How to Cite | Publication History | PlumX Article Matrix
Tissue Engineering: New Paradigm of Biomedicine
Department of Molecular Biology and Genetic Engineering, C.B.S.H., G. B. Pant University of Agriculture and Technology, Pantnagar- 263145, Uttarakhand, India.
Corresponding Author's Email: sg.mbge@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2766
ABSTRACT: Tissue engineering is a multidisciplinary field of biomedicine that is being used to develop a new tissue or restore the function of diseased tissue/organ. The main objective of tissue engineering is to overcome the shortage of donor organs. Tissue engineering is mainly based on three components i.e. cells, scaffold and growth factors. Among these three components, scaffold is a primary influencing factor that provides the structural support to the cells and helps to deliver the growth factors which stimulate the proliferation and differentiation of cells to regenerate a new tissue. The properties of a scaffold mainly depend upon types of biomaterial and fabrication techniques that are used to fabricate the scaffold. Biofabrication facilitates the construction of three-dimensional complex of living (cells) and non-living (signaling molecules and extracellular matrices polymers etc.) components. Biofabrication has potential application especially in skin and bone tissue regeneration due to its accuracy, reproducibility and customization of scaffolds as well as cell and signaling molecule delivery. In this review article, different types of biomaterials and fabrication techniques have been discussed to fabricate of a nanofibrous scaffold along with different types of cells and growth factor which are used for tissue engineering applications to regenerate a new tissue. Among different techniques to fabricate a scaffold, electrospinning is simple and cost effective technique that has been mainly focused in the review to produce nanofibous scaffold. On the other hand, a tissue might be repair itself and restore to its normal function inside the body by applying the principle of regenerative medicine.
KEYWORDS: Biofabrication; Biomaterials; Electrospinning; Natural Polymers; Synthetic Polymers; Tissue Engineering
Download this article as:| Copy the following to cite this article: Gautam S, Ambwani S. Tissue Engineering: New Paradigm of Biomedicine. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Gautam S, Ambwani S. Tissue Engineering: New Paradigm of Biomedicine. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/2kWAzUD |
Introduction
The failure or loss of a tissue or organ by severe disease, trauma and surgical interventions, is a frequent and devastating challenge in the health care industry. Organ or tissue transplantation is the first choice to restore or maintain the function of damaged tissue/organ.1 According to a medical survey, more than 8 million surgical treatments are performed to replace damage tissue or organ and the healthcare cost for transplantation is estimated more than $400 billion per year.1 Autograft, allograft and xenograft are conventional implants used for organ or tissue transplantation.2 Although autografts are used more frequently for tissue defects, but often these are limited for donor sites. Alternatively, allografts and xenogarft can be obtained in abundance, but these are related to high risk of disease transmission and immune rejection.3 Thus, there is an intense need of a potential solution which can overcome the limitations of conventional therapies. Biomedicine is the application of biology and physiology to clinical medicine. Tissue engineering is now emerging as a promising alternative approach of biomedicine to treat injured tissues or to replace the damaged tissue/organs.4
The term tissue engineering was initially described in National Science Foundation meeting in 1988.5 Later in 1993, Langer and Vacanti defined the early developments in tissue engineering and explained it as “an interdisciplinary field that applies the principles of engineering and life sciences toward the development of biological substitutes that restore, maintain or improve tissue or organ function”.6 Tissue engineering is mainly based on the three important components: cells, scaffold (3D polymeric matrix) and growth factors (signaling molecules).7 These three components interact with each other under suitable environmental conditions and regenerate a new tissue or organ. The components of tissue engineering are illustrated in Figure 1.
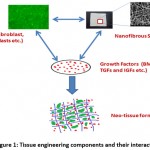 |
Figure 1: Tissue engineering components and their interaction |
Among these three components, scaffold is one of the most significant factor which accommodates the cells and creates favorable environment by supplying nutrients and growth factors for cells to proliferate and differentiate into specific tissue.8 A tissue engineered scaffold should be biodegradable, biocompatible, mechanically strong and mimic the morphological structure and chemical composition of extra cellular matrix (ECM), so that cells can adhere to the scaffold surface, proliferate and differentiate into new tissue.6 Some characteristic features of a tissue engineering scaffold are explained in Table 1.
Table 1: Properties of tissue engineering scaffold
| Scaffold characteristics | Remarks | References |
| Biodegradable | Scaffold should be degradable and the degradation rate of a scaffold should be comparable with the rate of new tissue formation.
The by-products of scaffold degradation should not be toxic in nature and must be capable to exit from the body without any intrusion in other organs. |
[9, 10] |
| Biocompatibility | Scaffold should not provoke any inflammation and prevent any adverse response of the surrounding tissue.
It should be vital to promote cell attachment, proliferation and differentiation. |
[1, 11] |
| Mechanical strength | Mechanical strength of a scaffold should be similar to the implantation site tissues. | [12] |
| Porosity | Scaffold should be highly porous with good pore connectivity.
It should provide adequate nutrient and oxygen supply and removal of waste product without compromising the mechanical strength. |
[13, 14] |
| Surface topography | Surface roughness, surface softness, stiffness, hydrophobicity and surface charge play a significant role in cell adhesion and proliferation.
Scaffold should be appropriate in surface chemistry and architecture parameters so that it can significantly influence cell behaviors such as adhesion, proliferation and differentiation. |
[15] |
The attributes of a tissue engineering scaffold, discussed in Table 1, depend upon the selected biomaterial for fabrication of the scaffold.
