How to Cite | Publication History | PlumX Article Matrix
Aiman Al-Qtaitat1* , Sinan S Farhan2
, Sinan S Farhan2  , Aiman Al-Maathidy1
, Aiman Al-Maathidy1  , Ghadeer Almuhaisen3
, Ghadeer Almuhaisen3  and Jihad Alzyoud4
and Jihad Alzyoud4 
1Department of Anatomy and Histology, Faculty of Medicine, Mutah University, Karak, Jordan
2Department of Pharmacy, Al-Rafidain University College, Baghdad, Iraq
3Department of Physiology and Pathology, Faculty of Medicine, Mutah University, Karak, Jordan
4Faculty of Allied Health Sciences, The Hashemite University, Zarqa, Jordan
Correspondent Author’s Email: aimanaq@mutah.edu.jo
DOI : http://dx.doi.org/10.13005/bbra/2778
ABSTRACT: Monosodium glutamate (MSG) has been recognized as flavor enhancer that adversely affects male reproductive systems. The present study was designed to investigate the potential protective effects of pomegranate juice on MSG induced histopathological changes in the seminiferous tubules of rats. Fifty adult male albino rats were divided into five groups of ten rats each; Group I (Control group), received daily standard diet only for one month. Group II (Pomegranate group), received daily pomegranate juice only for one month. Group III (MSG group), received daily a single dose of 60 mg/kg body weight of MSG for one month. Group IV (MSG and Pomegranate group), received daily a single dose of 60 mg/kg of MSG concomitant with pomegranate juice for one month. Group V (MSG withdrawal group), received daily a single dose of 60 mg/kg body weight of MSG for one month then leaved for another one month. The testis was subjected to histological study, using light and electron microscopes, and the cauda epididymis was used for caudal sperm count. Results: MSG induced toxicity in testicular tissues. Pomegranate juice resulted in improving the MSG induced changes, and it had the ability to increase sperms number and to reduce sperms abnormalities. Supplementation of pomegranate juice could ameliorate the MSG induced testicular toxicity. Thus, it could have a role in improving male fertility.
KEYWORDS: Monosodium Glutamate; Punica Granatum; Pomegranate Juice and Seminiferous Tubules; Sperm; Testicular Morphology
Download this article as:| Copy the following to cite this article: Al-Qtaitat A, Farhan S. S, Al-Maathidy A, Almuhaisen G, Alzyoud J. Potential Protective Effect of Pomegranate (Punica Granatum) Juice on Monosodium Glutamate Induced Seminiferous Tubules Changes in Adult Male Albino Rats: Histological Study. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Al-Qtaitat A, Farhan S. S, Al-Maathidy A, Almuhaisen G, Alzyoud J. Potential Protective Effect of Pomegranate (Punica Granatum) Juice on Monosodium Glutamate Induced Seminiferous Tubules Changes in Adult Male Albino Rats: Histological Study. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2PzyQSe |
Introduction
Consumption of healthy and nutritious diet is important to enjoy a good quality of life. Food additives, natural or artificial, is a common practice in food industry worldwide to amplify the taste and flavor so making food edible, pleasurable and attractable.1 Monosodium glutamate (MSG), a glutamic acid in the form of sodium salt, is a food additive flavor that has characteristic umami taste described by Ikeda 1908 and it is manufactured by bacterial fermentation of sugar cane, sugar beet or molasses.2 MSG is classified as “generally recognized as safe” without a specific acceptable daily intake (ADI) by several reputable international organizations such as the food and drug administration (FDA) in the United States, European Commission’s, food and agriculture organization (FAO).3 However, since the potential role of MSG in the “Chinese Restaurant Syndrome (CRS)” this recognized safety has been questioned by researchers. Moreover, ensured safety might encourage MSG overuse, intentionally or unintentionally, in human diet which needs to highlight its potential harmful effect and ensured it is used wisely.4-6 Additionally, safety concerns, in the form of several adverse effects seen in animal studies investigating MSG effect on different organs, haven’t been seen in human clinical trials.7, 8
