Manuscript accepted on : 27-Sep-19
Published online on: 30-09-2019
Plagiarism Check: Yes
Reviewed by: Abhishek Shrestha
Second Review by: Sowmi S
Impact of Camel's Milk on Aluminum Chloride (Alcl3) - Induced Toxicity in Rats
Hala A. Abdolhaleem1, Magda Abd Elaziz2, Mostafa M. Bashandy3 and Wafai Z. A. Mikhail4* 
1Dairy Department, Food Technology Research Institute, Agricultural Research Center, Ministry of Agriculture, Giza, Egypt
2Dairy Chemistry Department., Animal production Research Institute, Agricultural Research Center, Ministry of Agriculture, Dokki -Giza, Egypt
3Clinical Pathology Department, Faculty of Veterinary Medicine, Cairo University, Giza, Egypt
4Department of Natural Resources, Faculty of African Postgraduate Studies, Cairo University, Giza Egypt
Corresponding Author Email: wafai47@hotmail.com
DOI : http://dx.doi.org/10.13005/bbra/2782
ABSTRACT: The present study was carried out to evaluate the efficiency of camel's milk to ameliorate the toxicity of aluminum chloride AlCl3 on some hematological parameters; hepatic,renal functions andlipids profile; as well ashistopathological alterations of some organs. Forty rats (8 / group) were divided into 5 treatment groups:Group1: Normal rats (negative control); Group2: AlCl3induced toxicity rats (positive control); Group3: AlCl3induced toxicity rats fed with raw camel milk; Group4: AlCl3 induced toxicity rats fed with heat treated camel milk; andGroup5: AlCl3 induced toxicity rats fed with sweet acidophilus camel milk.Rats were treatedby 5ml camel’s milk 10 min before the administration of 1 ml AlCl3 (0.5 mg / kgbody weight); and had their respective doses daily for 30 successive days orally. AlCl3 oral administration resulted in a significant decrease in red blood cells count (RBC's), significant increase in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH); while hemoglobin (Hb), hematocrite (Hct), platelets(plt), reticulocytes (Ret), mean corpuscular hemoglobin concentration (MCHC) did not revealed significant changes; the obtained anemia was macrocytic normochromic. The lipids profile; hepatic and renal functions showed non significant changes between different groups; however, histopathological examination showed variable alterations of varying severity in some organs; besides their response to camel's milk administration. Camel’s milk administration in groups 3, 4, 5 alleviated the toxic effect of AlCl3 with variable degrees between different groups.
KEYWORDS: Aluminum Chloride; Camel's Milk; Kidney And Liver Functions; Lipids Profile; Macrocytic Normochromic Anemia; Red Blood Cells
Download this article as:| Copy the following to cite this article: Abdolhaleem H. A, Elaziz M. A, Bashandy M. M, Mikhail W. Z. A. Impact of Camel's Milk on Aluminum Chloride (Alcl3) - Induced Toxicity in Rats. Biosci Biotech Res Asia 2019;16(3). |
| Copy the following to cite this URL: Abdolhaleem H. A, Elaziz M. A, Bashandy M. M, Mikhail W. Z. A. Impact of Camel's Milk on Aluminum Chloride (Alcl3) - Induced Toxicity in Rats. Biosci Biotech Res Asia 2019;16(3). Available from: https://bit.ly/33Z0mMR |
Introduction
Aluminium (Al), the third most abundant element of the Earth’s crust, is found in combination with oxygen, silicon, fluorine and other elements in the soil, rocks, clays and gems; nonessential and toxic metal in humans.1 With the industrialization and consequent pollution, Al is increasingly taken into our bodies through foods, air, water, and even drugs.2 Food is the primary common source of Al; include yellow cheese, salt, herbs, spices, tea leaves, food additives.3 The use of Al and its compounds in processing(cooking utensils and containers); wrappings, packaging(Al foil); storage of food products (almost 95% beverage cans); cosmetics; and toothpaste may contribute to its presence.4,5 Al compounds are widely used in medicine e.g., antacids, phosphate binders, buffered aspirins, vaccines and allergen injections.6 Al has been recognized to be toxic for humans and animals and is involved in the aetiology of some diseases.7 Chronic exposure to Al ions may result in mood changes, dysmnesia, convulsions, muscular weakness, pathological fractures of bones. Al accumulates mainly in bones, spleen, liver and lungs.8 Daily injections of Al into rats produced severe anaemia within 2-3 weeks.9 Al has a direct effect on hematopoiesis.10 Al-induced damage to body organs has been reported in several studies which pointed out the toxic effect of AlCl3 such as hepatotoxicity, nephrotoxicity, and neurotoxicity11.AlCl3administration lead to increased activity of liver enzymes in the serum12; accumulating of Al in the kidneys destroys renal tubular cells, causing renal toxicity13; and Al-induced LPO “peroxidation of lipid” could arise from an alteration of lipid metabolism14