Biomaterials Used for Scaffold Fabrication
The selection of biomaterials for scaffold fabrication plays a pivotal role in tissue engineering. A wide range of biomaterials are used to fabricate the scaffold which usually includes natural polymers, synthetic polymers, composites and ceramics (Table 2). Naturally originated polymers are being widely used for scaffold fabrication owing to their similarities with ECM such as enzyme-controlled degradability, inherent cellular interaction and good biological performance. Collagen is a natural polymer and a major component of ECM of bone, tendon, skin, blood vessels, cartilage and heart valve.16 Due to this reason, collagen has been widely exploited for different types of tissue engineering applications. For example, bilayered collagen gels seeded with human fibroblasts and human keratinocytes have been used as the ‘dermal’ matrix of an artificial skin product under the name of Apligraf®.17 Further, collagen scaffold in the form of gel, sponge and electrospun nanofibers have been used for skin,18 cartilage19 and bone20 tissue engineering. Besides this, gelatin,21 silk,22-25 chitosan,26 alginate27 and chondroitin sulphate28 etc. have also been applied for scaffold fabrication to regenerate various kind of tissues. However, there are some drawbacks of natural polymers which include low mechanical strength, batch to batch variation, immune rejection and risk of pathogen transmission.29
Synthetic polymers offer several notable advantages over natural polymers and thus have potential use in tissue repair. Synthetic polymers can be tailored for specific applications due to controllable properties.19 They exhibit a wide range of mechanical strength and physical properties such as degradation rate, tensile strength and elastic modulus.30 Because to these properties, a variety of synthetic polymers such as PLA,31 PGA,32 PCL,33 PVA34 and PLGA35 etc. have been applied for various kind of tissue repair and regeneration. Apart from several beneficial properties, synthetic polymers also associated with some drawbacks, include lack of cell recognition site for cell attachment and proliferation, and generate acidic products in degradation procedure which produce inflammatory effect on surrounding tissues. However, the disadvantages associated with synthetic and natural polymers can be overcome by using two or more polymers (Synthetic/natural) in combination in order to afford higher mechanical strength, excellent cell attachment and tunable degradation.36 Therefore, various types of composite materials have been applied for fabricating the scaffold for tissue regeneration. Composite scaffold of hydroxyapatite/chitosan37 and PLGA/collagen38 were used for the treatment of osteochondral defects. PCL/gelatin/collagen type, I39 PCL/gelatin40 and PLGA/collagen41 composite scaffolds were used for skin and cartilage tissue regeneration respectively. Bioactive ceramics such as hydroxyapatite,42, 43 calcium phosphate44 and bioactive glass provoke osteoconductive and osteoinductive,14, 45 thus these are the attractive candidates for bone tissue engineering. However, they have limited biocompatibility and biodegradability which is not sufficient for any tissue regeneration. These problems are overcome by blending the ceramic with natural or synthetic polymers which improved the scaffold properties for tissue regeneration.46 Further, several metallic materials are also frequently used for implantation in orthopedic and dental surgery to provide support for healing bones or replace damaged bone. Metallic materials such as stainless steel 316 L (ASTM F138), Co based alloys (mainly ASTM F799 and ASTM F75), magnesium alloys (AZ31, AZ91, WE43 and LAE442) and titanium alloys (Ti-6Al-4V, F136 and ASTM F67) are used widely in joint implants and bone defects.47, 48 Moreover, besides all above discussed biomaterials, decellularized matrix is also being focused by scientists due to its bio-origin which mimic architecture of native ECM49 and consists of various kinds of biopolymers50, 51 required for tissue regeneration. Decellularized matrix has been exploited for different tissue engineering applications e.g., small intestinal submucosa,52 heart valves53 and urinary bladder54 etc. Generally used biomaterials for tissue engineering scaffold are summarized in Table 2.
Table 2: Various biomaterials used for tissue engineering Scaffold
| Origin | Properties | Polymers | Applications |
| Natural polymers | Biocompatible, biodegradable,
good in cell adhesion and proliferation properties Poor mechanical strength |
Collagen, Hyaluronic acid, chitosan, gelatin, fibrin, silk and alginic acid etc. | Skin , cartilage, vessels, heart etc. tissue scaffold, Drug Delivery etc. |
| Synthetic polymers | Biocompatible
Lack of cell recognition sites High mechanical strength
|
Poly(vinyl alcohol) (PVA), poly(lactic acid ) (PLA), poly(ethylene-oxide) (PEO) and poly( caprolactone) (PCL) etc. | Skin, cartilage, tendon, bladder , liver tissue scaffold, Drug Delivery etc. |
| Composite | Biocompatible and biodegradable
Good in cell adhesion and proliferation properties High mechanical strength
|
PCL/gelatin, PCL/chitosan
PCL/gelatin/chitosan, Collagen/chitosan and Poly(lactic acid)/ tricalcium phosphate composite etc. |
Cartilage, skin, nerve, bone, blood vessels tissue scaffold and drug delivery etc. |
| Ceramics | Bioinert, brittle and bioresorable,
High resistance to wear Low toughness |
Hydroxyapatite,
Tricalcium phosphate (TCP) and Calcium metaphosphate etc. |
Low- weight- bearing bone implants, Bone drug delivery, dental restoration etc. |
| Metals | Dense, too strong, Ductile, may corrode | Stainless steel
Titanium Alumina etc. |
Dental restoration, Load bearing bone implants etc. |
| Decellulai-zed matrix | Simple and economic for scaffolding
Retains the original architecture of tissue which influences the more significantly cellular behavior |
Collagen and elastin etc. from Cadaver Tissues | Urinary bladder, heart valves, nerves, liver tendon and ligament tissue etc. |
Fabrication Techniques for Tissue Engineering Scaffold
The success of a scaffold for tissue regeneration primarily depends upon two parameters i.e. composition (synthetic or natural origin) and architecture (designing) of scaffold. The complexity of scaffold architecture includes porosity, pore size, interconnectivity, and surface topography etc. which influence cell proliferation properties over the scaffold, greatly depends upon the scaffold fabrication technique. There are various technique for scaffold fabrication, e.g., ‘foaming’,55 ‘phase separation’,56 ‘solvent casting and particulate leaching’,57 ‘self assembly’,58 ‘solid free form fabrication technique’,59 freeze drying60 and ‘electrospinning’.61, 62, 63 Nowadays, a novel area called as “biofabrication” in which latest 3D printing technologies are exploited with the aim to spatially incorporate different cells, biomaterials and molecules into a matrix to form an artificial tissue. In our body the native ECM mainly composed of nanoscale collagen fibers. Therefore, creation of a nano-featured environment is believed to be one of the promising conditions for efficient cell attachment and proliferation, and differentiated into new tissue.1 There are only few techniques to produce the nanofibrous scaffold which are discussed in Table 3.
