small number of children with culture proven tuberculosis that did not have severe disease but consistently failed to react to tuberculin for no apparent reason.37 This was subsequently reinforced by another study38 as well this study. One possible reason for this could be due to underdeveloped immune sensitivity to the tuberculin in children. Secondly, Mantoux cannot be taken as a gold standard to rule out tuberculosis. Therefore, a strategy of screening only those in contact with sputum positive cases is likely to miss about one third of infected individuals. This advocates the need of a more appropriate screening strategy with the inclusion of all household contacts of persons diagnosed with Mtb infection. The base of contact survey has to be widened under ongoing RNTCP (Revised National Tuberculosis Control Program) agenda of Govt. of India, if children have to be brought in the main stream of “STOP TB STRATEGY”.
An additional aspect that needs to be evaluated is the role of these sputum negative children in further spread of disease, since they are believed not to contribute significantly to the transmission of infection and are usually ignored. This feature needs to be seriously looked at and is an avenue for further research. A possible explanation for the transmission of infection from sputum negative patients is that they may not be negative in the strict sense of the term. A sputum sample must contain over 10,000 AFB per ml to be detected on Ziehl-Nielsen staining, whereas culture of specimens has been able to detect the organism even when bacillary density is as low as 100 per ml.
Major drawback of this study is that we did not perform interferon-γ response assays (IGRA), on the subjects to ascertain Mtb infection status. IGRAs were intended to shun distress of cross-reactivity with BCG and to alleviate concerns about boosting effects after repeated tuberculin test.42 However, the sensitivity and cost-effectiveness of IGRAs in TB-endemic settings is still a topic of debate and concern.43-45 Participants in this study belonged to lower economic status that could not afford the test. Additional studies and follow ups in contacts of sputum positive as well as sputum negative individuals are needed to define the prevalence of tuberculosis in the population.
Conclusion
Sputum positivity has strong association with infection as well as disease nonetheless the number of infected and diseased children in sputum smear negative group is not negligible. Age less than 3 years , low socioeconomic status, severe malnutrition ,symptomatic children and contact with sputum smear positive adults significantly increased the disease rate in children . it is noteworthy that 36.67% of diseased children were Mantoux negative so Mantoux is not a gold standard test to rule out tuberculosis.
Acknowledgements
Authors are highly thankful to Prof. Aziz Khan for his invaluable assistance in carrying out the statistical analysis for this manuscript.
Author contributions
Conflict of interest
The authors declare that there is no conflict of interests.
Funding
This research received no specific grant from any funding agency in the public, commercial or not for- profit sectors.
Compliance with ethical standards
The research involved human participants. All procedures performed were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from parents/guardians of children in the household, and approval was taken from the Human Ethics Committee of the Faculty of Medicine, AMU, Aligarh. Complete confidentiality was ensured and was maintained throughout the study.
References
- Mohajan, HK. Tuberculosis is a Fatal Disease among Some Developing Countries of the World. American Journal of Infectious Diseases and Microbiology 2015; 3(1) : 18-31.
- Seddon JA and Shingadia D. Epidemiology and disease burden of tuberculosis in children: a global perspective. Infection and Drug Resistance. 2014; 7: 153-65.
CrossRef - World Health Statistics report 2015. WHO Press, World Health Organisation, Geneva Available at http://www.who.int/gho/publications/world_health_statistics/2015/en/
- Global Tuberculosis Report 2016. WHO Press, World Health Organisation, Geneva, Available at http://www.who.int/tb/publications/global_report/en/
- Raviglione MC, Snider DE and Kochi A. Global epidemiology of tuberculosis. Morbidity and mortality of a worldwide epidemic. JAMA. 1995; 273: 220-6.
CrossRef - Arora VK and Gupta R. Directly observed treatment for tuberculosis. Indian J Pediatr. 2003; 70: 885-9.
