How to Cite | Publication History | PlumX Article Matrix
Mohamed O. Badry1* , Tarek A. A. Radwan2
, Tarek A. A. Radwan2 , Fatma A. A. Ayed2
, Fatma A. A. Ayed2 and Mohamed G. Sheded2
and Mohamed G. Sheded2
1Department of Botany and Microbiology, Faculty of Science, South Valley University, Qena 83523, Egypt
2Department of Botany, Faculty of Science, Aswan University, Aswan 81528, Egypt
Corresponding Author E-mail: mohamedowis@svu.edu.eg
DOI : http://dx.doi.org/10.13005/bbra/2775
ABSTRACT: The present study was undertaken to survey the floristic composition in the islands and shorelines in Aswan Reservoir, south of the River Nile at Aswan Governorate, Egypt. Four elements of vegetation were analyzed: floristic composition, lifespan, life form, and phytogeographical affinities. A total of 165 species were recorded belonging to 134 genera in 45 families of vascular plants, of which six species were new to the flora of Aswan and Nubia (Amaranthus spinosus, Doellia bovei, Eleocharis parvula, Haematoxylum campechianum, Polygonum aviculare, and Pithecellobium dulce). The most represented families are Leguminosae, Poaceae, and Compositae. Species richness is highest in low-lying areas (shorelines) liable to flooding, compared to those of the islands in the river. The recorded flora consists of 50.91% perennials and 49.09% annuals. Therophytes and phanerophytes were the predominant life forms. Phytogeographical analysis revealed the prevalence of the pantropical (28.48%), palaeotropical (17.57%), and cosmopolitan (16.36%) plant species. Monoregional chorotype was represented by 29 species (17.58%) of the recorded flora with the Sudano-Zambezian species (11.52%) being the highest chorotype, while pure Mediterranean species were very poorly represented (3.63%). Biregional chorotype was represented by 25 species (15.15%), while the pluriregional chorotype was accounted for 2.43% of recorded species.
KEYWORDS: Aswan Old Dam; Diversity; High Dam; Lifeform spectrum; Philae Island; Vascular flora; Water fluctuation
Download this article as:| Copy the following to cite this article: Badry M. O, Radwan T. A. A, Ayed F. A. A, Sheded M. G. Floristic Diversity of Riparian Plants in Aswan Reservoir at the Extreme South of the River Nile, Upper Egypt: A Closed Ecological System. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Badry M. O, Radwan T. A. A, Ayed F. A. A, Sheded M. G. Floristic Diversity of Riparian Plants in Aswan Reservoir at the Extreme South of the River Nile, Upper Egypt: A Closed Ecological System. Biosci Biotech Res Asia 2019;16(3). Available from: https://www.biotech-asia.org/?p=34159 |
Introduction
Egypt is a riverain oasis generated by the River Nile in a region that was otherwise would have remained a vast barren desert with no vegetation 1,2. The Nile River runs for about 6700 km through ten countries in north-eastern Africa and ends in Egypt where it flows for about 1200 km from Aswan to the Mediterranean coast 3,4. The Nile River system has been subjected to extensive schemes of river control as the implementation of dams and barrages across the river and its tributaries, leading to changes in the natural hydrobiology with undoubted impact on the biota, especially flora and vegetation 1.
In 1964, Aswan High Dam was established seven kilometers to the south of the old dam (Aswan Low Dam) in upper Egypt, and brought the River Nile under full control, resulting in a smaller water body between the two dams, known as the Aswan Reservoir. A lower annual amplitude of water levels exhibited in Aswan Reservoir than in Lake Nasser, nevertheless there is an obvious diurnal fluctuation with a great amplitude of about 3 meters in the reservoir associated to the daily routine of water inflow via the High Dam turbines which have great effects on the plant life associated with the river 5.
Across the reservoir shoreline, several small dendritic side areas (khors) are present throughout mostly on the eastern side and a few small ones on the west, in addition to several granitic islands and associated superficial areas of water, typically the remains of part of the First Cataract of the Nile, flooded upon| completion of the original Aswan Dam 6. River Nile ecosystem along the reservoir is usually split up into three habitats: slope, water-edge, and open-water of the Nile Bank. Each of them has a distinctive flora. In addition, the vegetation of this reservoir is mainly on the shorelines and its different types of habitat are controlled by two main factors: moisture content and soil formation 6.
Around the world, riverain habitats have significantly modified from their own natural condition. One of the main reasons behind these changes is usually Dams, mainly because of their alteration of water and sediment regimes 7,8.
Fluctuations in hydrological patterns are important drivers for ecological systems 9. Likewise, Dams have a potential effect on hydrochory in different ways, such as: modifying the hydrologic regime, influencing seed dispersal distance, its deposition sits along channel margins, and the availability and suitability of streamside habitat for seed germination and seedling establishment, they function as a physical barrier to the downstream movement of plant propagules, trapping and storing seeds in reservoirs and resulting in retention and high rates of seed mortality 10,11.
To best of our knowledge, no study examined the plant diversity in River Nile Reservoir systems. Aswan Reservoir provides a model to address community assemblage in a closed ecohydrological system. This study is the first to survey the floristic diversity of the riparian and aquatic plants in the Aswan Reservoir area, Aswan Governorate in Egypt. The current study aims to identify the floristic diversity, life forms, lifespan, and phytogeographic relationships of the aquatic and riparian plant species of the Aswan Reservoir area.
Material and Methods
Study Area
This study was performed in the Aswan Reservoir area, Aswan Governorate, between Aswan High Dam and Aswan Low Dam from September 2017 to January 2019. The study area located between latitudes 23° 58′ 20″ and 24° 02′ 19″ N and longitudes 32° 51′ 50″ and 32° 54′ 8″ E, with 7.2 km length and an average width of 1.05 km.
The wild vegetation was sampled in 11 localities, which were divided into three zones (Eastern bank, Western bank and Middle islands) representing the inhabited areas (for practicing cultivation) and uninhabited areas (natural vegetation). A total of 255 quadrates (1 × 1 m2) located randomly within 27 stands were selected in the study area (Table 1, Fig. 1).
Table 1: Locations of the studied areas in Aswan Reservoir with their coordinates, human impact, number of stands, and quadrates.