Fresh camel milk and its products are a good bioactive adjuvant for the people living in the arid and semiarid areas. Awareness and utilization of camel milk as health adjuvant are gradually increasing as the camel milk has been found to have unique properties of its proteins, fatty acids, richer in microminerals and vitamin C compared to milks of other animal species, such as bovine milk. Fresh and fermented camel milk are reported for improving immunity and provides particular health benefits to the consumer depending on the unique bioactive substances in milk.15 It has a high concentration of iron which makes it panacea for those who have iron-deficiency anaemia. Camel milk is unique from other ruminant milk in terms of composition as well as functionality. Moreover, it is also used for its potential therapeutic properties such as efficacy against diabetes and cancer as well as having anti-hypertensive properties.16
This study aims to evaluate the effectiveness of camel’s milk towards the toxicity of aluminium chloride AlCl3 on some hematologic parameters; hepatic, renal functions; lipids profile; and histopathology of some organs.
Materials and Methods
Materials
Aluminium Chloride anhydrous 98%; M.W. 133.34 ( Alpha Chemika India)
El-Gomhouria Co. For Trading Chemicals And Medical Appliances (Egypt).
Dromedary camel milk fresh and frozen (Animal Production Research Institute, Agricultural Research Center, Dokki- Giza).
AST/ GOT- ALT/ GPT, total and direct bilirubin, total cholesterol, triglycerides, HDL- cholesterol, urea, creatinine, and haemoglobin it’s of Biolabo, France.
Lactobacillus acidophilus DSM 20079 (Egypt Microbial Culture Collection, Microbiological Resource Centre [Cairo Mircen] Faculty of Agriculture, Ain Shams University, Shobra Khayma, Cairo, Egypt).
Animals and Experimental Diets
Forty male Sprague – dawely albino rats were obtained from Animal House at Food Technology Research Institute, Agricultural Research Center, Giza, Egypt. Rats weighing (200-250 g) were housed in plastic cages under standard condition temperature (25-27℃, humidity 30-70% and 12 hour light / dark cycles) and fed with standard pellet diet and water ad libitum. All rats were fed on basal diet for one week before starting the experiment (acclimatization period ). The basal diet consisted of corn starch (60%), casein (20%) ,corn oil ( 10%), cellulose (5%), salt mixture (4%), and vitamin mixture (1%).17
Animal Grouping And Experimental Design
The rats were divided into five groups comprising 8 rats in each group as follows:
Group1: Negative control rats (normal, fed with basal diet only).
Group2: Positive control rats (AlCl3 induced toxicity).
Group3: AlCl3 induced toxicity rats fed with raw camel milk.
Group4: AlCl3 induced toxicity rats fed with heat treated camel milk (72℃ for 15 sec. and cooling)
Group5: AlCl3 induced toxicity rats fed with sweet acidophilus camel milk (10% of starter culture added to heat treated camel milk).
Daily AlCl3 oral dose given to rats was 1 ml AlCl3 (0.5 mg / kg body weight).18 Rats were treated by 5ml camel’s milk 10 min before the administration of1 ml AlCl3.Rats received a single dose of the selected treatment daily and had their respective doses for 30 successive days orally by using a cavage needle. Twenty Four hours after the last administration fresh blood samples were collected from orbital plexus venous into heparinized test tubes for haematological analysis. A second blood fraction was collected without anticoagulant into centrifuge tubes and the serum was separated into eppendorf tubes and stored at -20℃ for analysis. Then the animals were sacrificed and some organs were collected for histopathological examination.