Several MSG adverse effects have been revealed in different in vivo animal studies and in clinical trials.9 MSG is a source of glutamate which is an important neurotransmitter in central nervous system10 when taken in excess might result in neurological toxicity.11 An in vitro study revealed nerve cell death caused by higher MSG doses.12 Abnormal liver changes were observed in rats after higher doses of MSG for six-week,13 also in mice following ten weeks treatments.14 A two-month study on rats treated with MSG showed changes in testis seminiferous tubules and interstitial tissue.15 Adult rats kidney showed some toxic effect following MSG supplementation.16 Another study on rats showed that MSG result in changes in fertility parameters such as testicular tissue and sex related hormones after six weeks.17 A one-month duration of low doses of MSG did not result in variations in sex hormones and antioxidant hormones in rodents.18 In female rats, MSG supplementation has showed some degenerative changes affecting the oviduct.19 While in male rats MSG resulted in an increased abnormal sperms and reduced its count.20 Another study on dwarf goats showed decrease sex hormones levels in blood following a 4-week of MSG treatment.21
In human, Jiangsu Nutrition Study (JIN) results showed elevated hemoglobin level in males with increased MSG in their diet.22 Five days of high MSG doses intake were associated with headache, nausea and an increase in blood pressure parameters with no pain in mastication muscles.23 MSG side effects were more apparent when higher doses were administered for long periods in animal studies. In humans such side effects were less apparent due to mode of intake, and the differences in both models (human vs animal), thus, necessitating further research.9 In the China Health and Nutrition Survey (CHNS) a conclusion was made regarding a link between consumption of MSG containing food and the increased in weight.24 Orally taken MSG was associated with symptoms similar to Chinese restaurant syndrome includes, headache, muscle tightness, numbness/tingling, general weakness and flushing.25
A number of products were used to moderate the oxidative effects of MSG on testis, for example, Propolis, a bee product, was investigated for its beneficial influence on male rat testis function and structure against MSG chemotoxicity by moderating the oxidative stress effect.26 The nutritive value of the pomegranate fruit was documented and that a 250 ml glass of pomegranate juice (PJ) provides approximately 50% of an adult’s recommended daily allowance of the vitamins A, C and E. Pomegranate contains a number of polyphenols, including anthocyanins, minor flavonoids and punicalagin, which is the most important member of the ellagitannins family. The punicalagin is the largest polyphenol among the pomegranate ellagitannins and it is responsible for most of the antioxidant activity of the PJ.27 The potent antioxidant effects of polyphenols have been demonstrated in clinical and experimental studies.28, 29 Scientific research has highlighted the relationship between oxidative stress that result in reactive oxygen species (ROS) production and male infertility.30, 31 pomegranate fruit is rich with antioxidant ingredients which could be recommended as protective constituent of a healthy diet against the adverse effect of stressful agents as MSG.32-34
This preclinical animal study was aimed to assess the potential protective effect of PJ against monosodium glutamate induced morphological alterations in adult male rats reproductive system using histology methods and semen analysis.
Material and Methods
Pomegranate Processing
Fresh pomegranates were washed, crushed, and squeezed. The juice was filtered, pasteurized, and stored at -18°C.
Chemicals
Monosodium glutamate crystalline powder (99% NT purity, Sigma chemical Company, ST Louis, MO, USA) was used to prepare a stock solution by dissolving 60 g of MSG crystals in 100 ml of distilled water. A working dose of 60 mg/Kg (15) was administered by orogastric tube to adjust the proper dose and according to the dose schedule per group and as per their respective weight.
Study Design
This study was approved by the Scientific and Ethics Committee at Faculty of Medicine Mutah University. Fifty adult male Sprague-Dawley rats (150-200 gm) were housed in cages under standard laboratory conditions with dark and light cycle. They were permitted free access to standard diet and water ad libitum. In the experiment the animals were randomly divided into 5 groups each has 10 rats: Group I (Normal control): received daily standard diet only for one-month, free access to water with no treatment throughout the study period. Group II (Positive control/Pomegranate juice): received daily pomegranate juice 2 ml / day via gastric gavage only for one month. Group III (MSG treated): received daily a single dose of 60 mg/kg body weight of MSG for one month based on the previously identified toxic dose in vivo. Group IV (MSG and Pomegranate treated / Protected group): received daily a single dose of 60 mg/kg of MSG concomitant with pomegranate juice 2 ml/day for one month. Group V (MSG Withdrawal group): received daily a single dose of 60 mg/kg body weight of MSG for one month then leaved without any treatment for another one month.