CrossRef - Chadha VK, Jagannatha PS, Nagaraj AV, Narayana Prasad D and Anantha N. A comparative study of tuberculin reactions to 1TU and 1TU of PPD- RT23. Indian J Tuberc. 2000; 47: 15-20.
- Chadha VK, Kumar P, Gupta J, et al. The annual risk of tuberculous infection in the eastern zone of India. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2004; 8: 537-44.
- An evaluation of infant growth: the use and interpretation of anthropometry in infants. WHO Working Group on Infant Growth. Bull WHO. 1995; 73: 165-74.
- Sharma R. Revision of Prasad’s social classification and provision of an online tool for real-time updating. South Asian Journal of Cancer. 2013; 2: 157.
CrossRef - Jordan TJ, Sunderam G, Thomas L and Reichman LB. Tuberculin Reaction Size Measurement by the Pen Method Compared to Traditional Palpation. Chest. 1987; 92: 234-6.
CrossRef - Kline TS, Kannan V and Kline IK. Lymphadenopathy and aspiration biopsy cytology. Review of 376 superficial nodes. Cancer. 1984; 54: 1076-81.
CrossRef - Bailey TM, Akhtar M and Ali MA. Fine needle aspiration biopsy in the diagnosis of tuberculosis. Acta Cytol. 1985; 29: 732-6.
- Ahuja GK, Mohan KK, Prasad K and Behari M. Diagnostic criteria for tuberculous meningitis and their validation. Tubercle and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 1994; 75: 149-52.
CrossRef - Jain R, Sawhney S, Bhargava DK and Berry M. Diagnosis of abdominal tuberculosis: sonographic findings in patients with early disease. AJR American journal of roentgenology. 1995; 165: 1391-5.
CrossRef - Kedar RP, Shah PP, Shivde RS and Malde HM. Sonographic findings in gastrointestinal and peritoneal tuberculosis. Clinical Radiology. 1994; 49: 24-9.
CrossRef - Treatment of childhood tuberculosis: Consensus Statement of IAP Working Group. Indian Academy of Pediatrics. Indian Pediatrics. 1997; 34: 1093-6.
- Guwatudde D, Nakakeeto M, Jones-Lopez EC, et al. Tuberculosis in Household Contacts of Infectious Cases in Kampala, Uganda. Am J Epidemiol. 2003; 158: 887-98.
CrossRef - Chapman JS and Dyerly MD. Social and other factors in intrafamilial transmission of tuberculosis. The American Review of Respiratory Disease. 1964; 90: 48-60.
- Lienhardt C, Fielding K, Sillah J, et al. Risk factors for tuberculosis infection in sub-Saharan Africa: a contact study in The Gambia. Am J Respir Crit Care Med. 2003; 168: 448-55.
CrossRef - Lienhardt C, Sillah J, Fielding K, et al. Risk factors for tuberculosis infection in children in contact with infectious tuberculosis cases in the Gambia, West Africa. Pediatrics. 2003; 111: e608-14.
CrossRef - Almeida LM, Barbieri MA, Da Paixão AC and Cuevas LE. Use of purified protein derivative to assess the risk of infection in children in close contact with adults with tuberculosis in a population with high Calmette-Guérin bacillus coverage. The Pediatric Infectious Disease Journal. 2001; 20: 1061-5.
CrossRef - Comstock GW. Epidemiology of tuberculosis. The American Review of Respiratory Disease. 1982; 125: 8-15.
- Andersen S and Geser A. The distribution of tuberculous infection among households in African communities. Bull WHO. 1960; 22: 39-60.
- Narain R, Nair SS, Rao GR and Chandrasekhar P. Distribution of tuberculous infection and disease among households in a rural community. Bull WHO. 1966; 34: 639-54.
- Grzybowski S, Barnett GD and Styblo K. Contacts of cases of active pulmonary tuberculosis. Bulletin of the International Union Against Tuberculosis. 1975; 50: 90-106.