| Sites | Human impact | No. of
Stands |
No. of
Quadrates |
Coordinates | |||
| Latitude
(N) |
Longitude
(E) |
||||||
| Eastern bank | 1 | El Shallal | Inhabited | 10 | 100 | 24°01ꞌ47.55″ | 32°53ꞌ52.22″ |
| 2 | Bute El-Hasaya | Uninhabited | 1 | 10 | 24°00ꞌ54.29″ | 32°53ꞌ30.81″ | |
| 3 | Maezana Belal | Uninhabited | 1 | 10 | 24°00ꞌ30.17″ | 32°53ꞌ21.88″ | |
| 4 | High Dam Colony | Inhabited | 3 | 20 | 23°59’03.69″ | 32°52ꞌ55.01″ | |
| 5 | Philae Port | Uninhabited | 1 | 10 | 24°02ꞌ02.03″ | 32°53ꞌ09.15″ | |
| Western bank | 6 | El Mahgar Valley | Uninhabited | 3 | 30 | 24°00ꞌ17.62″ | 32°52ꞌ14.72″ |
| 7 | Tingar | Inhabited | 2 | 30 | 24°59ꞌ37.85″ | 32°52ꞌ12.28″ | |
| Middle islands | 8 | Awad | Inhabited | 1 | 5 | 24°01ꞌ43.26″ | 32°52ꞌ24.80″ |
| 9 | Heisa | Inhabited | 2 | 10 | 24°00ꞌ19.88″ | 32°52ꞌ32.08″ | |
| 10 | Bigga | Inhabited | 1 | 10 | 24°01ꞌ15.69″ | 32°52ꞌ24.80″ | |
| 11 | Agilkia | Uninhabited | 2 | 20 | 24°01ꞌ17.31″ | 32°53ꞌ22.44″ | |
| Total | 27 | 255 | |||||
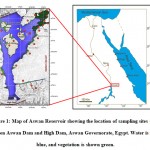 |
Figure 1: Map of Aswan Reservoir showing the location of sampling sites (red) between Aswan Dam and High Dam, Aswan Governorate, Egypt. Water is shown blue, and vegetation is shown green. |
Species identification
Plant specimens were collected seasonally during intensive floristic surveys of the study area. The collected taxa were identified and named according to the available literature 12–20 ,and were updated according to 21 and the Kew Garden plantlist website (http://www.theplantlist.org). Life-form categories were identified 22–24. Plant chorological affinities were defined 25–30. Collections were pressed, dried, and deposited in the ASW at Aswan University in Egypt (herbarium acronyms 31).
Similarity and dissimilarity between each pair of sites within the study area estimated 32. Species richness (alpha-diversity) was calculated as the average number of species per stand 33.
Results
Floristic composition
A total of 165 taxa of vascular plants were recorded from 28 vegetation stands in the study area, belonging to 135 genera in 45 families. Among them, six species are considered new records to the riverain flora in Aswan and Nubia (Amaranthus spinosus L., Doellia bovei (DC.) Anderb., Eleocharis parvula (Roem. & Schult.) Link ex Bluff, Nees & Schauer, Haematoxylum campechianum L., Polygonum aviculare L., and Pithecellobium dulce (Roxb.) Benth.) (Table 2).
Table 2: List of the recorded plant species in Aswan Reservoir area along with their families, life span, life form, chorotypes, and presence percentage.
| Family | Species | Life span | Life form | Chorotype | P% |
| Aizoaceae | Trianthema portulacastrum L. | Annual | Hydrophyte | PAN | 66.6 |
| Amaranthaceae | Aerva javanica (Burm.f.) Juss. ex Schult. | Perennial | Chamaephyte | SU-ZA + SA-AR | 33.3 |
| Alternanthera sessilis (L.) R.Br. ex DC. | Annual | Hydrophyte-Helophyte | COSM | 66.6 | |
| Amaranthus blitum subsp. oleraceus (L.) Costea | Annual | Therophyte | COSM | 33.3 | |
| A. spinosus L.* | Annual | Therophyte | NEO | 33.3 | |
| Chenopodium album L. | Annual | Therophyte | PAL | 33.3 | |
| C. murale L. | Annual | Therophyte | COSM | 16.6 | |
| Salsola imbricata Forssk. subsp. imbricata | Perennial | Chamaephyte | SU-ZA + SA-AR + IR-TR | 66.6 | |
| Apiaceae | Cyclospermum leptophyllum (Pers.) Sprague | Annual | Therophyte | PAN | 33.3 |
| Ammi majus L. | Annual | Therophyte | ME | 16.6 | |
| Apocynaceae | Calotropis procera ( Aiton ) W.T.Aiton | Perennial | Phanerophyte | SA-SI | 83.3 |
| Leptadenia arborea (Forssk.) Schweinf. | Perennial | Phanerophyte | SU-ZA | 83.3 | |
| Nerium oleander L. | Perennial | Phanerophyte | ME | 33.3 | |
| Oxystelma esculentum (L. f.) Sm. | Perennial | Hemicryptophyte | SU-ZA + SA-SI | 16.6 | |
| Arecaceae | Hyphaene thebaica (L.) Mart. | Perennial | Phanerophyte | SU-ZA | 83.3 |
| Phoenix dactylifera L. | Perennial | Phanerophyte | SU-ZA + SA-SI | 83.3 | |
| Boraginaceae | Echium rauwolfii Delile | Annual | Therophyte | SU-ZA | 16.6 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Brassicaceae | Brassica nigra (L.) W.D.J.Koch | Annual | Therophyte | COSM | 16.6 |
| B. tournefortii Gouan | Annual | Therophyte | ME + IR-TR | 33.3 | |
| Lepidium didymum L. | Annual | Therophyte | COSM | 100 | |
| L. coronopus (L.) Al-Shehbaz | Annual | Therophyte | ME + ER-SR + IR-TR | 66.6 | |
| Eruca sativa Mill. | Annual | Therophyte | SA-SI | 33.3 | |
| Rorippa palustris (L.) Besser | Annual | Therophyte | COSM | 83.3 | |
| Casuarinaceae | Casuarina equisetifolia L. | Perennial | Phanerophyte | PAN | 16.6 |
| Ceratophyllaceae | Ceratophyllum demersum L. | Perennial | Hydrophyte | COSM | 66.6 |
| Convolvulaceae | Convolvulus arvensis L. | Perennial | Hemicryptophyte | PAN | 16.6 |
| Cuscuta pedicellata Ledeb. | Annual | Parasite | COSM | 100 | |
| Ipomoea cairica (L.) Sweet | Perennial | Phanerophyte | PAL | 33.3 | |
| I. carnea Jacq. | Perennial | Phanerophyte | PAN | 16.6 | |
| I. eriocarpa R. Br. | Annual | Therophyte | PAN | 16.6 | |
| Compositae | Ageratum conyzoides L. | Annual | Therophyte | PAN | 100 |
| Bidens pilosa L. | Annual | Therophyte | PAN | 100 | |
| Doellia bovei (DC.) Anderb.* | Perennial | Chamaephyte | ME | 16.6 | |
| Erigeron bonariensis L. | Annual | Therophyte | COSM | 16.6 | |