All administrations were given orally by gavage. Body weight of each animal was determined at the start of the treatments and at time when sacrificed. The animals were sacrificed by decapitation after ether anesthesia. The cauda epididymis was chipped in 1 ml of normal physiological saline and the clear fluid was used for semen analysis using Neubauer’s hemocytometer at a 1: 19 dilutions. Sperm count, motility, viability, and abnormality were assessed as described in WHO laboratory manual.35 The testicles were dissected out from each rat, weighed then cut into two specimens and subjected to histological and semen analysis. Optical microscopy: specimen was fixed in Boun`s fixative solution and histologically processed to obtain 6µm thick paraffin sections. Tissue sections were dehydrated in an ascending grade of alcohol (ethanol), cleared in xylene ready for staining. Some sections were stained with Haematoxylin and Eosin (H&E) stain and Periodic acid Schiff (PAS) stain for light microscopic examination.36 Other sections were labelled using activated Caspase 3 (apoptotic marker) for Immunohistochemical analysis. Transmition electron microscopy: specimen were immediately fixed in 3% glutaraldehyde solution and each specimen was processed and examined using transmission electron microscope.
Statistical Analysis
Analyses were performed using IBM SPSS Statistics v.19. Biochemical parameters were compared by one way-ANOVA and multiple comparisons were done with Tukey’s Post-hoc tests. P<0.05 was accepted as statistically significant.
Results
Optical Microscopy
Haematoxylin and Eosin (H&E), and periodic acid Schiff (PAS) stained sections of testicular tissue in group I and II revealed normal architecture of seminiferous tubules lined with spermatogenic cells and Sertoli cell that rest on a basal lamina (Figure 1 A&B). The lumen could be easily delineated in almost all tubules and the majority of them are occupied by the late spermatids that acquired darkly stained elongated heads and long tails. Sertoli cells were clearly identified with characteristic oval nuclei (Figure 2 A&B). Group II sections revealed evident increase in the number of sperms in the lumen of the tubules (Figure 1 B). Each tubule is surrounded by normal basal lamina forming the boundary tissue (Figure 2 B). Group III stained sections showed severe to moderate damaged of seminiferous tubules manifested as hyaline material and widening of the spaces between seminiferous tubules. Some seminiferous tubules manifested grade damage that included disorganization of spermatogenesis cells and edematous with congestion of interstitial tissues. Damaged germ cells which separated from the basal lamina and contains vacuoles within the spermatogonia with loss of late spermatids. Sloughing and exfoliation of spermatocytes, spermatids and immature germ cells appeared into the lumen of the seminiferous tubules. Moreover, the tubules were surrounded by thin irregular, destructed and a split basal lamina (Figure 1 C-F and Figure 2 C&D). Group IV revealed more or less normal architecture of seminiferous tubules except focal and mild vacuolation of spermatogenic cells and Sertoli cells. The tubules were surrounded by well-defined basal lamina with evident amelioration of degenerative changes (Figure 1 G&H and Figure 2 E). Group V revealed shrunken, complete destruction and degeneration of spermatogenic cells as evident by loss of germ cells, necrotic tissues were present in most of the seminiferous tubules and vacuolated hyaline material involving interstitial tissue. Destruction of most spermatogenic cell layers with absence of spermatozoa was clearly recognized. The seminiferous tubules contain Sertoli cells only resting on thick and darkly stained basal lamina (Figure 1 I&J and Figure 2 F).
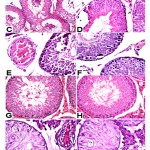 |
Figure 1: Histological sections of rat testis. |
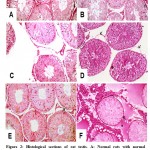 |
Figure 2: Histological sections of rat testis. |
Immunohistochemical
Examination of immunohistochemical stained sections of the MSG treated rats (group III) the relative intensity of activated Caspase – 3 expression was significantly higher in the testicular germ cells, primary spermatocytes and predominantly in the spermatogonia when compared with the control groups (Figure 3). In the protected group (group IV) sections revealed more or less normal reaction as compared with the control group (Figure 3 D). Atrophied tubules lined with Sertoli cell only, and with complete degeneration of spermatogenic cells were seen in the testes of withdrawal group (Figure 3 E).