Table 3: Techniques employed to fabricate nanofibrous scaffold for tissue engineering applications
| Process | Advantages | Disadvantages | Example |
| Phase separation | Simple and easy process
Tailorable mechanical properties, pore size, and interconnectivity |
Low yield
Limited to few polymers Unable to produce continuous and oriented fibers |
PU [64] and PLA [65] |
| Self-assembly | Produce lowest ECM scale fiber diameter (5-8 nm)
|
Low yield
complex process Limited to few polymers Unable to produce continuous and oriented fibers |
PA nanofiber [66] |
| Electrospinning | Simple and cost effective
Used for a wide range of polymers Produce the continuous nanofibers with alignments |
Use of toxic solvent
Use of high-voltage apparatus |
PGA [67] and PLLA [68] |
Electrospinning Technique for Nanofibrous Scaffold Fabrication
Among the above mentioned techniques, only elctrospinning is a simple and cost effective technique which produce nanofibrous scaffold that mimic the structural similarity with the native ECM of our body. It produces the continuous nanofibers with control over the fiber orientation.1, 69 First time, the technique was introduced by Zelency in 191470 and patented by Formhals in 1934.71 The electrospinning technique is governed by many parameters which include flow rate of polymeric solution, electric field, viscosity, distance between tip of needle to collector, surface tension, conductivity, humidity and temperature.72 Each of these parameters significantly affect the morphology and diameter of nanofibers fabricated during electrospinning process and by proper manipulation of these parameters, nanofibers of desired morphology and diameters can be obtained.73
In brief, the electrospinning apparatus consists of mainly three components: high voltage power supply, a syringe pump holding a syringe with a metallic needle and electrically grounded collector (Figure 2). Polymeric solution is filled in the syringe for its electrospinning. The electric field is applied on the needle of the syringe to produce charges in the polymer droplet which held at the tip of needle with its surface tension. The droplet present at the tip of needle become elongate as the intensity of electric field is increased, and forms a conical shape which is recognized as Taylor cone.
 |
Figure 2: Schematic representation showing major components of the electrospinning setup; pictures of the syringe pump and high voltage power supply has been taken from the laboratory electrospinning setup |
At a critical value of electric field, the surface tension of polymer droplet is overcome by repulsive electrostatic force overcomes and a charged jet is evicted from the tip of Taylor cone. The evicted jet undergoes an instability and elongation process, which convert the jet into very long and thin. During the jet travelling, the solvent becomes evaporate and it collects on the collection target in the form of nanofibers.74 Electrospinning is emerged as attractive approach for tissue engineering application because it produce nanofibrous scaffold which illustrate high porosity with tremendous pore interconnectivity, high surface area to volume ratio and mimic nano-feature environment similar to native ECM. These are the some favorable characteristics which influence cellular growth and function over the scaffold.1 Therefore, electrospun nanofibrous scaffolds from different biomaterials are being widely explored for various kinds of tissue engineering applications. Some examples of electrospun nanofibrous scaffold used in tissue engineering applications are illustrated in Table 4.