| Pluchea dioscoridis (L.) DC. | Perennial | Phanerophyte | SU-ZA + SA-SI | 100 | |
| Lactuca sativa L. | Annual | Therophyte | ME + IR-TR | 33.3 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Lactuca serriola L. | Annual | Therophyte | ME + IR-TR | 33.3 | |
| Blumea viscosa (Mill.) V.M.Badillo | Annual | Therophyte | PAN | 33.3 | |
| Laphangium luteoalbum (L.) Tzvelev | Annual | Therophyte | COSM | 83.3 | |
| Pulicaria undulata (L.) C.A.Mey. | Perennial | Chamaephyte | SU-ZA + SA-SI | 100 | |
| Senecio aegyptius L. | Annual | Therophyte | SU-ZA | 16.6 | |
| Sonchus oleraceus L. | Annual | Therophyte | COSM | 66.6 | |
| Symphyotrichum squamatum (Spreng.) G.L.Nesom | Perennial | Chamaephyte | NEO | 16.6 | |
| Xanthium strumarium L. | Annual | Therophyte | SU-ZA | 16.6 | |
| Cucurbitaceae | Citrullus colocynthis (L.) Schrad. | Annual | Hemicryptophyte | PAL | 50 |
| Cucumis melo L. | Annual | Therophyte | PAL | 16.6 | |
| Cucurbita pepo L. | Annual | Therophyte | PAN | 16.6 | |
| Luffa cylindrica (L.) M.Roem. | Annual | Phanerophyte | PAN | 16.6 | |
| Cyperaceae | Cyperus alopecuroides Rottb. | Perennial | Geophyte-Helophyte | PAN | 16.6 |
| C. difformis L. | Annual | Therophyte | PAN | 16.6 | |
| C. laevigatus L. | Perennial | Geophyte-Helophyte | PAN | 66.6 | |
| C. longus L. | Perennial | Helophyte | ME | 100 | |
| C. michelianus subsp. pygmaeus (Rottb.) Asch. & Graebn. | Annual | Therophyte | PAL | 33.3 | |
| C. rotundus L. | Perennial | Geophyte-Helophyte | PAN | 83.3 |
| Family | Species | Life span | Life form | Chorotype | P% | |
| Eleocharis geniculata (L.) Roem. & Schult. | Annual | Helophyte | PAN | 66.6 | ||
| E. parvula (Roem. & Schult.) Link ex Bluff. Nees & Schauer* | Perennial | Hemicryptophyte | COSM | 50 | ||
| Fimbristylis bisumbellata (Forssk.) Bubani | Annual | Therophyte | PAL | 16.6 | ||
| Euphorbiaceae | Euphorbia forsskalii J.Gay | Annual | Therophyte | SU-ZA + SA-SI | 33.3 | |
| E. heterophylla L. | Annual | Therophyte | COSM | 16.6 | ||
| E. hirta L. | Annual | Therophyte | SU-ZA | 100 | ||
| E. peplus L. | Annual | Therophyte | COSM | 83.3 | ||
| Ricinus communis L. | Perennial | Phanerophyte | PAN | 16.6 | ||
| Haloragaceae | Myriophyllum spicatum L. | Perennial | Hydrophyte | ME + IR-TR | 16.6 | |
| Juncaceae | Juncus rigidus Desf. | Perennial | Geophyte-Helophyte | COSM | 16.6 | |
| Lamiaceae | Mentha longifolia (L.) L. | Perennial | Chamaephyte | PAL | 33.3 | |
| M. pulegium L. | Perennial | Therophyte | ME + IR-TR | 16.6 | ||
| Leguminosae | Acacia farnesiana (L.) Willd. | Perennial | Phanerophyte | NEO | 83.3 | |
| A. laeta R. Br. ex Benth. | Perennial | Phanerophyte | SU-ZA | 16.6 | ||
| A. nilotica (L.) Delile | Perennial | Phanerophyte | SU-ZA | 33.3 | ||
| A. tortilis subsp. raddiana (Savi) Brenan | Perennial | Phanerophyte | SU-ZA | 50 | ||
| A. seyal Delile | Perennial | Phanerophyte | SU-ZA + SA-SI | 50 | ||
| Alhagi graecorum Boiss. | Perennial | Chamaephyte | PAL | 50 | ||
| Family | Species | Life span | Life form | Chorotype | P% |
| Astragalus vogelii (Webb) Bornm. | Annual | Therophyte | SU-ZA + SA-SI | 16.6 | |
| Cajanus cajan (L.) Millsp. | Perennial | Phanerophyte | SU-ZA | 16.6 | |
| Dalbergia sissoo DC. | Perennial | Phanerophyte | PAN | 33.3 | |
| Haematoxylum campechianum L.* | Perennial | Phanerophyte | PAN | 33.3 | |
| Indigofera oblongifolia Forssk. | Perennial | Chamaephyte | SU-ZA | 16.6 | |
| Lablab purpureus (L.) Sweet | Perennial | Chamaephyte | SU-ZA | 16.6 | |
| Leucaena leucocephala (Lam.) de Wit | Perennial | Phanerophyte | PAN | 50 | |
| Lotus arabicus L. | Annual | Therophyte | SU-ZA + SA-SI | 33.3 | |
| Medicago sativa L. | Perennial | Hemicryptophyte | PAN | 16.6 | |
| Melilotus indicus (L.) All. | Annual | Therophyte | COSM | 33.3 | |
| Pithecellobium dulce (Roxb.) Benth.* | Perennial | Phanerophyte | PAN | 16.6 | |
| Sesbania sesban (L.) Merr. | Perennial | Phanerophyte | PAL | 100 | |
| Senna didymobotrya (Fresen.) H.S.Irwin & Barneby | Perennial | Phanerophyte | PAN | 33.3 | |
| S. italica Mill. | Perennial | Chamaephyte | SU-ZA + SA-SI | 16.6 | |
| S. occidentalis (L.) Link | Perennial | Chamaephyte | PAN | 50 | |
| Tephrosia purpurea (L.) Pers. subsp. apollinea (Delile) Hosni & El Karemy | Perennial | Chamaephyte | PAN | 83.3 | |
| Trifolium alexandrinum L. | Annual | Therophyte | PAL | 16.6 | |
| T. resupinatum L. | Annual | Therophyte | ME + IR-TR | 50 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Trigonella hamosa Del. ex Smith | Annual | Therophyte | ME + IR-TR | 33.3 | |
| Vicia faba L. | Annual | Therophyte | ME + IR-TR | 16.6 | |
| Lythraceae | Lawsonia inermis L. | Perennial | Phanerophyte | SU-ZA | 16.6 |
| Ammannia baccifera L. | Annual | Therophyte | PAL | 66.6 | |
| Malvaceae | Abutilon pannosum (G.Forst.) Schltdl. | Perennial | Chamaephyte | SU-ZA | 100 |
| Bombax ceiba L. | Perennial | Phanerophyte | PAL | 16.6 | |
| Corchorus olitorius L. | Annual | Therophyte | PAL | 33.3 | |
| Hibiscus sabdariffa L. | Annual | Therophyte | PAN | 16.6 | |
| Malva parviflora L. | Annual | Therophyte | PAN | 50 | |
| Sida alba L. | Perennial | Hemicryptophyte | PAL | 16.6 | |
| Meliaceae | Khaya senegalensis (Desv.) A.Juss. | Perennial | Phanerophyte | SU-ZA | 16.6 |
| Molluginaceae | Glinus lotoides L. | Annual | Therophyte | PAL | 66.6 |