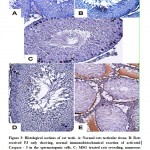 |
Figure 3: Histological sections of rat testis |
Ultrastructural
Ultrastructural examination of the testes of the control rats (Group I) showed that germ cells had cellular characteristics typical of those seen in active spermatogenesis. Sertoli cell of the control rats showed, distinct nuclear and cytoplasmic characteristics consistent with an active secretory state. The nuclei were irregular with deep indentation. Adjacent to the nuclei, the cytoplasm was characterized by cisternae of RER in close association with mitochondria and ribosomes scattered throughout the cytoplasm. The cell membranes showed close association with adjacent spermatocytes and spermatids. The spermatogonia displayed round or oval configuration with prominent nuclei. The adluminal compartment of seminiferous tubules contained round spermatids characterized by well-defined nuclei with distinct nuclear membranes. The lumen of seminiferous tubules revealed normal mature sperms as evident by normal heads and tails structure surrounded with mitochondrial sheath (Figure 4 A1-4). Group II revealed under TEM examination revealed almost similar structure as in control group, but the number of sperms was evidently increased (Figure 4 B1-4). Electromicrograph of MSG treated group (III) showed degenerative changes in seminiferous tubules. The cytoplasm of Sertoli cell showed evident cytoplasmic vacuolation and large lipid droplet. Spermatogonia and spermatocytes showed morphological changes typical of apoptosis as evident by cell shrinkage, excessive chromatin condensation, cytoplasmic vacuolation and organelles were relatively sparse. Abnormal spermatids were also common in monosodium glutamate treated rats as evident by abnormal shape nuclei with acrosomal defects, nuclear degeneration and sparse cytoplasmic organelles. The lumen of the tubules revealed few abnormal sperms surrounded by abnormal mitochondrial sheath (Figure 4 C1-4). MSG and PJ treated group (IV) electron images examination of seminiferous tubules showed a significant improvement as evident by normal Sertoli cells resting on almost normal basal lamina, normal spermatogonia and spermatocytes, normal round spermatids with intact acrosomal cap, and the lumen of the tubules illustrating normal and adequate sperms (Figure 4 D1-4). Withdrawal group ultrastructural examination of seminiferous tubules revealed atrophy of seminiferous tubules lined with Sertoli cell resting on thick irregular basal lamina and filled with vacuolated remnant of degenerated spermatogenic cells (Figure 4 E1-4).
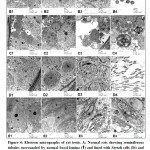 |
Figure 4: Electron micrographs of rat testis |
Semen Analysis
Seminal fluid assessment for sperm morphology, count and motility in different groups is an important step in the evaluation of testicular function and structure in male rats. Analysis showed variations in the sperm count in different groups compared to control group I. An average increase in sperm count was seen in PJ supplemented group (group II) while both MSG treated group (group III) and MSG-withdrawal group (group V) showed a marked significant decrease in sperm count. Supplementing MSG group with PJ (group IV) has improved sperm count albeit very low compared to control group (group I). Statistical significant difference is present between all groups with p value < 0.001). Multiple comparisons showed that Group IV shows statistical significant increase in sperm count compared with groups III and V, with p value < 0.001 (Table 1). Sperm motility results have shown that PJ supplemented group (group II) had increased significantly in sperm motility compared to control (group I), while all groups treated with MSG (group III, IV and V) showed significant decrease in sperm motility, and the difference was statistically significant with p value < 0.001. Sperm tail defect count results showed variations among all groups with the highest increase was among the MSG treated group (III), and the lowest score was among the MSG group supplemented with PJ (group IV). However, there was no significant difference between all groups with (Table 1). Sperm vitality results showed significant variations among all groups with highest values among PJ supplemented rats (II) and the lowest was among MSG treated groups (III, IV, V). PJ supplemented MSG group (IV) showed better values compared with MSG model groups, and a significant difference was measured between all groups with value < 0.001 (Table 1). It can be seen that histological findings were supported by seminal fluid assessment in the form of abnormal shape and significant decreased in number of sperms in both MSG treated groups, p value < 0.001 (Table 1).