Surface Functionlization of Nanofibrous Scaffold
Sometimes, scaffold fabricated by electrospinning is not sufficient for providing all desirable properties to the cells for regenerating into a specific tissue. There is often necessary to modify the scaffold to enhance the some specific properties, for better cell attachment and proliferation to regenerate a new specific new tissue. Several techniques have been used to modify the nanofibrous scaffold but physical adsorption and covalent surface bonding are commonly applied techniques to modify the scaffold.86 A variety of biomolecules have been used to modify the nanofibrous scaffold to increase their bioactivities. Collagen, laminin and many other molecules have been used for coating synthetic nanofibrous scaffold by physical absorption method which reflected the enhanced cell attachment, spreading, viability, and phenotype preservation87
Table 4: Nanofibrous scaffolds for different tissue engineering applications
| Origin | Electrospun scaffold | Tissue Engineering Applications | References |
| Natural polymers | Collagen Type II | Cartilage | [75] |
| Silk Fibroin | Skin | [76] | |
| Collagen Type I | Wound dressing | [77] | |
| Chitosan | Bone | [78] | |
| Synthetic polymers | PLGA | Skin, cartilage | [79, 80] |
| PVA | Wound dressings | ||
| PCL | Nerve | [81] | |
| PLGA | Cardiac tissue | [82] | |
| Composite | PCL/gelatin | Nerve | [36] |
| PCL/gelatin/collagen Type I | Skin | [39] | |
| PCL/PU | Vascular tissue | [83] | |
| PLGA/collagen/elastin | Vascular graft | [84] | |
| Hydroxyaptite/chitosan | Bone | [37] | |
| PVA/Gum tragacanth/PCL | skin | [85] |
Similarly, many researchers have also used physical absorption method to coat the nanofibrous scaffold by hydroxyapatite to stimulate the expression of osteogenic genes by osteoblastic cells.88, 89 Besides physical adsorption, many biomolecules are bounded covalently to the surface of nanofibrous scaffolds to enhance the surface properties. It has been observed in a study that covalent bonding of laminin and collagen to nanofibrous scaffold improved the suitability of the scaffold for neural tissue.87 In some cases, both covalent bonding and physical absorption can also be used to biofunctionalize the scaffold surface. Chen et al. modified the surface of PLLA electrospun scaffold by SBF coating using physical adsorption method and then, further functionalized the scaffold by hydrolysis of PLLA scaffold in NaOH aqueous solution.88 In addition to surface functionalization, biomolecules can also be incorporated directly into the polymer solution during electrospinning. Antibiotics have been incorporated into electrospun scaffold during electrospinning process for their sustained release and bioactivity retention.90 Vitamins91 and nanoparticles92 have been loaded directly into the polymer solution to functionalize the scaffold.
Cells Used for Tissue Engineering
The cells selected for new tissue regeneration should be able to respond to surrounding environment, differentiate into new tissue and integrate with native tissue. For the treatment of injured tissues or to regenerate a new tissue, cells are taken from same patient or another individual. On the basis of cell source, the cells can be differentiated into autologous, allogenic and xenogenic cells. Autologous cells are isolated from the same individual while if the cells are isolated from another individual of same species are called allogenic cells. Xenogenic cells are obtained from the individual of another species. Over the last decade, adult stem cells and embryonic stem cells have been investigated as a potential source to regenerate or repair damaged tissues.93, 94 Embryonic stem cells are derived from pre-implantation embryo and have the capacity to differentiate into any tissue or organ95, 96 but the use of embryonic stem cells is restricted due to some ethical and political considerations. Adult stem cells such as mesenchymal stem cells (MSC) have capability to differentiate into a wide range of tissue includes, cartilage, bone, muscle, tendon, fat and connective tissue, furthermore these cells are less problematic in terms of ethical issues which make them very attractive and promising source for tissue engineering applications.97
Growth Factors and Nutrients for Tissue Engineering
Growth factors are polypeptides signaling molecules which transmit signals to modulate cellular activities such as cellular adhesion, migration, proliferation, differentiation, and gene expression.98 Commonly used growth factors which influence the tissue regeneration are insulin-growth factor I (IGF I), bone morphogenetic proteins (BMPs), transforming growth factor-β (TGF-β), basic fibroblast growth factor (FGF-2), Platelet-derived growth factor (PDGF), polypeptide growth factor etc. For tissue engineering applications, these signaling molecules are used singly or in combine form to enhance tissue regeneration. Normally, Dulbecco’s modified Eagle’s (DMEM) media supplemented with FBS, penicillin and streptomycin antibiotics are used for cell.
Conclusions
Tissue engineering is one of the most exciting research areas which are growing exponentially with time to overcome the shortage of donor organ. The success of tissue engineering mainly depends upon the chemical composition and surface architecture of scaffold that can obtain specific, desired and timely responses from surrounding cells and tissues in a host. A wide range of natural polymer, synthetic polymer and ceramics have been used to fabricate a scaffold. Nowadays, more than one biomaterial is being used to fabricate a scaffold sothat it can mimic the chemical composition of native tissue. Surface functionalization strategies are being further applied to improve the surface properties of scaffold. In addition, highly optimized nanofibrous scaffold fabrication technologies are being applied to synthesize a more efficient tissue engineering scaffold. Recently, biofabrication techniques are offering a definite and structured strategy for tissue generation. However, there are still considerable constraints with biofabrication for the development of clinically appropriate constructs. Thus, further advances in material designing and biofabrication techniques are increasing the possibility of production of good quality implants.
References
- Murugan R., Ramakrishna S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Engineering, 2007; 13, 1845-1866.
CrossRef - Kumbar S. G., Nukavarapu S. P., James R., Nair L. S. Laurencin C. T. Electrospunpoly(lactic acid-co-glycolic acid) scaffolds for skin tissue engineering. Biomaterials, 2008; 29, 4100-4107.
CrossRef - Priya S. G., Jungvid H., and Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Engineering Part B: Reviews, 2008; 14, 105-118.
CrossRef - Sah M. K., Pramanik K. Surface modification and characterization of natural polymers for orthopaedic tissue engineering: a review. Journal of Biomedical Engineering and Technology, 2012; 9, 101-121.
CrossRef - Skalak R., Fox C. F. Tissue engineering: proceedings of a workshop, held at Granlibakken, Lake Tahoe, California, February, 1988;26-29, 1988.
- Langer R., Vacanti J. P. Tissue engineering. Science, 1993; 260, 920-926.