| Moringaceae | Moringa oleifera Lam. | Perennial | Phanerophyte | PAN | 16.6 |
| Myrtaceae | Eucalyptus camaldulensis Dehnh. | Perennial | Phanerophyte | AUS | 33.3 |
| Psidium guajava L. | Perennial | Phanerophyte | PAN | 100 | |
| Syzygium cumini (L.) Skeels | Perennial | Phanerophyte | PAL | 33.3 | |
| Nyctaginaceae | Boerhavia repens L. | Annual | Chamaephyte | PAL | 33.3 |
| Bougainvillea glabra Choisy | Perennial | Phanerophyte | PAN | 16.6 | |
| Onagraceae | Epilobium hirsutum L. | Perennial | Hydrophyte-Helophyte | PAL | 16.6 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Oxalidaceae | Oxalis corniculata L. | Annual | Geophyte-Helophyte | COSM | 50 |
| Papaveraceae | Argemone mexicana L. | Annual | Therophyte | PAN | 33.3 |
| Pedaliaceae | Sesamum indicum L. | Annual | Therophyte | PAL | 16.6 |
| Plantaginaceae | Plantago lagopus L. | Annual | Therophyte | ME + IR-TR | 16.6 |
| P. major L. | Perennial | Hemicryptophyte | COSM | 33.3 | |
| Plantaginaceae | Veronica anagallis-aquatica L. | Perennial | Geophyte – Helophyte | COSM | 66.6 |
| Poaceae | Arundo donax L. | Perennial | Hydrophyte | ME + IR-TR | 50 |
| Avena fatua L. | Annual | Therophyte | COSM | 16.6 | |
| Cenchrus biflorus Roxb. | Annual | Therophyte | PAL | 66.6 | |
| Chloris pycnothrix Trin. | Annual | Therophyte | SU-ZA | 16.6 | |
| Cynodon dactylon (L.) Pers. | Perennial | Geophyte | PAN | 100 | |
| Dactyloctenium aegyptium (L.) Willd. | Annual | Therophyte | PAL | 33.3 | |
| Dichanthium annulatum (Forssk.) Stapf | Annual | Geophyte | PAL | 100 | |
| Digitaria sanguinalis (L.) Scop. | Annual | Therophyte | PAN | 33.3 | |
| Echinochloa colona (L.) Link | Annual | Therophyte | PAN | 33.3 | |
| Eleusine indica (L.) Gaertn. | Annual | Therophyte | PAL | 16.6 | |
| Eragrostis cilianensis (All.) Vignolo ex Janch. | Annual | Therophyte | ME + SU-ZA + IR-TR | 33.3 | |
| Imperata cylindrica (L.) Raeusch. | Perennial | Geophyte | PAN | 100 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Lolium perenne L. | Perennial | Therophyte | ME + IR-TR | 33.3 | |
| Panicum coloratum L. | Perennial | Geophyte | SU-ZA | 16.6 | |
| P. repens L. | Perennial | Geophyte | COSM | 33.3 | |
| Paspalidium geminatum (Forssk.) Stapf | Perennial | Geophyte | PAL | 66.6 | |
| Paspalum distichum L. | Perennial | Geophyte | PAN | 50 | |
| Phragmites australis (Cav.) Trin. ex Steud. | Perennial | Geophyte-Helophyte | PAL | 100 | |
| Poa infirma Kunth | Annual | Therophyte | ME | 33.3 | |
| Polypogon monspeliensis (L.) Desf. | Annual | Therophyte | COSM | 50 | |
| Setaria viridis (L.) P.Beauv. | Annual | Therophyte | COSM | 16.6 | |
| Sorghum × drummondii (Nees ex Steud.) Millsp. & Chase | Annual | Geophyte-Helophyte | SU-ZA | 16.6 | |
| S. virgatum (Hack.) Stapf | Annual | Geophyte-Helophyte | SU-ZA | 16.6 | |
| Polygonaceae | Emex spinosa (L.) Campd. | Annual | Therophyte | PAN | 33.3 |
| Polygonum aviculare L.* | Annual | Therophyte | ME + IR-TR | 50 | |
| Persicaria decipiens (R.Br.) K.L.Wilson | Perennial | Geophyte-Helophyte | PAN | 66.6 | |
| P. senegalensis (Meisn.) Soják | Perennial | Geophyte-Helophyte | PAN | 66.6 | |
| Rumex dentatus L. | Annual | Therophyte | PAN | 50 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Portulacaceae | Portulaca oleracea L. | Annual | Therophyte | PAL | 83.3 |
| Potamogetonaceae | Potamogeton perfoliatus L. | Perennial | Hydrophyte | COSM | 33.3 |
| P. crispus L. | Perennial | Hydrophyte | COSM | 50 | |
| Stuckenia pectinata (L.) Börner. | Perennial | Hydrophyte | COSM | 50 | |
| Primulaceae | Anagallis arvensis L. | Annual | Therophyte | ME | 83.3 |
| Rhamnaceae | Ziziphus spina-christi (L.) Desf. | Perennial | Phanerophyte | ME + IR-TR | 83.3 |
| Rubiaceae | Oldenlandia capensis L.f. | Annual | Therophyte | PAL | 33.3 |
| Salicaceae | Salix tetrasperma Roxb. | Perennial | Phanerophyte | PAL | 50 |
| Sapindaceae | Cardiospermum halicacabum L. | Annual | Therophyte | PAN | 16.6 |
| Solanaceae | Solanum nigrum L. | Annual | Therophyte | COSM | 83.3 |
| Physalis angulata L. | Annual | Therophyte | PAN | 100 | |
| Datura innoxia Mill. | Annual | Therophyte | PAN | 66.6 | |
| Withania somnifera (L.) Dunal | Perennial | Chamaephyte | PAL | 16.6 | |
| Tamaricaceae | Tamarix aphylla (L.) H.Karst. | Perennial | Phanerophyte | SA-AR + SU-ZA + IR-TR | 16.6 |
| T. senegalensis DC. | Perennial | Phanerophyte | SU-ZA + SA-SI | 100 | |
| Typhaceae | Typha domingensis Pers. | Perennial | Helophyte | PAN | 50 |
| Urticaceae | Forsskaolea tenacissima L. | Perennial | Hemicryptophytes | SU-ZA + SA-AR | 33.3 |
| Verbenaceae | Lantana camara L. | Perennial | Phanerophyte | PAN | 100 |
| Phyla nodiflora (L.) Greene | Perennial | Hemicryptophyte | PAN | 100 |
| Family | Species | Life span | Life form | Chorotype | P% |
| Zygophyllaceae
|
Balanites aegyptiaca (L.) Delile | Perennial | Phanerophyte | SU-ZA + SA-SI | 33.3 |
| Fagonia indica Burm.f. | Perennial | Chamaephyte | SA-AR | 16.6 | |
| Tribulus terrestris L. | Annual | Therophyte | PAN | 50 |
Legend: (*) = new records, P %= The mean presence percentages for each species. Chorotypes abbreviations: AUS: Australian, COSM: Cosmopolitan, ER-SR=Euro-Siberian, IR-TR: Irano-Turanian, ME: Mediterranean, NEO: Neotropical, PAL: Palaeotropical, PAN: Pantropical, SA-AR= Saharo-Arabian, SA-SI: Saharo-Sindian, SU-ZA: Sudano-Zambezian.