All the experiment was approved by the ethical committee of Cairo University in accordance with the guidelines of the National Institute of Health(NIH) for the care and use of laboratory animals in scientific investigations.
Haematological Studies
The evaluated hematological parameters in this study included estimation of red blood cells (RBC’s), hemoglobin concentration (Hb), hematocrite (Hct), mean corpuscular volume (MCV), mean corpuscular hemoglobin ( MCH), mean corpuscular hemoglobin concentration (MCHC), reticulocytes counts (Retics),and blood platelets(plt).These parameters were performed according to theadopted routine hematological procedures.19
Serum Biochemical Studies
Serum sample was examined for the enzymatic activities of alanine aminotransferase (ALT), aspartate aminotransferase (AST), and the concentration of serum bilirubin, blood urea, creatinine, and lipid profile (cholesterol, triglycerides and HDL cholesterol). These parameters were determined by spectrophotometric method using commercial diagnostic kits supplied by Biolabo, France and followed the manufacturer’s instructions.
Histopathological Studies
For histopathological sections, the liver, kidney were carefully dissected and collected as whole organ specimens for all experimental animals. The organs were fixed in 10% neutral buffered formalin solution and paraffin sections were prepared and stained with hematoxylin and eosin.20
Statistical Analysis
Data were analyzed by means of one way(ANOVA) using the software statistical program (SPSS, ver.16, USA). Data are expressed as the mean ± SE, and results were statistically significant at p≤ 0.5.21
Results
I-Clinical Signs
Rapid heartbeat, rat hair loss, outer ear hematoma, blood thinning, laziness, weight loss and diarrhoea.
II- Haematological Parameters
Results of erythrogram, blood platelets and reticulocytes as shown in Table(1)and Figs 1-3, revealed a significant difference in Hb, RBC’s, Hct, MCV, MCH, MCHCbetween different groups, while platelets and reticulocyte count showed no differences. Referring to the obtained data we can deduce that:
Relative to control group 1
RBC’s of groups 2, 3, 4, and 5 is lower by 30.9%, 9.7%, 27.5%, 15.2% respectively.
MCV of groups 2, 3, 4, and 5 is higher by 46.9%, 2.5%, 10.5%, 12.6% respectively.
MCH of groups 2, 3, 4, and 5 is higher by 46.2%, 2.02%, 24.4%, 9.2% respectively.
Relative to AlCl3 group 2
RBC’s of groups 3, 4, and 5 is higher by 30.6%, 4.8%, 22.6% respectively.
MCV of groups 3, 4, and 5 is lower by 30.2%, 24.8%, 23.3% respectively.
MCH of groups 3, 4, and 5 is lower by 30.2%, 14.9%, 25.3% respectively.
The following bar charts is showing the degree of alterations in some haematological parameters that induced by oral administration of AlCl3 (group2), and the alleviating degree of different oral camel milk treatments by (groups 3, 4, 5) compared to the control (group1).
Table 1: Results of erythrogram, blood platelets and reticulocyte count in control and treated Rats
| Biochemical test | G1 | G2 | G3 | G4 | G5 | Prop. | LSD |
| Hemoglobin (g /dL) | 17.05a±0.389 | 17.31a±0.611 | 15.55b±0.455 | 15.33b±0.374 | 15.53b±0.348 | 0.0292 S at p≤ 0.05 | 1.470 |
| RBC’s (X106 mm3) | 8.33a±0.327 | 5.76b±0.152 | 7.52a±0.455 | 6.04b±0.262 | 7.06ab±0.678 | 0.0104 S at p≤ 0.05 | 1.410 |
| Hematocrite (%) | 39.13ab±0.848 | 39.90a±1.408 | 35.88bc±1.050 | 35.33c±0.974 | 36.70abc±0.734 | 0.0424 S at p≤ 0.05 | 3.351 |
| MCV (fl) | 47.20b±2.077 | 69.34a±2.152 | 48.40b±3.884 | 52.17b±6.018 | 53.16b ±4.115 | 0.0064 S at p≤ 0.01 | 11.10 |