CrossRef - Parveen S., Krishnakumar K., Sahoo S. K. New Era in Health Care: Tissue Engineering. Journal of Stem Cell and Regenerative Medicine, 2006; 1, 8-24.
- Sharma C., Dinda A. K., Mishra, N. C. Synthesis and Characterization of Glycine Modified Chitosan-Gelatin-Alginate Composite Scaffold for Tissue Engineering Applications. Journal of Biomaterials and Tissue Engineering, 2012a; 2, 133-142.
CrossRef - O’Brien F. J. Biomaterials & scaffolds for tissue engineering. Materials Today, 2011;14, 88-95.
CrossRef - Hutmacher D. W. Scaffold design and fabrication technologies for engineering tissues state of the art and future perspectives. Journal of Biomaterials Science; Polymer Edition, 2001; 12, 107-124.
CrossRef - Ito Y., Zheng J., Imanishi Y. Enhancement of cell growth on a porous membrane coimmobilized with cell-growth and cell adhesion factors. Biomaterials, 1997; 18, 197-202.
CrossRef - Mitragotri S., Lahann, J. Physical approaches to biomaterial design. Nature Materials, 2009; 8, 15-23.
CrossRef - Karande T. S., Ong J. L., Agrawal C. M. Diffusion in musculoskeletal tissue engineering scaffolds: Design issues related to porosity, permeability, architecture, and nutrient mixing. Annuals of Biomedical Engineering, 2004; 32, 1728-1743.
CrossRef - Karageorgiou V., Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials, 2005; 26, 5474-5491.
CrossRef - Chang H. I., Wang, Y. Cell responses to surface and architecture of tissue engineering scaffolds. Regenerative Medicine and Tissue Engineering—Cells and Biomaterials, InTech: Rijeka, Croatia, 2011; 569-588.
CrossRef - Liu C., Xia Z., Czernuszka J. T. Design and Development of Three-Dimensional Scaffolds for Tissue Engineering. Chemical Engineering Research and Design, 2007; 85, 1051-1064.
CrossRef - Eaglstein W. H., Falanga V. Tissue engineering and the development of Apligraf®,a human skin equivalent. Clinical Therapeutics, 1997; 19, 894-905.
CrossRef
- Chandler L. A., Ma C., Gonzalez A. M., Doukas J., Nguyen T., Pierce G. F., Phillips M. L. Matrix-enabled gene transfer for cutaneous wound repair. Wound Repair and Regeneration, 2000; 8, 473-479.
CrossRef - Fujisato T., Sajiki T., Liu Q., Ikada Y. Effect of basic fibroblast growth factor on cartilage regeneration in chondrocyte-seeded collagen sponge scaffold, Biomaterials, 1996; 17, 155-162.
CrossRef - Xiao Y., Qian H., Young W.G., Bartold P. M. Tissue engineering for bone regeneration using differentiated alveolar bone cells in collagen scaffolds. Tissue Engineering, 2003;9, 1167-1177.
CrossRef - Vashi A.V., Abberton K. M., Thomas G. P., Morrison W. A., O’Connor A. J., Cooper- White J. J., Thompson E. W. Adipose tissue engineering based on the controlled release of fibroblast growth factor-2 in a collagen matrix. Tissue Engineering, 2006; 12, 3035-3043.
CrossRef - Bhattacharjee M., Miot S., Gorecka A., Singh K., Loparic M., Dickinson S., Das A., Bhavesh N. S., Ray A. R., Martin I., Ghosh S. Oriented lamellar silk fibrous scaffolds to drive cartilage matrix orientation: Towards annulus fibrosus tissue engineering. Acta Biomaterialia, 2012; 8, 3313-3325.
CrossRef - Mandal B. B, Kundu S. C. Non-bioengineered silk gland fibroin protein: characterization and evaluation of matrices for potential tissue engineering applications. Biotechnology and Bioengineering, 2008a; 100, 1237-1250.
CrossRef - Sah M. K., Pramanik K. Regenerated Silk Fibroin from B. mori Silk Cocoon for Tissue Engineering Applications. International Journal of Environment Science and Development, 2010; 1, 404-408.
CrossRef - Mandal B. B, Kundu, S. C. Non-bioengineered silk fibroin protein 3D scaffolds for potential biotechnological and tissue engineering applications. Macromolecular Bioscience, 2008b; 8, 807-818.
CrossRef - Lee J. Y., Nam S. H., Im S.Y., Park Y. J., Lee Y. M., Seol Y. J., Chung C. P., Lee, S. J. Enhanced bone formation by controlled growth factor delivery from chitosan-based biomaterials. Journal of Controlled Release, 2002; 78, 187-197.
CrossRef - Ghosh S., Jassal M. Use of Polysaccharide fibres for modern wound dressing. Indian Journal of Fibre and Textile Reserach,2002; 27, 434-450.
- Cai S., Liu Y., Zheng Shu X., Prestwich, G. D. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials, 2005; 26, 6054-6067.
CrossRef - Malafaya P. B., Silva G. A., Reis, R. L. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Advanced Drug Delivery Reviews, 2007; 59, 207-233.
CrossRef - Dhandayuthapani B., Yoshida Y., Maekawa T., Kumar, D. S. Polymeric scaffolds in tissue engineering application: a review. International Journal of Polymer Science, 2011; doi:10.1155/2011/290602.