Table 1: Sperms structure and function scores in different studied groups.
| Sperm character | I- Control | Group II (PJ) | Group III (MSG) | Group IV (MSG&PJ Protected) | Group V MSG withdrawal |
| Sperm Count | 533 ± 221.4 | 905 ± 90.1 | 5.6 ± 4.7* | 43.2 ± 2.7* | 0.51 ± 0.7* |
| Motile Sperms | 10.8 ± 2.4 | 13.7 ± 2.4 | 1.4 ± 1.6* | 2.0 ± 1.6* | 1.1 ± 1.1* |
| Sperm Tail Defect | 17.6 ± 3.0 | 20.5 ± 2.2 | 31.5 ± 23.1 * | 12.9 ± 2.2 | 22.0 ± 23.4 |
| Sperm Vitality | 76.3 ± 4.3 | 71.4 ± 3.8 | 4.1 ± 3.0* | 15.9 ± 2.7* | 4.4 ± 4.9* |
| Normal Sperms | 17.7 ± 2.3 | 23.0 ± 5.1 | 1.8 ± 1.6* | 4.8 ± 1.3* | 0.9 ± 1.1* |
Values are means ± SD (n=10). *: significant at p < 0.001 compared to control. Φ: X103.
Discussion
MSG has been reported to cause toxic effects on several organs functions such as ovary, testis, brain, pancreas, kidney and liver, however, these effects varies in different studies depending on the route of administration, dosage and animal model used.37 Effects include metabolic disturbances in different chemicals as serum insulin, fatty acids and triglycerides, transaminases and pathological changes in ovaries and fallopian tube and testis.9, 13-18, 20, 21 In human, MSG intake was associated with mild disturbances contrary to preclinical animal studies.38 Histological findings in this study were comparable with other studies that were carried out on the testes of different animals treated with MSG. For example, administration of MSG for two months in rats caused degeneration in testicular tissue.15 MSG six weeks consumption produced changes in fertility parameters, such as testicular tissue and sex related hormones.17 A one-month duration of low doses of MSG did not result in variations in sex hormones and antioxidant hormones in rodents.18 Also, in male rats MSG resulted in increased abnormal sperms and reduced its count.20 Our results showed very low sperm count which could be explained in part by direct toxic effect of MSG and in part by a decrease testosterone production which lead into decrease spermatogenesis.39 MSG toxic effect over testicular tissue was suggested to be mediated by three pathways; neural pathway including hypothalamus and pituitary gland that lead to disturbance in sex hormone production40 or via directly targeting corresponding receptors in testis41 or via the production of reactive oxygen species (ROS) as a result of low vitamin C which leading to injury to testicular tissue.42
It has been found that the principal cause of idiopathic male infertility is an underlying pathological condition known as oxidative stress.32 ROS produced by sperms are necessary factors during the process of developing sperm ability to fertilize oocytes, however, imbalances in ROS metabolism will lead to increase in lipid peroxidation cause membrane damage and decreased energy production and subsequently leading to abnormal sperms structure and function.43, 44 However, testicular tissue contains numerous intrinsic antioxidant enzymes such as peroxidases and dismutase’s, additionally, antioxidant chemical factors such as vitamins C and E to protect its spermatogenic function from oxidative stress damage.45-47 The current study demonstrated that monosodium glutamate induced a reduction in the concentrations of all spermatogenic cell types, including spermatogonia, spermatocytes and spermatids without any reduction in testicular tissue weight, which may indicate that ROS were the most important mechanism for this pathology.48 On the other hand, the daily consumption of PJ over a period of one month resulted in a significant increase in the spermatogenic cell concentration, which could be due to a reduction in the ROS parameters. PJ possesses strong antioxidant properties due to its high content of polyphenols particularly ellagitannins (EA) which can easily pass through the mitochondrial membrane.49-52 Animal study revealed that the daily consumption of PJ a source of anti-oxidative stress over a period of 50 days caused a dropping in ROS parameters and an improvement in normal sperms parameters.53 Another in vivo study demonstrated that a daily consumption of PJ at various doses for six weeks period resulted in a significant increase in serum glutathione peroxidase (GSH-Px) and catalase activities in rats and a significant improvement in epididymal sperm function and structure parameters.54
Conclusion
The current experimental investigations have shown that MSG are capable of producing degenerative changes in rats testicular tissue, as well as causing a decrease in number and vitality of testicular sperms, leading to infertility. However, daily consumption of PJ counteracts MSG degenerative changes, cause recovery of testicular tissue, and therefore improving male infertility.