| MCH (pg) | 20.57c ±0.917 | 30.08a ±0.882 | 20.98c ±1.699 | 25.58b±1.474 | 22.46bc ±1.595 | 0.0031 S at p≤ 0.01 | 4.493 |
| MCHC g /dL | 43.57a ±0.055 | 43.38a ±0.126 | 43.34a ±0.193 | 43.41a±0.252 | 42.32b ±0.436 | 0.0256 S at p≤ 0.05 | 0.767 |
| Platelets(x109/L) | 409.75a ±22.295 | 292.25a ±19.598 | 384.50a ±58.506 | 399.00a ±29.637 | 318.00a ±56.006 | 0.2587 ns | 130.9 |
| Reticulocyte count % | 3.00a ±0.220 | 2.90a ±0.058 | 3.20a ±0.071 | 3.13a ±0.155 | 3.08a ±0.103 | 0.4054 ns | 0.341 |
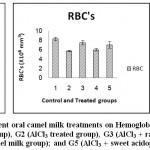 |
Figure 1: Effect of Aluminum and different oral camel milk treatments on Hemoglobin concentration; Red Blood Cells count; Hematocrite%. G1 (control group), G2 (AlCl3 treated group), G3 (AlCl3 + raw camel milk group), G4 (AlCl3 + heat treated camel milk group); and G5 (AlCl3 + sweet acidophilus camel milk). |
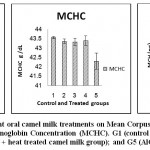 |
Figure 2: Effect of Aluminum and different oral camel milk treatments on Mean Corpuscular Volume (MCV), Mean Corpuscular Hemoglobin (MCH), Mean Corpuscular Hemoglobin Concentration (MCHC). G1 (control group), G2 (AlCl3 treated group), G3 (AlCl3 + raw camel milk group), G4 (AlCl3 + heat treated camel milk group); and G5 (AlCl3 + sweet acidophilus camel milk). |
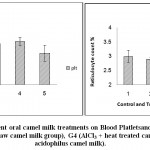 |
Figure 3: Effect of Aluminum and different oral camel milk treatments on Blood Platletsand Reticulocyte count. G1 (control group), G2 (AlCl3 treated group), G3 (AlCl3 + raw camel milk group), G4 (AlCl3 + heat-treated camel milk group); and G5 (AlCl3 + sweet acidophilus camel milk). |
III-Biochemical Parameters
III-1. Renal And Liver Functions
The results of renal function (urea and creatinine) and liver function (ALT, AST and bilirubin) tests have been summarized in Table (2) and Figs 4-6. The obtained values clarified that there were no significant differences between the different treated groups in comparison with the control one.
Table 2: Results of kidney and liver functions in control and treated rats
| Biochemical test | 1 | 2 | 3 | 4 | 5 | Prop | LSD |
| Urea mg/dl | 68.15a ±6.400 | 71.15a ±6.313 | 66.05a ±3.650 | 67.28a ±5.726 | 72.30a ±6.261 | 0.2762 ns | 6.75 |
| Creatinine mg/dl | 1.73a ±0.333 | 1.80a ±0.252 | 1.73a ±0.189 | 1.74a ±0.314 | 1.65a ±0.328 | ns | 0.41 |
| AST/GOTU/ml | 142.92a ±14.282 | 143.15a ±9.971 | 127.15a ±13.779 | 128.28a ±19.305 | 69.33b±0.394 | 0.0171 S at p≤ 0.01 | 43.68 |
| ALT/GPT U/ml | 52.32a ±9.021 | 50.09a ±3.499 | 34.95a ±7.804 | 45.48a ±7.212 | 34.74a ±4.974 | 0.3362 ns | 22.70 |
| Total bilirubin mg/dl | 0.20a ±0.033 | 0.20a ±0.018 | 0.18a ±0.015 | 0.18a ±0.026 | 0.18a ±0.034 | ns | 0.05 |
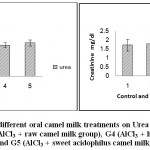 |
Figure 4: Effect of Aluminum and different oral camel milk treatments on Urea andCreatinine. G1 (control group), G2 (AlCl3 treated group),G3 (AlCl3 + raw camel milk group), G4 (AlCl3 + heat-treated camel milk group); and G5 (AlCl3 + sweet acidophilus camel milk). |
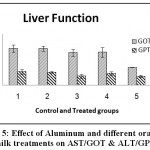 |
Figure 5: Effect of Aluminum and different oral camel milk treatments on AST/GOT & ALT/GPT. |
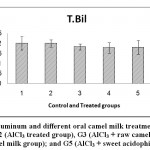 |
Figure 6: Effect of Aluminum and different oral camel milk treatments on Total Bilirubin.G1 (control group), G2 (AlCl3 treated group), G3 (AlCl3 + raw camel milk group), G4 (AlCl3 + heat treated camel milk group); and G5 (AlCl3 + sweet acidophilus camel milk). |
III-2.Lipogram Test
Compared to the control group, values of lipogram (total cholesterol, triglyceride and HDL), showed no significant difference in all treated groups as shown in Table (3), Fig.7.