CrossRef
- Uyama S., Kaufmann P. M., Takeda T., Vacanti J. P. Delivery of whole liver-equivalent hepatocyte mass using polymer devices and hepatotrophic stimulation. Transplantation, 1993; 55, 932-935.
CrossRef - Uyama S., Kaufmann P. M., Kneser U., Fiegel H. C., Pollok J. M., Kluth D., Vacanti J. P., Rogiers X. Hepatocyte Transplantation using biodegradable matrices in ascorbic acid-deficient rats: comparison with heterotopically transplanted liver grafts. Transplantation, 2001; 71, 1226-1231.
CrossRef - Gomes S.R., Rodrigues G., Martins G.G., Roberto M. A., Mafra M., Henriques C. M., Silva J.C. In vitro and in vivo evaluation of electrospun nanofibers of PCL, chitosan and gelatin: A comparative study. Mater. Sci. Eng. C. 2015; 46:348-58.
CrossRef - Kneser U., Kaufmann P. M., Fiegel H. C., Pollok J. M., Kluth D., Herbst H., Rogiers X. Long-term differentiated function of heterotopically transplanted hepatocytes on three-dimensional polymer matrices. Journal of Biomedical Materials Research, 1999; 47, 494-503.
CrossRef - Hasirci V., Berthiaume F., Bondre S. P., Gresser J. D., Trantolo D. J., Toner M., Wise D. L. Expression of liver-specific functions by rat hepatocytes seeded in treated poly(lactic-co-glycolic) acid biodegradable foams. Tissue Engineering, 2001; 7, 385-394.
CrossRef - Ghasemi-Mobarakeh L., Prabhakaran M. P., Morshed M., Nasr-Esfahani M. H., Ramakrishna S. Electrospun poly(ɛ-caprolactone)/gelatin nanofibrous scaffolds for nerve tissue engineering. Biomaterials, 2008; 29, 4532-4539.
CrossRef - Zhang Y., Venugopal J. R., El-Turki A., Ramakrishna S., Su B., Lim C.T. Electrospun biomimetic nanocomposite nanofibers of hydroxyapatite/chitosan for bone tissue engineering. Biomaterials, 2008; 29, 431-4322.
CrossRef - Ngiam M., Liao S., Patil A. J., Cheng Z., Chan C. K., Ramakrishna S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behavior for bone tissue engineering. Bone, 2009; 45, 4-16.
CrossRef - Gautam S., Chou C. F., Dinda A. K., Potdar P. D., Mishra N. C. Surface modification of nanofibrous polycaprolactone/gelatin composite scaffold by collagen type I grafting for skin tissue engineering. Materials Science and Engineering C, 2014a; 34, 402-409.
CrossRef - Ren K., Wang Y., Sun T., Yue W., Zhang H. Electrospun PCL/gelatin composite nanofiber structures for effective guided bone regeneration membranes. Mater. Sci. Eng. C. 2017;78:324-32.
CrossRef - Chen G., Sato T., Ushida T., Hirochika R., Shirasaki Y., Ochiai N., Tateishi, T. The use of a novel PLGA fiber/collagen composite web as a scaffold for engineering of articular cartilage tissue with adjustable thickness. Journal of Biomedical Materials Research Part A, 2003; 67, 1170-1180.
CrossRef - Neffe A. T., Loebus A., Zaupa A., Stoetzel C., Muller F. A., Lendlein A. Gelatin functionalization with tyrosine derived moieties to increase the interaction with hydroxyapatite fillers. Acta Biomaterialia, 2011; 7, 1693-1701.
CrossRef - Dan Y., Liu O., Liu Y., Zhang Y.Y., Li S., Feng X.B., Shao Z.W., Yang C., Yang S.H., Hong J.B. Development of novel biocomposite scaffold of chitosan-gelatin/nanohydroxyapatite for potential bone tissue engineering applications. Nanoscale Res. Lett. 2016:11(1):487.
CrossRef - Thomas P. S., Thomas S., Bandyopadhyay S., Wurm A., Schick C. Polystyrene/calcium phosphate nanocomposites: Dynamic mechanical and differential scanning calorimetric studies. Composite Science and Technology, 2008; 68, 3220-3229.
CrossRef - Rao S.H., Harini B., Shadamarshan R.P., Balagangadharan K., Selvamurugan N. Natural and synthetic polymers/bioceramics/bioactive compounds-mediated cell signalling in bone tissue engineering. Int. J. Biol. Macromol. 2018; 110:88-96.
CrossRef - Bolder S. B. T., Verdonschot N., Schreurs B. W., Buma P. Acetabular defect reconstruction with impacted morsellized bone grafts or TCP/HA particles. A study on the mechanical stability of cemented cups in an artificial acetabulum model. Biomaterials, 2002; 23, 659-666.
CrossRef - Witte F., Kaese V., Switzer H., Meyer-Lindenberg A., Wirth C. J., Windhag H. In vivo corrosion of four magnesium alloys and the associated bone response. Biomaterials, 2005; 26, 3557-3563.
CrossRef - Zhang E., Xu L., Yu G., Pan F., Yang K. In vivo evaluation of biodegradable magnesium alloy bone implant in the first 6 months implantation. Journal of Biomedical Materials Research Part A, 2009; 90, 882-893.
CrossRef - Ramalingam, M., and Tiwari, A. (2010). Spatially controlled cell growth using patterned biomaterials. Advanced Materials Letters, 1, 179-187
CrossRef - Neffe A. T., Scharnagl N., Behl M., Lendlein A. Biomaterials in Regenerative Medicine, BioTOPics,2012; 43, 8-10.