Acknowledgements
The authors gratefully acknowledge use of the services and facilities of Al-Rafidain University College.
Funding Sources
None.
Conflict of Interest
The authors declare no conflict of interest
References
- Rangan C, Barceloux DG. Food additives and sensitivities. Disease-a-month. 2009;55(5):292-311.
CrossRef - Jinap S, Hajeb P. Glutamate. Its applications in food and contribution to health. Appetite. 2010; 55(1): 1-10.
CrossRef - Walker R, Lupien JR. The safety evaluation of monosodium glutamate. The Journal of nutrition. 2000; 130(4):1049S-52S.
CrossRef - Hamza RZ, AL-Harbi MS. Monosodium glutamate induced testicular toxicity and the possible ameliorative role of vitamin E or selenium in male rats. Toxicology reports. 2014; 1: 1037-45.
CrossRef - Kolawole OT. Assessment of the effects of monosodium glutamate on some biochemical and hematological parameters in adult wistar rats. American Journal of BioScience. 2013;1: 11-5.
CrossRef - Bellisle F, Monneuse M, Chabert M, Larue-Achagiotis C, Lanteaume M, Louis-Sylvestre J. Monosodium glutamate as a palatability enhancer in the European diet. Physiology & behavior. 1991;49(5): 869-73.
CrossRef - Zerasky K. Nutrition and healthy eating; monosodium glutamate: is it harmful. Available@ mayo clinic com. 2010.
- Geha RS, Beiser A, Ren C, Patterson R, Greenberger PA, Grammer LC, et al., Review of alleged reaction to monosodium glutamate and outcome of a multicenter double-blind placebo-controlled study. The Journal of nutrition. 2000;130(4):1058S-62S.
CrossRef - Husarova V, Ostatnikova D. Monosodium glutamate toxic effects and their implications for human intake: a review. JMED Research 2013, 1-12 (2013).
CrossRef - Zhou Y, Danbolt N. Glutamate as a neurotransmitter in the healthy brain. Journal of neural transmission. 2014;121(8): 799-817.
CrossRef - Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1(8): 623-34.
CrossRef - Xiong JS, Branigan D, Li M. Deciphering the MSG controversy. International journal of clinical and experimental medicine. 2009;2(4): 329.
- Eweka A, Igbigbi P, Ucheya R. Histochemical studies of the effects of monosodium glutamate on the liver of adult Wistar rats. Annals of medical and health sciences research. 2011;1(1): 21-30.
CrossRef - Bhattacharya T, Bhakta A, Ghosh S. Long term effect of monosodium glutamate in liver of albino mice after neo-natal exposure. Nepal Med Coll J.2011;13(1): 11-6.
- Alalwani AD. Monosodium glutamate induced testicular lesions in rats (histological study). Middle East Fertility Society Journal. 2014;19(4): 274-80.
CrossRef - Dixit SG, Rani P, Anand A, Khatri K, Chauhan R, Bharihoke V. To study the effect of monosodium glutamate on histomorphometry of cortex of kidney in adult albino rats. Renal failure. 2014;36(2): 266-70.
CrossRef - Dong HV, Robbins WA. Ingestion of monosodium glutamate (MSG) in adult male rats reduces sperm count, testosterone, and disrupts testicular histology. Nutrition Bytes. 2015;19(1).
- Ibegbulem CO, Chikezie PC, Ukoha AI, Opara CN. Effects of diet containing monosodium glutamate on organ weights, acute blood steroidal sex hormone levels, lipid profile and erythrocyte antioxidant enzymes activities of rats. Journal of Acute Disease. 2016;5(5): 402-7.