Table 3: Results of lipogram in control and treated rats
| Biochemical test | 1 | 2 | 3 | 4 | 5 | Prop. | LSD |
| Chol. mg/dl | 57.64a ±10.121 | 85.91a ±21.465 | 80.72a ±20.327 | 68.14a ±5.194 | 68.08a ±18.329 | ns | 54.61 |
| TG mg/dl | 66.14a ±7.050 | 73.94a ±18.827 | 77.72a ±20.266 | 42.84a ±4.741 | 71.69a ±15.483 | ns | 47.84 |
| HDL mg/dl | 46.21a ±23.918 | 48.58a ±19.935 | 59.28a ±16.866 | 83.19a ±5.656 | 70.35a ±6.718 | ns | 53.68 |
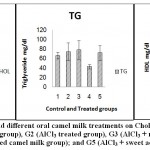 |
Figure 7: Effect of Aluminum and different oral camel milk treatments on Cholesterol(Chol.), Triglyceride(TG) and HDL. G1 (control group), G2 (AlCl3 treated group), G3 (AlCl3 + raw camel milk group), G4 (AlCl3 + heat-treated camel milk group); and G5 (AlCl3 + sweet acidophilus camel milk) |
IV-Bacterial Count
Lactobacillus acidophilus count was 1.7×107(Cfu/ml).
V-Histopathlogical Examination
Hepatic and renal of control, AlCl3, and AlCl3 +camel milk treated rats showed variable histopathological alterations of varying severity and extent. Histopathological examination of rat’s organs proved that camel milk has the capacity to reduce the aluminium toxicity in the liver, kidney.
Liver
Microscopical examination of liver sections from the control group showed no evidence of histological abnormalities. The examination revealed regular hepatocytes architecture with distinct central vein, polygonal hepatocytes arranged in strands running radially from the central vein with blood sinusoids in between these hepatic strands (Fig.8-a). On the other hand, liver sections from rats administered AlCl3 showed distorted liver architecture. focal hepatic necrosis associated with inflammatory cells infiltration and cytoplasmic vacuolization of hepatocytes were observed (Fig.8-b). Administration of camel milk (the 3 different treatments) prior to AlCl3 improved to a large extent the hepatic damage induced by AlCl3. The examined sections revealed normal architecture of the liver (Figs 8-c; d; e).
Kidneys
Kidney sections from control group revealed the normal histological structure of renal parenchyma from renal cortex and medulla (Fig.9-a). Microscopical examination of AlCl3 treated group showed necrobiotic changes of renal tubular epithelium, interstitial inflammatory cells infiltration, atrophy of glomerular tuft and distension of Bowman’s space (Fig.9-b). The different treatments of camel milk restoring the normal histological structure of renal tissue ( Figs 9- c; d; e).