Dicots were represented by 39 families (86,67 %) and 119 taxa (72,12%), while monocots represented by 6 families (13,33%) and 46 taxa (27,88%). Leguminosae (26 species=15.76%), Poaceae (25 species= 15.15%), and Compositae (14 species= 8.48%), were the most species-rich families. Cyperaceae and Amaranthaceae were represented by 5.45% (9 species) and 4.24% (7 species), respectively. Both Brassicaceae and Malvaceae were represented by 3.64% (6 species each), while Convolvulaceae, Euphorbiaceae, and Polygonaceae were represented by 3.03% (5 species each). Apocynaceae, Cucurbitaceae, and Solanaceae were represented by 2.42% (4 species each). Four families were represented by three species (1.82%), meanwhile, seven families were represented by two species (1.21%). On the other hand, 21 families were poorly represented, having one species each (0.61%) (Fig. 2). The most common genera with a larger number of species were Cyperus L. with six species (3.64%), Acacia Mill. with five species (3.03%), Euphorbia L. with four species (2.42%, Ipomoea L. and Senna Mill. with three species each (1.82%). Regarding the lifespan, the majority of the recorded species during this survey were perennials with 84 species of the total recorded species (50.91%), followed by the annuals with 81 species (49.09%) (Fig. 3B, D).
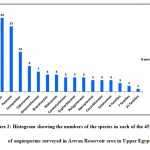 |
Figure 2: Histogram showing the numbers of the species in each of the 45 families of angiosperms surveyed in Aswan Reservoir area in Upper Egypt. |
Kidney
Comparing our floristic results with the earlier recorded from Aswan and Nubia 6,34–37, six species have been recorded for the first time and represent new additions to the floristic structure of study area (Amaranthus spinosus L., Doellia bovei (DC.) Anderb., Eleocharis parvula (Roem. & Schult.) Link ex Bluff, Nees & Schauer, Haematoxylum campechianum L., Polygonum aviculare L., and Pithecellobium dulce (Roxb.) Benth.) (Table 2).
Biological spectrum of species
Ten lifeforms were recorded in the current study. Therophytes were the most abundant lifeform of plants between Aswan High Dam and Aswan low Dam (72 species= 43.64%), followed by phanerophytes (37 species= 22.42%), Chamaephytes (16 species= 9.70%), Geophytes-Helophytes (11 species= 6.67%), Hemicryptophytes (9 species= 5.45%), Hydrophytes and Geophytes with (7 species each= 4.24%), Helophytes (3 species= 1.82%), and Hydrophytes-Helophytes (2 species= 1.21%). While Parasites were represented by a single species Cuscuta pedicellata Ledeb (0.61%) (Table 2, Fig. 3A).
Phytogeographical affinities
Phytogeographical analysis of the 165 plant species surveyed in this study revealed that pantropical (47 species= 28.48%), palaeotropical (29 species=17.57%), and cosmopolitan (27 species=16.36%) chorotypes constituted the main bulk of the recorded flora (103 species=62%) of the total recorded flora. Mono-regional chorotype was represented by 29 species (17.58%), of which 19 species were Sudano-Zambezian. On the other hand, the bi-regional chorotype was represented by 25 species (15.15% of the total flora). Mediterranean-Irano-Turanian represented by 13 species (7.88%), while 11 species (6.66%) originally came from the chorotype comprising the Saharo-Sindian and Sudano-Zambezian and only two species originated from Sudano-Zambezian and Saharo-Arabian regions (Aerva javanica (Burm.f.) Juss. ex Schult. and Forsskaolea tenacissima L.). Pluri-regional phytochoria were represented by 2.43% (4 species) of the total recorded species. (Fig. 3C).
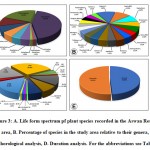 |
Figure 3: A. Life form spectrum pf plant species recorded in the Aswan Reservoir area, B. Percentage of species in the study area relative to their genera, C. Chorological analysis, D. Duration analysis. For the abbreviations see Table 2. |
Kidney
Similarity coefficient between the investigated islands
Regarding the entire flora of the study area, sites of El Shallal, Bute El-Hasaya, Tingar, and El Mahgar valley have the highest value of species richness (139, 77, 76 and 75 species, respectively). On the other hand, EL-Heisa, Philae Port and Awad islands have the lowest value of species richness (22, 22 and 19 species, respectively). There is a high similarity between the floristic composition of the following sites: Bute El-Hasaya vs High Dam Colony (96.00%) Awad island vs El Mahgar Valley (82.98%), El-Hasaya vs Maezana Belal (81.63%), Bute El-Hasaya vs. Awad island (81.01%), and EL-Heisa vs El Mahgar Valley (80.41%). However, the lowest similarity was between Bigga island vs Tingar (23.52%), Awad island vs El Shallal (22.78%), and Agilkia island vs Tingar (22.58%) (Table 3).
Table 3: The number and percentage of plant species belonging to the main floristic chorotypes and their relevant percent (%) recorded in Aswan Reservoir area, Awan Governorate, Egypt.
| Chorotype | No. of plant species | Percentage (%) |
| Cosmopolitan | 27 | 16.36 |
| Neotropical | 3 | 1.82 |
| Palaeotropical | 29 | 17.58 |
| Pantropical | 47 | 28.48 |
| Total | 106 | 64.24 |
| Monoregional | ||
| AUS | 1 | 0.61 |
| ME | 6 | 3.64 |
| SA-AR | 1 | 0.61 |
| SA-SI | 2 | 1.21 |
| SU-ZA | 19 | 11.52 |
| Total | 29 | 17.58 |
| Biregional | ||
| ME+IR-TR | 13 | 7.88 |
| SU-ZA+ SA-SI | 11 | 6.66 |
| SU-ZA+SA-AR | 2 | 0.61 |
| Total | 25 | 15.15 |
| Pleuriregional | ||
| ME+ SU-ZA+IR- TR | 1 | 0.61 |
| SU-ZA + SA-AR + IR-TR | 2 | 1.21 |
| ME + ER-SR + IR-TR | 1 | 0.61 |
| Total | 4 | 2.43 |
Discussion
The current study attempted to survey plant species distribution and diversity in the islands and shorelines with dams and associated reservoir in the south of the River Nile at Aswan Governorate between the High Dam and Aswan Dam (Low Dam). During the botanical surveys, a total of 165 taxa were recorded belonging to 134 genera in 45 families of vascular plants, of them, 119 plants belong were dicots and 46 plants were monocots. Compared to the floristic composition of other Nile ecosystems in Aswan, the quantity of species recorded in this study is within the range, since 94 species of angiosperms were recorded in the first Cataract 35, 206 species were recorded in seven islands in the Nile stream north of Aswan dam until reaching Edfu 36, and 162 species were recorded in ten River Nile islands in the area between Aswan and Esna 38. However, the floristic composition of these areas, vary with respect to the dominant plant families.
Interestingly, fieldwork and herbarium studies revealed that out of the 165 species recorded, six species were considered new to the flora of Aswan and Nubia (Table 2). The addition of these new species to the riverain flora in Aswan and Nubia from the study area can be related to the following factors: (i) a very little floristic surveys had been done in Aswan reservoir area, and (ii) human impact on the inhabited islands of the study area which resulted in the presence of seeds of ruderal weeds within the crop seeds which were derived from other agricultural areas in Egypt where the plants, seeds, manure, and agricultural equipment originated.