CrossRef - Eweka AO, Eweka A, Om’Iniabohs FA. Histological studies of the effects of monosodium glutamate of the fallopian tubes of adult female Wistar rats. North American journal of medical sciences. 2010;2(3): 146-149.
- Onakewhor JU, Oforofuo IA, Singh SP. Chronic Administration of Monosodium Glutamate Induces Oligozoospermia and Glycoen Accumulation in Wistar Rat Testes. African Journal of Reproductive Health. 2017;2(2): 193-195.
- Ochiogu I, Ogwu D, Uchendu C, Okoye C, Ihedioha J, Mbegbu E. Serum luteinising hormone, testosterone and total cholesterol levels, libido and testicular histomorphology of male West African Dwarf goats orally or subcutaneously treated with monosodium L-glutamate. Veterinarni Medicina. 2015;60(5): 253-260.
CrossRef - Shi Z, Yuan B, Taylor AW, Dal Grande E, Wittert GA. Monosodium glutamate intake increases hemoglobin level over 5 years among Chinese adults. Amino acids. 2012;43(3): 1389-97.
CrossRef - Shimada A, Baad-Hansen L, Castrillon E, Ghafouri B, Stensson N, Gerdle B, et al., Differential effects of repetitive oral administration of monosodium glutamate on interstitial glutamate concentration and muscle pain sensitivity. Nutrition. 2015;31(2): 315-23.
CrossRef - He K, Du S, Xun P, Sharma S, Wang H, Zhai F, et al. Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS)–. The American journal of clinical nutrition. 2011;93(6): 1328-36.
CrossRef - Yang WH, Drouin MA, Herbert M, Mao Y, Karsh J. The monosodium glutamate symptom complex: assessment in a double-blind, placebo-controlled, randomized study. Journal of Allergy and Clinical Immunology. 1997;99(6): 757-62.
CrossRef - Khaled FA, Yousef MI, Kamel KI. The protective role of propolis against the reproductive toxicity of mono-sodium glutamine in male rabbits. Intenational Journal of Chemical Study. 2016;4(2): 04-9.
- Seeram N, Lee R, Hardy M, Heber D. Rapid large scale purification of ellagitannins from pomegranate husk, a by-product of the commercial juice industry. Separation and purification technology. 2005;41(1): 49-55.
CrossRef - Hermann DD. Naturoceutical agents in the management of cardiovascular disease. American Journal of Cardiovascular Drugs. 2002;2(3): 173-96.
CrossRef - Seeram N, Aviram M, Volkova N, Zhang Y, Henning S, Nair M, et al., editors. Dietary polyphenols derived from pomegranates are potent antioxidants: Evaluation in various in vitro models of antioxidation. ABSTRACTS OF PAPERS OF THE AMERICAN CHEMICAL SOCIETY; 2004: AMER CHEMICAL SOC 1155 16TH ST, NW, WASHINGTON, DC 20036 USA.
- Sharma RK, Agarwal A. Role of reactive oxygen species in male infertility. Urology. 1996:48(6): 835-50.
CrossRef - Ko EY, Sabanegh ES, Agarwal A. Male infertility testing: reactive oxygen species and antioxidant capacity. Fertility and sterility. 2014;102(6): 1518-27.
CrossRef - El Ghazzawy IF, Meleis AE, Farghaly EF, Solaiman A. Histological study of the possible protective effect of pomegranate juice on bisphenol-A induced changes of the caput epididymal epithelium and sperms of adult albino rats. Alexandria Journal of Medicine. 2011;47(2): 125-37.
CrossRef - Martínez-Soto JC, Domingo JC, Cordobilla B, Nicolás M, Fernández L, Albero P, et al., Dietary supplementation with docosahexaenoic acid (DHA) improves seminal antioxidant status and decreases sperm DNA fragmentation. Systems biology in reproductive medicine. 2016;62(6): 387-95.
CrossRef - Peña S, Gummow B, Parker A, Paris D. 141 Antioxidant Supplementation Alleviates DNA Damage in Boar Sperm Induced by Tropical Heat Stress. Reproduction, Fertility and Development. 2018;30(1): 210-1.
CrossRef - Organisation WH (1999). WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction: Cambridge university press.
- Bancroft JD, Floyd AD, Suvarna SK (2013). Bancroft’s Theory and Practice of Histological Techniques.