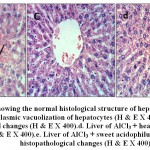 |
Figure 8: a.Liver of control rat [group1] showing the normal histological structure of hepatic lobule (H & E X 400).b. Liver of AlCl3 treated rat [group2] showing cytoplasmic vacuolization of hepatocytes(H & E X 400).c. Liver of AlCl3 + raw camel milk rat [group3] showing no histopathological changes (H & E X 400).d. Liver of AlCl3 + heat treated camel milk rat [group4] showing no histopathological changes (H & E X 400).e. Liver of AlCl3 + sweet acidophilus camel milk rat [group5] showing no histopathological changes (H & E X 400). |
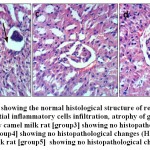 |
Figure 9: a. Kidney of control rat [group1] showing the normal histological structure of renal parenchyma(H&E X 400). b. Kidney of AlCl3 treated rat [group2] showing interstitial inflammatory cells infiltration, atrophy of glomerular tuft and distension of Bowman’s space (H&E X 400). c. Kidney of AlCl3 + raw camel milk rat [group3] showing no histopathological changes (H & E X 400). d. Kidney of AlCl3 + heat treated camel milk rat [group4] showing no histopathological changes (H & E X 400). e. Kidney of AlCl3 + sweet acidophilus camel milk rat [group5] showing no histopathological changes (H & E X 400). |
Discussion
Aluminium in its metallic form has many useful roles, such as in construction, packaging, and transport vehicles. Aluminium salts are included in pharmaceuticals, processed foods, and vaccines to enhance their qualities. Many cities use aluminium sulfate or poly aluminium chloride to clarify their drinking water. According to the World Health Organization, aluminium additives are the main source of exposure for most humans.22
Camel milk is full of balanced nutritional constituents and also displays a wide variety of biological actions that influence growth and development of particular body organs, metabolic responses towards nutrients absorption, digestion and fight against diseases.23
The present study was carried out to evaluate the efficiency of camel’s milk to ameliorate the toxicity of AlCl3 on some hematologic parameters; hepatic, renal functions; lipids profile; and histopathology of liver and kidney.
Our results showed a significant decrease in red blood cells count (RBC’s), significant increase in mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) 5.76±0.152, 69.34±2.152, 30.08±0.882 respectively in AlCl3 group compared with the control group values that were 8.33±0.327, 47.20±2.077, 20.57±0.917 respectively; while hemoglobin (Hb), hematocrite (Hct), platelets(plt), reticulocytes (Ret), mean corpuscular haemoglobin concentration (MCHC) did not revealed significant changes between two groups; the obtained anemia was macrocytic normochromic. Similar results more or less were reported in a previous studies24whichdenoted that AlCl3had led to a significant decrease (P< 0.05) in red blood cells count (RBCs), hemoglobin (Hb), mean corpuscular hemoglobin concentration (MCHC), hematocrite (Hct) and iron level, and a significant increase in the mean cell volume (MCV) and no significant change in the platelets (plt); while another study18 showed that oral AlCl3 treatment caused a significant decrease (P< 0.05) in red blood cells count (RBCs), blood hemoglobin (Hb), and hematocrite (Hct), whereas the values of mean corpuscular volume (MCV), mean hemoglobin concentration (MHC), mean corpuscular haemoglobin concentration (MCHC) didn’t change. The differences between records may be due to the experimental design adopted by the investigator. Administration of camel’s milk with AlCl3 significantly increased red blood cells count (RBCs), and decreased mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) compared with the results in rats orally administered AlCl3 alone. Thus brought the altered blood parameters to near-normal levels. The obtained macrocytic normochromic anemia (megaloblastic – pernicious anemia) may be due to vitamin B12 defficiency.25 The term macrocytosis is a disease in which not enough red blood cells are produced therefore red blood cells (RBC) are larger than normal so macrocytosis is reported in terms of mean corpuscular volume (MCV).
Vitamin B12 or cobalamin is important in normal blood formation. It is well established that gastric acid secretion is needed for dietary vitamin B12absorption from foods bound to proteins, and the presence of gastric acid is required for the peptic enzymes, mainly pepsin, allowing its reassociation with intrinsic factor (IF) and eventual absorption in the terminal ileum. Accordingly, alterations in the gastric acid secretion may lead to malabsorption of vitamin B12. The origin of these alterations could be either natural, such as ageing process, or iatrogenic, such as partial resection of the stomach or acid suppressive therapy which lead to low stomach acid with a high or alkaline pH thus foods aren’t broken down, B12 is not released. That is the way by which aluminum antagonize B12; the antagonism may not be direct but, as a result of excessive intake. For example Al- containing antacids are helpful in relieving symptoms of gastritis by neutralizing gastric acids and inhibit smooth muscle contraction, thus inhibiting gastric emptying,henceantacids may lead to malabsorption of vitamin B12.26
Our results showed that the hepatic and renal functions; lipids profile had no significant changes between different groups. It has been reported that animals exposed to AlCl3 displayed hepatic necrosis, which was indicated by an increase in serum levels of liver enzymes including AST and ALT; increase in total bilirubin in the serum increase in serum urea and creatinine; increased serum cholesterol and triglyceride levels.27 In our study, The lack of significant changes is due to a mild change in organs not lead to significant increase in current laboratory test, so the differences from other studies probably according to the magnitude of AlCl3 dose.20mg/kg body weight7; 50 mg/kg body weight28; 100 mg/kg body weight.29 However, histopathological examination showed variable alterations of varying severity in different organs. Disagreement with our results may be attributed to several reasons like experimental design, chemicals, camel milk source, the animal model, dosage and the way by which both AlCl3 and camel milk was administered, the exposure frequency and duration, environmental conditions and methods of analysis.