- Anandhan S., Bandyopadhyay S. Polymer Nanocomposites: From Synthesis to Applications. Nanocomposites and Polymers with Analytical Methods. 2011; 630-636.
CrossRef - Cozad M. J., Bachman S. L., Grant, S. A. Assessment of decellularized porcine diaphragm conjugated with gold nanomaterials as a tissue scaffold for wound healing. Journal of Biomedical Materials Research A, 2011; 99, 426-434.
CrossRef - Rieder E., Kasimir M. T., Silberhumer G., Seebacher G., Wolner E., Simon P., Weigel G. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. Journal of Thoracic and Cardiovascular Surgery, 2004; 127, 399-405.
CrossRef - Gilbert T. W., Stolz D. B., Biancaniello F., Simmons-Byrd A., Badylak S. F. Production and characterization of ECM powder: implications for tissue engineering applications. Biomaterials, 2005; 26, 1431-1435.
CrossRef - Kim T. K., Yoon J. J., Lee D. S., Park T. G. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials, 2006a; 27, 152-159.
CrossRef - Schugens C., Maquet V., Grandfils C., Jérôme R., Teyssie, P. Polylactide macroporous biodegradable implants for cell transplantation. II. Preparation of polylactide foams by liquid-liquid phase separation. Journal Biomedical Materials Reserach, 1996;30, 449-461.
CrossRef - Mikos A. G., Thorsen A. J., Czerwonka L. A., Bao Y., Langer R., Winslow D. N., Vacanti, J. P. Preparation and characterization of poly (L-lactic acid) foams. Polymer, 1994;35, 1068-1077.
CrossRef - Das A., Ghosh S., Ray A. K. Unveiling the self-assembly behavior of copolymers of AAc and DMAPMA in situ to form smart hydrogels displaying nanogels-within-macrogel hierarchical morphology. Polymer, 2011; 52, 3800-3810.
CrossRef - Sachlos E., Czernuszka J. T. Making tissue engineering scaffolds work. Review: the application of solid freeform fabrication technology to the production of tissue engineering scaffolds. European Cells and Materials, 2003; 5, 39-40.
CrossRef - Kumar, A., Mishra, R., Reinwald, Y. and Bhat, S. Cryogels: Freezing Unveiled by Thawing. Materials Today, 2010; 13, 42-44.
CrossRef - Ma Z., Kotaki M., Inai R., Ramakrishna, S. Potential of nanofiber matrix as tissue-engineering scaffolds. Tissue Engineering, 2005a; 11, 101-109.
CrossRef - Sharma Y., Tiwari A., Hattori S., Terada D., Sharma A. K., Ramalingam M., Kobayashi, H. Fabrication of conducting Electrospun nanofibers scaffold for three-dimensional cells culture. International Journal of Biological Macromolecules, 2012b; 51, 627-631.
CrossRef - Yang G., Li X., He Y., Ma J., Ni G., Zhou S. From nano to micro to macro: Electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018; 81:80-113.
CrossRef - Guan J., Fujimoto K., Sacks M., Wagner W. Preparation and characterization of highly porous biodegradable polyurethane scaffolds for tissue applications. Biomaterials, 2005; 26, 3961-3971.
CrossRef - Chen J., Ling S., Tsay R. A morphological study of porous polylactide scaffolds prepared by thermal induced phase separation. Journal of the Taiwan Institute of Chemical Engineers, 2010; 41, 229-238.
CrossRef - Beniash E., Hartgerink J. D., Storrie H., Stendahl J. C., Stupp S. I. Self-assembling peptide amphiphile nanofiber matrices for cell entrapment. Acta Biomaterialia, 2005; 1, 387-397.
CrossRef - Boland E. D., Telemeco T. A., Simpson D. G., Wnek G. E., Bowlin G. L. Utilizing acid pretreatment and electrospinning to improve biocompatibility of poly (glycolic acid) for tissue engineering. Journal of Biomedical Materials Research Part B: Applied Biomaterials, 2004; 71, 144-152.
CrossRef - Yang F., Murugan R., Wang S., Ramakrishna S. Electrospinning of nano/micro scale poly (L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials, 2005; 26, 2603-2610.
CrossRef - Repanas A., Andriopoulou S., Glasmacher B. The significance of electrospinning as a method to create fibrous scaffolds for biomedical engineering and drug delivery applications. J.Drug Deliv. Sci. Tech. 2016; 31:137-46.
CrossRef - Zeleny J. The electrical discharge from liquid points and a hydrostatic method of measuring the electric intensity at their surfaces. Physical Review, 1914; 3, 69-91.
CrossRef - Formhals A. (1934). US Patent 1975504.
- Bhardwaj N., Kundu S. C. Electrospinning: a fascinating fiber fabrication technique. Biotechnology Advances, 2010; 28, 325-347.
CrossRef - Chong E. J., Phan T. T., Lim I. J., Zhang Y. Z., Bay B. H., Ramakrishna S., Lim, C. T. Evaluation of Electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution. Acta Biomaterialia, 2007; 3, 321-330.
CrossRef - Huang Z. M., Zhang Y. Z., Kotaki M., Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology, 2003; 63, 2223-2253.
CrossRef - Matthews J. A., Boland E. D., Wnek G. E., Simpson D. G., Bowlin G. L. Electrospinning of collagen type II: A feasibility study. Journal of Bioactive and Compatible Polymers, 2003; 18, 125-134.