- Henry-Unaeze HN. Update on food safety of monosodium l-glutamate (MSG). Pathophysiology. 2017;24(4): 243-9.
CrossRef - Hosaka H, Kusano M, Zai H, Kawada A, Kuribayashi S, Shimoyama Y, et al. Monosodium glutamate stimulates secretion of glucagon‐like peptide‐1 and reduces postprandial glucose after a lipid‐containing meal. Alimentary pharmacology & therapeutics. 2012;36(9): 895-903.
CrossRef - Ochiogu I, Ogwu D, Uchendu C, Okoye C, Ihedioha J, Mbegbu E. Effects of monosodium-L-glutamate administration on serum levels of reproductive hormones and cholesterol, epididymal sperm reserves and testicular histomorphology of male albino rats. Acta Veterinaria Hungarica. 2015;63(1): 125-39.
CrossRef - Gill SS, Mueller RW, Mcguire PF, Pulido OM. Potential target sites in peripheral tissues for excitatory neurotransmission and excitotoxicity. Toxicologic pathology. 2000;28(2): 277-84.
CrossRef - Gong S, Xia F, Wei J, Li X, Sun T, Lu Z, et al. Harmful effects of MSG on function of hypothalamus-pituitary-target gland system. Biomedical and environmental sciences: BES. 1995;8(4): 310-7.
- Nayanatara A, Vinodini N, Damodar G, Ahemed B, Ramaswamy C, Shabarinath M, et al. Role of ascorbic acid in monosodium glutamate mediated effect on testicular weight, sperm morphology and sperm count in rat testis. J Chin Clin Med. 2008;3(1): 1-5.
- LECTOR C. Oxidative stress and role of antioxidants in normal and abnormal sperm function. Frontiers in Bioscience. 1996;1:e78-86.
CrossRef - Agarwal A, Virk G, Ong C, du Plessis SS. Effect of oxidative stress on male reproduction. The world journal of men’s health. 2014;32(1): 1-17.
CrossRef - Koksal I, Usta M, Orhan I, Abbasoglu S, Kadioglu A. Potential role of reactive oxygen species on testicular pathology associated with infertility. Asian journal of andrology. 2003;5(2): 95-100.
- Turner TT, Lysiak JJ. Oxidative stress: a common factor in testicular dysfunction. Journal of andrology. 2008;29(5): 488-98.
CrossRef - Lanzafame FM, La Vignera S, Vicari E, Calogero AE. Oxidative stress and medical antioxidant treatment in male infertility. Reproductive biomedicine online. 2009;19(5): 638-59.
CrossRef - Alkan I, Simsek F, Haklar G, Kervancioglu E, Ozveri H, Yalcin S, et al. Reactive oxygen species production by the spermatozoa of patients with idiopathic infertility: relationship to seminal plasma antioxidants. The Journal of urology. 1997;157(1):140-3.
CrossRef - Seeram NP, Lee R, Heber D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clinica Chimica Acta. 2004;348(1-2): 63-8.
CrossRef - Larrosa M, Tomás-Barberán FA, Espín JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. The Journal of nutritional biochemistry. 2006;17(9): 611-25.
CrossRef - Cerdá B, Llorach R, Cerón JJ, Espín JC, Tomás-Barberán FA. Evaluation of the bioavailability and metabolism in the rat of punicalagin, an antioxidant polyphenol from pomegranate juice. European journal of nutrition. 2003;42(1): 18-28.
CrossRef - Manach C, Mazur A, Scalbert A. Polyphenols and prevention of cardiovascular diseases. Current opinion in lipidology. 2005;16(1): 77-84.
CrossRef - Türk G, Sönmez M, Aydin M, Yüce A, Gür S, Yüksel M, et al., Effects of pomegranate juice consumption on sperm quality, spermatogenic cell density, antioxidant activity and testosterone level in male rats. Clinical nutrition. 2008;27(2): 289-96.
CrossRef - Mansour SW, Sangi S, Harsha S, Khaleel MA, Ibrahim A. Sensibility of male rats fertility against olive oil, Nigella sativa oil and pomegranate extract. Asian Pacific journal of tropical biomedicine. 2013;3(7): 563-8.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