Exposure to high concentrations of Al (500 mg/kg body weight) can result in its accumulation in the liver and in turn to alterations in the liver function.30 Transaminases are intracellular enzymes, released into the circulation after damage and necrosis of hepatocytes, the induced histological changes in the liver are attributed to free radicals production and oxidative stress.31 Moreover, it was found that the induction rate of serum bilirubin was associated with free radical production.32
It was recorded that the kidney may be exposed to high concentrations of Al during the normal process of renal excretion making the kidney vulnerable to Al-mediated toxicity, which is dependent on the route of exposure13. Alternatively, a study33 declared that the increase in urea serum concentration in Al treated animals may be due toAl -mediated changes in liver function.
A study showed that oral administration of AlCl3 induced a significant increase in plasma level of total lipids, total cholesterol and triglycerides.7 Accumulation of AlCl3 in the liver may lead to a disturbance of lipid metabolism and in turn to the elevation in lipid profile. On the other hand are search demonstrated that treatment of rats with camel’s milk prior to AlCl3 resulted in decreased cholesterol and triglyceride levels in the serum compared to the AlCl3-intoxicated group.27
In our experiment camel’s milk administration in groups 3, 4, 5 alleviated the toxic effect of AlCl3 with variable degrees between different groups. This alleviation could be attributed to the positive health effects of camel milkas antioxidative, anti-microbial, antihypertensive or immuno-modulatory and anti-thrombotic.34 Both caseins and whey proteins of camel milk possess bioactive peptides with significant radical-scavenging activities(e.g.lactoferrin) and thus herald a fascinating opportunity for their potential as nutraceuticals or therapeutic peptides for prevention and treatment of oxidative stress-associated diseases.35 Numerous vitamins exhibit powerful antioxidant action such as D, E, A, C and vitamins of B group e.g. folic acid and B12 are found in dromedary camel milk36. Camel milk is rich in Mg and Zn.15 Magnesium (Mg) protects cells from heavy metals such as aluminium. Mg protects cells against oxyradical damage; assists in the absorption and metabolism of vitamins B, C and E; and is essential for the biosynthesis of glutathione37. Additionally, camel’s milk is rich in zinc (Zn), More than 300 enzymes require Zn for their activity, and can prevent cell damage through activation of the antioxidant system.38 Some authors reported that the major nutrients of milk were left unchanged by pasteurization; Suliman et al., (2013) showing that in general heat has very little effect on mineral content with exception of Zn39; while another concluded that pasteurization does not destroy zinc in camel milk.40
Development of the technology for special purpose dairy products based on camel milk using probiotic starter cultures that have the ability to destroy toxic metabolites and synthesize vitamins.41 Lactic acid bacteria (LAB) and more particularly lactobacilli were reported to bind heavy metals and thus represent a promising approach for decontamination of heavy metals in food and water and perhaps gastrointestinal tract as well.42
Conclusion
Indeed, it could be concluded that oral administration camel’s milk administration at a dose of 5ml camel’s milk 10 min before the administration of AlCl3 in alleviated its toxic effect. Moreover, it improved to a large extent the histological changes induced by AlCl3 in such a way that more or less normal architecture of the liver and kidney was observed. We can suggest that the best treatment was raw camel milk group; followed by sweet acidophilus milk group, and the last one was thermally treated camel milk. Therefore supplementation with camel milk may be useful as a protective therapy in cases of intoxication with aluminium.
Conflict of Interest
The authors declared that the present study was performed in absence of any conflict of interest.