CrossRef - Min B. M., Lee G., Kim S. H., Nam Y. S., Lee T. S., Park, W. H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro, Biomaterials, 2004a; 25, 1289-1297.
CrossRef - Rho K. S., Jeong L., Lee G., Seo B. M., Park Y. J., Hong S. D., Roh S., Cho J. J., Park W. H., Min B. M. Electrospinning of collagen nanofibers: effects on the behavior of normal human keratinocytes and early-stage wound healing. Biomaterials, 2006; 27, 1452-1461.
CrossRef - Shin S. Y., Park H. N., Kim K. H., Lee M. H., Choi Y. S., Park Y. J., Lee Y. M., Rhyu I. C., Han S. B., Lee S. J., and Chung C. P. Biological evaluation of chitosan nanofiber membrane for guided bone regeneration. Journal of Periodontology, 2005; 76, 1778-1784.
CrossRef - Li W. J., Laurencin C. T., Caterson E. J., Tuan R. S., Ko F. K. Electrospun nanofibrous structure: a novel scaffold for tissue engineering. Journal of Biomedical Materials Research, 2002; 60, 613-621.
CrossRef - Li W. J., Danielson K. G., Alexander P. G., Tuan R. S. Biological response of chondrocytes cultured in three-dimensional nanofibrous poly (ϵ-caprolactone) scaffolds. Journal of Biomedical Materials Research Part A, 2003; 67, 1105-1114.
CrossRef - Jia J., Duan Y. Y., Wang S. H., Zhang S. F., and Wang Z. Y. Preparation and characterization of antibacterial silver-containing nanofibers for wound dressing applications. Journal of US-China Medical Science, 2007; 4, 52-54.
- Zong X., Bien H., Chung C. Y., Yinc L., Fang D., Hsiao B. S. Electrospun fine-textured scaffolds for heart tissue constructs. Biomaterials, 2005; 26, 5330-5338.
CrossRef - Williamson M. R., Black R., Kielty C. PCL–PU composite vascular scaffold production for vascular tissue engineering: attachment, proliferation and bioactivity of human vascular endothelial cells. Biomaterials, 2006; 27, 3608-3616
CrossRef - Stitzel J., Liu J. S., Lee J., Komura M., Berry J., Soker S. Controlled fabrication of a biological vascular substitute. Biomaterials, 2006; 27, 1088-1094.
CrossRef - Zarekhalili Z., Bahrami S.H., Ranjbar-Mohammadi M., Milan P.B. Fabrication and characterization of PVA/Gum tragacanth/PCL hybrid nanofibrous scaffolds for skin substitutes. Int. J. Biol. Macromol. 2017;94:679-90.
CrossRef - Beachleyand V., Wen X. Polymer nanofibrous structures: Fabrication, biofunctionalization, and cell interactions. Progress in Polymer Science, 2010; 35, 868-892.
CrossRef - Koh, H. S., Yong T., Chan C. K., Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials, 2008. 29, 3574-3582.
CrossRef - Chen J., Chu B., Hsiao B. S. Mineralization of hydroxyapatite in electrospun nanofibrous poly (L-lactic acid) scaffolds. Journal of Biomedical Materials Research Part A, 2006; 79, 307-317.
CrossRef - Ito Y., Hasuda H., Kamitakahara M., Ohtsuki C., Tanihara M., Kang I. K. Kwon O. H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. Journal of Bioscience and Bioengineering, 2005; 100, 43-49.
CrossRef - Kim K., Luu Y. K., Chang C., Fang D., Hsiao B. S., Chu B., Hadjiargyrou M. Incorporation and controlled release of a hydrophilic antibiotic using poly (lactide-co-glycolide)-based electrospun nanofibrous scaffolds. Journal of Controlled Release, 2004; 98, 47-56.
CrossRef - Taepaiboon P., Rungsardthong U., Supaphol P. Vitamin-loaded electrospun cellulose acetate nanofiber mats as transdermal and dermal therapeutic agents of vitamin A acid and vitamin E. European Journal of Pharmaceutics and Biopharmaceutics, 2007; 67, 387-397.
CrossRef - Erisken C., Kalyon D. M., Wang H. A hybrid twin screw extrusion/electrospinning method to process nanoparticle-incorporated electrospun nanofibers. Nanotechnology, 2008; 19, 165302.
CrossRef - Fung Y. C. Bone and Cartilage. Biomechanics: Mechanical Properties of Living Tissues. 1993; 500-544.
CrossRef - Cancedda R., Dozin B., Giannoni P., Quarto R. Tissue engineering and cell therapy of cartilage and bone. Matrix Biology, 2003; 22, 81-91.
CrossRef - Ringe J., Kaps C., Burmester G. R., Sittinger M. Stem cells for regenerative medicine: advances in the engineering of tissues and organs. Naturwissenschaften, 2002;89, 338-351
CrossRef - Martin I., Padera R. F., Vunjak-Novakovic G., Freed L. E. In vitro differentiation of chick embryo bone marrow stromal cells into cartilaginous and bone-like tissues. Journal of Orthopaedic Research, 1998; 16, 181-189.
CrossRef - Risbud M. V., Sittinger M. Tissue engineering: advances in vitro cartilage generation. Trends in Biotechnology, 2002; 20, 351-356.
CrossRef - Babensee J. E., McIntire L. V., Mikos A. G. Growth factor delivery for tissue engineering. Pharmaceutical Research, 2000; 17, 497-504.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