- Saiman L, San Gabriel P, Schulte J, Vargas MP, Kenyon T and Onorato I. Risk factors for latent tuberculosis infection among children in New York City. Pediatrics. 2001; 107: 999-1003.
CrossRef - Joel DR, Steenhoff AP, Mullan PC, et al. Diagnosis of paediatric tuberculosis using sputum induction in Botswana: programme description and findings. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2014; 18: 328-34.
CrossRef - Stevens H, Ximenes RAA, Dantas OMS and Rodrigues LC. Risk factors for tuberculosis in older children and adolescents: a matched case–control study in Recife, Brazil. Emerging Themes in Epidemiology. 2014; 11: 20.
CrossRef - Singh M, Mynak ML, Kumar L, Mathew JL and Jindal SK. Prevalence and risk factors for transmission of infection among children in household contact with adults having pulmonary tuberculosis. Arch Dis Child. 2005; 90: 624-8.
CrossRef - Jesson J and Leroy V. Challenges of malnutrition care among HIV-infected children on antiretroviral treatment in Africa. Médecine et Maladies Infectieuses. 2015; 45: 149-56.
CrossRef - Lloyd AVC. Tuberculin Test in Children with Malnutrition. British Medical Journal. 1968; 3: 529-31.
CrossRef - Sm N, Aj B, S A, E W and B K. Paediatric Tuberculosis. The Lancet infectious diseases. 2008; 8: 498-510.
CrossRef - Park’s Textbook of Preventive and Social Medicine by K. Park — Reviews, Discussion, Bookclubs, Lists.
- Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet. 1999; 353: 444-9.
CrossRef - Underwood BR, White VLC, Baker T, Law M and Moore-Gillon JC. Contact tracing and population screening for tuberculosis–who should be assessed? Journal of Public Health Medicine. 2003; 25: 59-61.
CrossRef - Steiner P, Rao M, Victoria MS, Jabbar H and Steiner M. Persistently negative tuberculin reactions: their presence among children with culture positive for Mycobacterium tuberculosis (tuberculin-negative tuberculosis). American Journal of Diseases of Children (1960). 1980; 134: 747-50.
CrossRef - Seth V, Singhal PK, Semwal OP, Kabra SK and Jain Y. Childhood tuberculosis in a referral centre: clinical profile and risk factors. Indian Pediatrics. 1993; 30: 479-85.
- Murray CJL, Ortblad KF, Guinovart C, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet. 2014; 384: 1005-70.
CrossRef - Shah I and Chilkar S. Clinical profile of drug resistant tuberculosis in children. Indian Pediatrics. 2012; 49: 741-4.
CrossRef - Jain SK, Ordonez A, Kinikar A, et al. Pediatric Tuberculosis in Young Children in India: A Prospective Study, Pediatric Tuberculosis in Young Children in India: A Prospective Study. BioMed Research International, BioMed Research International. 2013; 2013, 2013: e783698.
CrossRef - Menzies D. Interpretation of repeated tuberculin tests. Boosting, conversion, and reversion. Am J Respir Crit Care Med. 1999; 159: 15-21.
CrossRef - Machingaidze S, Wiysonge CS, Gonzalez-Angulo Y, et al. The utility of an interferon gamma release assay for diagnosis of latent tuberculosis infection and disease in children: a systematic review and meta-analysis. The Pediatric Infectious Disease Journal. 2011; 30: 694-700.
CrossRef - Menzies D, Pai M and Comstock G. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann Intern Med. 2007; 146: 340-54.
CrossRef - Oxlade O, Schwartzman K and Menzies D. Interferon-gamma release assays and TB screening in high-income countries: a cost-effectiveness analysis. The International Journal of Tuberculosis and Lung Disease: The Official Journal of the International Union Against Tuberculosis and Lung Disease. 2007; 11: 16-26.

This work is licensed under a Creative Commons Attribution 4.0 International License.