Table 4: Matrix of similarity coefficient, calculated between each pair of sites surveyed within the Aswan Reservoir area Awan Governorate, Egypt.
| Site Name | Sh | Bu | Ma | Hi | Ph | Mv | Ti | Aw | He | Bi | Ag |
| Sh | 62.96 | 61.24 | 48,12 | 24.84 | 42.05 | 64.18 | 22.78 | 26.08 | 42.85 | 47.05 | |
| Bu | (68) | 81.63 | 96.00 | 79.50 | 59.81 | 53.42 | 81.01 | 79.50 | 70.32 | 68.44 | |
| Ma | (64) | (60) | 50.84 | 65.21 | 41.38 | 41.09 | 67.41 | 65.21 | 53.09 | 50.84 | |
| Hi | ( 45 | (30) | (30) | 42.85 | 24.39 | 42.19 | 44.77 | 42.85 | 32.96 | 31.25 | |
| Ph | (20) | (20) | (20) | (15) | 30.92 | 30.61 | 73.17 | 68.18 | 46.15 | 42.85 | |
| Mv | (45) | (39) | (33) | (21) | (16) | 51.65 | 82.98 | 80.41 | 66.10 | 63.41 | |
| Ti | (69) | (53) | (52) | (32) | (18) | (39) | 29.47 | 28.57 | 23.52 | 22.58 | |
| Aw | (18) | (17) | (16) | (13) | (12) | (12) | (14) | 78.05 | 51.61 | 47.76 | |
| He | (21) | (20) | (20) | (17) | (12) | (17) | (18) | (16) | 58.46 | 54.28 | |
| Bi | (39) | (41) | (36) | (20) | (19) | (28) | (37) | (14) | (19) | 70.33 | |
| Ag | (44) | (44) | (42) | (20) | (16) | (28) | (40) | (13) | (18) | (32) | |
| Total number | 139 | 77 | 70 | 48 | 22 | 75 | 76 | 19 | 22 | 43 | 48 |
Bold numbers indicate the total number of species per site.
Bracketed numbers indicate the common species for each pair of sites.
Normal numbers indicate the quotient of similarity.
Sites abbreviated as follows: Sh: El Shallal, Bu: Bute El-Hasaya, Ma: Maezan Belal, Hi: High Dam Colony, Ph: Philae Port, Mv: El Mahgar valley, Ti: Tingar, Aw: Awad, He: EL-Heisa island, Bi: Bigga island, Ag: Agilkia island
Dam construction across a river is usually associated with catchment’s biological and hydromorphological features and causes great changes in limnological regime, including chemical and physical changes and which in turn lead to the growth of riparian and island plant communities with a remarkable increase in the number of plant taxa comparing with the natural environment pre-existing the dam construction 39,40.
Based on the number of species, three major families comprised 38.18% of the total flora surveyed in the study area (Leguminosae, Poaceae, and Compositae), these families were also reported as most frequent families in the floristic composition across the River Nile and the associated irrigation and drainage canals in Egypt 5,35,37,41–43. Moreover, Leguminosae, Poaceae, and Compositae were reported as the most frequent families in the floristic composition of the Nile islands at Aswan 44. These three families were reported as the most dominant in eastern Ethiopia and northern Zambia 45,46, as well as in the flora of the Mediterranean and North Africa 47. Moreover, the former three families were dominant in the floristic composition of the agro-ecosystem in Egypt 48,49. This can be attributed to their wide ecological range of tolerance, efficient seeds dispersal capabilities, migration efficiency in addition to local conditions of water depth 50,51.
The percentages of distribution of species and genera per families are both strongly directed towards the smallest size classes. Means of 1.2 species per genus, 3.6 species per family and 2.8 genera per family were recorded in the flora of the studied area. These results agree with the findings of 41 regarding the flora of riverain islands in Upper Egypt, where means of 1.3 species per genus, 3.04 species per family and 2.8 genera per family were concluded.
The current study showed that the floristic composition of the Aswan reservoir area exhibited a high degree of monotypism. A total of 21 families (12.73 %) were represented by a single species. Moreover, 116 genera (85.93 %) were monotypic. This may be due to the fact that a few numbers of plants tolerate harsh environments in these areas. In the meantime, other plants could not survive in the severe physical disturbance in Aswan Reservoir caused by the daily water fluctuation.
Distribution of life form in this study was mostly dominated by therophytes (72 species=43.64%%). This may be due to many factors such as short life cycle and high growth rate that enables them to resist substrate instability, their high ability to set seeds without the need of pollinator visit, ecological, genetic and morphological plasticity under high level of disturbance (water fluctuations, hot dry climate, topographic variation, biotic influence, and human activities) 52–57. The recorded life form spectra agree with many previous studies conducted in different riverine habitats in Egypt 41,43,58–60.
Regarding the lifespan (duration), the percentage of perennials (50.91%) exceeded that of annuals (49.09%). This trend matching the finding of 58, however, disagrees with the spectrum reported for Nubian flora and for the Egyptian flora in general 23,36,61. This may be due to the fact that perennial plants are adapted to the extreme habitat of the area (e.g., waterlogged, and water fluctuations over the year) 62.
Phytogeographical analysis of the 165 species surveyed in Aswan reservoir area showed that the pantropical, palaeotropical and cosmopolitan species, respectively were the most dominant in the study area (62.42% of the total flora). This agrees with the finding of 63 who reported that the major percentage of plants surveyed in the flora of Egypt belonged to the cosmopolitan, palaeotropical, and pantropical phytochoria. Similar results were obtained in different studies in the flora of Egypt concerned by the plants of River Nile and associated irrigation and drainage canals 36,41,60.
The Sudano-Zambezian chorotype was represented by 35 species (21.21% of the total flora recorded). While the Mediterranean chorotype was represented by 21 species (12.73%). These results were in line with other studies of the flora of Upper Egypt and Nubia, which reported that the Sudano-Zambezian elements exceed that of the Mediterranean ones in the entire flora 36,41. Moreover, 19 reported that the percentage of Sahelian and Sudanian taxa (sensu 64) is highest in Upper Egypt, while Mediterranean taxa are the lowest. This may be attributable to the narrow alluvial strips coupled with a dry and hot atmosphere in the study area which allows only a very limited movement of Mediterranean species to the Nubia 65.
The Irano-Turanian chorotype comprise 17 species (10.30%) including only 13 biregionals and 4 pluriregionals. The Saharo-Sindian elements represented by 13 species (7.88%) including 2 monoregional and 11 biregionals. The Saharo-Arabian elements represented by 5 species (3.03%) including one monoregional,2 biregionals, and 2 pluriregionals, while the remaining taxa are belonging to Australian, Euro-Siberian, and neotropical phytogeographical regions. This combination of different floristic chorotypes with variable numbers of species can be attributed to different factors such as human impact, history of agriculture, water fluctuations and capability of certain floristic elements to penetrate the study area from different adjacent phytogeographical regions 66,67.
In studying the spatial distribution of species in Aswan reservoir area, it was obvious that the number of species and their presence varied from site to another, even neighboring sites showed remarkable differences in their floristic composition. Species richness is highest in shorelines (El Shallal, Bute El-Hasaya, Tingar, and El Mahgar valley) liable to flooding, due to strong artificial and heterogeneity of these environments, compared to those of the islands in the river (EL-Heisa, Philae Port and Awad islands).
Regarding the similarity coefficient, the highest value was recorded between Bute El-Hasaya and High Dam Colony (96.00 %). This could be because of their close geographical position, and their exposure to the same conditions where they are uninhabited islands (Table 3). 68 reported that of the neighboring regions might have similarity their floristic composition if they were exposed to similar environmental conditions. However, the very low similarity was reported between Bigga island vs Tingar (23.52%), Awad island vs El Shallal (22.78%), and Agilkia island vs Tingar (22.58%), this may be due to the large distance between these sites. 69 stated that the influence of geographical distance on the floristic similarity between sites is probably related to the change of abiotic factors with the distance between them. Moreover, the dissimilarity maybe since each pair of these sites present in different habitat and have a different human impact.
Conclusion
The vegetation of Aswan Reservoir catchment area is highly diverse, characterized by 165 species representing 45 families of vascular plants. The high diversity in this section of River Nile may due to the combination of various environmental factors which is favorable for a wide range of plant species. Aswan Reservoir shows an ideal ecosystem as a study model, which provided many insights into how dams have an impact on vegetation structure over time and space. Anthropogenic activities in the study area influenced the species diversity, which has affected the number of species recorded in each site. Also, water fluctuation may be on the main reasons for the presence of many species and several newly recorded ones.
Conflict of Interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgements
We would like to express our deep gratitude to Mr. Mohamed Mahmoud & Mrs. Zainab Gaber, Department of Botany, Aswan University for their help during the field survey. We are grateful to Deanship of Scientific Research, Faculty of Aswan University, for supporting this research.
References
- Kassas, M. The River Nile ecological system: A study towards an international programme. Biol. Conserv. 1971; 4: 19–25.
CrossRef - Hefny, M. & El-Din Amer, S. Egypt and the Nile Basin. Aquat. Sci. 2005; 67: 42–50.
CrossRef - Swain, A. Ethiopia, the Sudan, and Egypt: The Nile River dispute. J. Mod. Afr. Stud. 1997; 35: 675–694.
CrossRef - El-Abassery, E. M. & Hassan, S. Nile Islands History and Future. In: Implementation of the CBD Programme of Work on Protected Areas: Progress and Perspectives. Abstracts of Poster Presentations at the Second Meeting of the Ad Hoc Open-ended Working Group on Protected Areas, 11–15 February 11. Rome, Italy: Secretariat of the Convention on Biological Diversity. 2008; pp 11.
- Springuel, I. & Murphy, K. J. Euhydrophytes of Egyptian Nubia. Aquat. Bot. 1990; 37: 17–25.
CrossRef - Springuel, I. The shoreline vegetation of the area between the two dams south of Aswan, Egypt. Proc. Egypt. Bot. Soc., Egypt 1985; 4: 1408–1421.
- Shafroth, P. B., Friedman, J. M., Auble, G. T., Scott, M. L. & Braatne, J. H. Potential Responses of Riparian Vegetation to Dam Removal: Dam removal generally causes changes to aspects of the physical environment that influence the establishment and growth of riparian vegetation. Bioscience 2002; 52: 703–712.
CrossRef - Nilsson, C. & Berggren, K. Alterations of riparian ecosystems caused by river regulation. Bioscience 2000; 50: 783–792.
CrossRef - Wantzen, K.M., Rothhaupt, K.-O., Mörtl, M., Cantonati, M., G.-Tóth, L. & Fischer, P. Ecological effects of water-level fluctuations in lakes: an urgent issue. Hydrobiologia 2008; 613: 1–4.
CrossRef - Merritt, D. M. & Wohl, E. E. Processes Governing Hydrochory along Rivers: Hydraulics, Hydrology, and Dispersal Phenology. Ecol. Appl. 2002; 12: 1071–1087.
CrossRef - Merritt, D. M. & Wohl, E. E. Plant dispersal along rivers fragmented by dams. River Res. Appl. 2006; 22: 1–26.
CrossRef - Täckholm, V. Students’ Flora of Egypt. Cairo: Cairo University press. 1974; pp 888.
- Boulos, L. Flora of Egypt: checklist. Cairo: Al Hadara Publishing. 1995; pp 283.
- Boulos, L. Flora of Egypt, Volume 1: Azollaceae – Oxalidaceae. Cairo: Al Hadara Publishing. 1999; pp 419.
- Boulos, L. Flora of Egypt, Volume 2: Geraniaceae–Boraginaceae. Cairo: Al Hadara Publishing. 2000; pp 352.
- Boulos, L. Flora of Egypt, Volume 3: Verbenaceae-Compositae. Cairo: Al Hadara Publishing. 2002; pp 373.
- Boulos, L. Flora of Egypt, Volume 4: Alismataceae-Orchidaceae. Cairo: Al Hadara Publishing. 2005; pp 617.
- Boulos, L. Flora of Egypt Checklist – Revised Annotated Edition. Cairo: Al Hadara Publishing. 2009; pp 410.
- El Hadidi, M. N. & Fayed, A. A. Materials for Excursion Flora of Egypt. Volume 15. Cairo: Taeckh. 1995; pp 233.
- El Hadidi, M. N. Flora Aegyptiaca. Volume. 1. Cairo: Palm press, 2000; pp 170.
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants. Bot. J. Linn. Soc. 2009; 161: 105–121.
CrossRef - Raunkiaer, C. The life forms of plants and statistical plant geography. Oxford: Clarendon press, 1934; pp 632.
- Hassib, M. Distribution of plant communities in Egypt. Bulletin of Faculty of Science, University of Fouad 1, 1951; 59–261.
- Ellenberg, H. & Mueller-Dombois, D. A key to Raunkiaer plant life forms with revised subdivisions. Berichte des Geobot. Institutes der Eidg. Techn. Hochschule, Stift. Rübel 1967; 37: 56–73.
- Zohary, M. Flora Palaestina: Equisetaceae to Moringaceae. Volume 1. Jerusalem: The Israel Academy of Science and Humanities, 1966; pp 364.
- Zohary, M. Flora Palaestina: Platanaceae to Umbelliferae. Volume 2. Jerusalem: Israel Academy of Science and Humanities 1972; pp 489.
- Wickens, G. E. The Flora of Jebel Marra (Sudan Republic) and Its Geographical Affinities. Kew Bulletin Additional Series V. London: HM Stationery Office 1976; pp 368.
- Feinbrun-Dothan, N. Flora Palaestina, Part 4: Alismataceae – Orchidaceae. Jerusalem: Israel Academy of Sciences and Humanities 1986; pp 461.
- Feinbrun-Dothan, N. Flora Palaestina, Part 3: Ericaceae – Compositae. (Jerusalem: Israel Academy of Sciences and Humanities 1978; pp 481.
- Pignatti, S. Flora d’Italia. Bologna, Italy: Edagricole 1982; pp 343.
- Thiers, B. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium 2017. Available at: http://sweetgum.nybg.org/science/ih/. (Accessed: 29th July 2018)
- Sørenson, T. A method of establishing groups of equal amplitude in plant sociology based on similarity of species content and its application to analyses of the vegetation on Danish commons. K. Danske Vidensk. Selsk. Skr. 1948; 5: 1–34.
- Shaltout, K. H. On the diversity of the vegetation in the western Mediterranean coastal region of Egypt. Proc. 4th Egypt. Conf. Bot., Ismaileyah, Egypt 1985; 355–1376.
- Rzóska, J. (ed): The Riverain Flora in Nubia. in The Nile, Biology of an Ancient River. Monographiae Biologicae, volume 29. Springer, Dordrecht: El Hadidi, M. N. 1976; pp 87–91.
CrossRef - Springuel, I. V. Studies on the Natural Vegetation of the Islands of the First Cataract at Aswan, Egypt. PhD. Thesis. Egypt.Botany Department, Faculty of Science, Assiut University 1981; pp 185.
- Shaheen, A. M., Sheded, M. G., Hamed, I. & Hamada, F. A. Botanical diversity of the flora of some islands in the Egyptian nubia. Proc. 1st Int. Conf. . Strat. Egypt. Herb., Egypt. J. Egypt. Bot. Soc., 2004; 161–182.
- Ali, M. M. Aquatic and shoreline vegetation of Lake Nubia, Sudan. Acta Bot. Croat. 2004; 63: 101–111.
CrossRef - Ali, A. H. Ecology and flora of plants of the Nile Islands in the area between Aswan and Esna. M.Sc. Thesis. Aswan University, Egypt 2014; pp 212.
- Ngcaba, P. & Maroyi, A. Floristic composition and diversity in tsitsa river catchment area, the eastern cape province, South Africa. J. Biol. Sci. 2017; 17: 288–297.
CrossRef - Rørslett, B. An integrated approach to hydropower impact assessment. I. Environmental features of some Norwegian hydro-electric lakes. Hydrobiologia 1988; 164: 39–66.
CrossRef - Hamed, S. T., Sheded, M. G. & Badry, M. O. Floristic Composition of Some Riverian Islands at Qena Governorate-Egypt. in 2nd International conference,Minia Univ. Egypt. J. Bot. 2012; 322: 299–322.
- Mashaly, I. A., El-Habashy, I. E., El-Halawany, E. F. & Omar, G. Habitat and plant communities in the Nile delta of Egypt II. irrigation and drainage canal bank habitat. Pakistan J. Biol. Sci. 2009; 12: 885–895.
CrossRef - Amer, W., Soliman, A. & Hassan, W. Floristic composition of Nile islands in Middle Egypt with special reference to the species migration route. J. Am. Sci., 2015; 11: 14–23.
- Ali, M. M. Studies on the shoreline vegetation of Aswan High Dam Lake (Lake Nasser) and impact of the lake on the desert. Unpublished MSc thesis, University of Assiut (Faculty of Science at Aswan), Egypt 1987; pp 237.
- Ǡfors, M. Weeds and weed management in small-scale cropping systems in northern Zambia. Department of Crop Production Science. Swedish University of Agricultural Sciences. Uppsala (Suecia) 1994; 21: pp 190.
- Tamado, T. & Milberg, P. Weed flora in arable fields of eastern Ethiopia with emphasis on the occurrence of Parthenium hysterophorus. Weed Res. 2000; 40: 507–521.
CrossRef - Quezel, P. Analysis of the Flora of Mediterranean and Saharan Africa. Ann. Missouri Bot. Gard. 1978; 65: 479–534.
CrossRef - El-Ghani, M. M. A. & Fawzy, A. M. Plant diversity around springs and wells in five oases of the western desert, Egypt. Int. J. Agric. Biol. 2006; 8: 249–255.
- Shaheen, A. M. Weed Diversity of Newly Farmed Land on the Southern Border of Egypt (Eastern and Western Shores of Lake Nasser). Pakistan J. Biol. Sci. 2002; 5: 802–806.
CrossRef - Alsherif, E. A., Ayesh, A. M. & Rawi, S. M. Floristic composition, life form and chorology of plant life at khulais region, western Saudi Arabia. Pakistan J. Bot. 2013; 45: 29–38.
- Kassas, M. Ecological consequences of water development project, Keynote paper. The Environmental Future 7. 1972; 215–246.
- Shaltout, K. H. & Al-Sodany, Y. M. Vegetation analysis of Burullus Wetland: A RAMSAR site in Egypt. Wetl. Ecol. Manag. 16, 421–439 (2008).
CrossRef - Baker, H. G. The Evolution of Weeds. Annu. Rev. Ecol. Syst. 5, 1–24 (1974).
CrossRef - Osman, A. K. E. & Abdein, M. A. E.-H. Floristic diversity of Wadi Ar’ar, Saudi Arabia. J. Taibah Univ. Sci., 2019; 13: 772–789.
CrossRef - Barbero, M., Bonin, G., Loisel, R. & Quézel, P. Changes and disturbances of forest ecosystems caused by human activities in the western part of the mediterranean basin. Vegetatio 1990; 87: 151–173.
CrossRef - Grime, J. P. Plant strategies and vegetation processes. Chichester, UK: John Wiley and Sons, 1979; pp 222.
- Kosinová, A. Weed communities of winter crops in Egypt. Preslia (Praha) 1975; 47: 58–74.
- Mashaly, I. A., El-Shahaby, O. A. & El-Ameir, Y. A. Floristic features of the canal bank habitats, Egypt. J. Environ. Sci., 2010; 39: 483–501.
- Elkordy, A., Elshikh, O. & Abdallah, N. Floristic diversity and vegetation analysis of riparian and aquatic plants of the cnals in the Sohag Governorate, Egypt. Phytol. Balc., 2019; 25: 81–95.
- Faried, A. & Amro, A. Floristic and community structure of some irrigation and drainage canals in Assiut, Egypt. Taeckh. 2016; 1–20.
- Khedr, A.-H. H., Cadotte, M. W., El-Keblawy, A. & Lovett-Doust, J. Phylogenetic diversity and ecological features in the Egyptian flora. Biodivers. Conserv., 2002; 11: 1809–1824.
CrossRef - Khedr, A. H. Floristic composition and phytogeography in a Mediterranean deltaic lake (Lake Burollos), Egypt. Ecol. Mediterr., 1999; 25: 1–11.
- El Hadidi, M. N.: Natural vegetation. In: The Agriculture of Egypt (Craig, G.n ed). Oxford: Oxford University Press. 1993; pp 39–62.
- Wickens, G. E. Some of the phytogeographical problems associated with Egypt. Publ. Cairo Univ. Herb. 1977; 7& 8: 223–230.
- Shaheen, A. M. Flora of date palm orchards in Egyptian Nubia. Proc. 2nd Inter. Conf. Biol. Sci. (ICBS), Fac. Sci., Tanta Univ., Egypt 2002; 2: 31–45.
- Mashaly, I. A. Ecological and floristic studies of Dakahlia-Damietta region. Unpublished Ph. D. Thesis. Mansoura University, Egypt 1987; pp 282.
- Shalaby, M. E. Studies on plant life at Kafr El-Sheikh province, Egypt. M. Sc. Thesis. Tanta University, Egypt 1995; pp 328.
- AlNafie, A. H. Phytogeography of Saudi Arabia. Saudi J. Biol. Sci. 2008; 15: 159–176.
CrossRef - Slik, J. W. F. et al. A floristic analysis of the lowland dipterocarp forests of Borneo. J. Biogeogr. 2003; 30: 1517–1531.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