Acknowledments
The author would thank all participants and their parents
Author Contributions
All authors contributed equally in all parts of this study.
References
- Schetinger, M.R.C.; Morsch V.M.; and Bohrer, D. Aluminum: Interaction with nucleotides and nucleotidases and analytical aspects of determination,2003;104, 99-138.
- Kim, M.S.; Lenore, S.; and Clesceri, L.S. Aluminum exposure: A study of an effect on cellular growth rate. Sci. Total ,2001;278(1-3),127-135.
- El-Demerdash, F.M. Antioxidant effect of vitamin E and selenium on lipid peroxidation, enzyme activities and biochemical parameters in rats exposed to aluminum. J. Trace Elem. Med. Biol., 2004; 18(1), 113-121.
- Greger, J.L. “Dietary and Other Sources of Aluminum Intake. In: Aluminium in Biology and Medicine.” Chichester: Wiley,1992; (Ciba Foundation Symposium no. 169). pp. 26-49.
- Abbasali, K.M.; Zhila, T.; and Farshad, N. Developmental toxicity of aluminum from high doses of AlCl3 in mice. J. Applied Res.,2005; 5(4), 575-579.
- Kaehny, W.D.; Hegg, A.P.; and Alfrey, A.C. Gastrointestinal absorption of aluminum from aluminum-containing antacids. New England Journal of Medicine, 1977;296(24), 1389-1390.
- Abdel-Wahab, W. AlCl3-induced toxicity and oxidative stress in liver of male rats: Protection by melatonin. Life Science Journal, 2012; 9(4), 1173-1182.
- Chen, J.; Wang, M.; Run, D.; and She, J. Early chronic aluminium exposure impairs long-term potentiation and depression to the rat dentate gyrus in vivo. Neuroscience, 2002;112 (4), 879-887.
- Mahieu, S.; Contini, M.; Gonzalez, M. et al. Aluminum toxicity. Hematological effects. Toxicology Letters, 2000;111(3), 235-242.
- Wills, M.R.; and Savory, J. Aluminum poisoning. Dialysis encephalopathy, osteomalacia and anemia. Lancet, 1983;2)8340), 29-34.
- Ibraheem, A.S.; Seleem, A.A.; El-Sayed, M.F.; and Hamad, B.H. Single or combined cadmium and aluminum intoxication of mice liver and kidney with possible effect of zinc. The Journal of Basic and Applied Zoology, 2016;77, 91–101.
- Al-Qhtani, S.A.; and Farran, S.K. The protective and therapeutic effect of resveratrol in improving renal and hepatic failure induced by aluminum chloride in experimental animals. J. Am. Sci.,2017;13(10), 26-31.
- Al Dera, H. S. Protective effect of resveratrol against aluminum chloride induced nephrotoxicity in rats. Saudi Medical Journal, 2016;37(4), 369-378.
- Belaïd-Nouira, Y.; Bakhta, H.; Bouaziz, M. et al.Study of lipid profile and parieto-temporal lipid peroxidation in AlCl3 mediated neurotoxicity. modulatory effect of fenugreek seeds.Lipids in Health and Disease, 2012;11, 16.
- Singh, R.; Mal, G.; Kumar, D. et al.Camel Milk: An important natural adjuvant.Agric. Res.,2017;6(4), 327–340.
- Sakandar, H.A.; Ahmad, S.; Perveen, R. et al. Camel milk and its allied health claims: A review. Progress in Nutrition,2018;20(Suppl.1), 15-29.
- Lane-Peter, W.A.; and Pearson, A.E. Dietary requirement in ” The Laboratory Animal Principals and Practice.” Academic press, London and New York,1971; p. 142.
- Al-Hashem, F. Camel’s milk protects against aluminum chloride-induced normocytic normocromic anemia, lipid peroxidation and oxidative stress in erythrocytes of white albino rats. American Journal of Biochemistry and Biotechnology, 2009a;5 (3), 126-136.
- Feldman, B.F.; Zinkl, J.G.; and Jain, N.C. “Schalm’s Veterinary Hematology.” 5th ed., Lippincott Williams & Wilkins,Philadelphia, London, 2000; 1120-1124.
- Bancroft, J. D.; and Gamble, M. “Theory and Practice of Histological Techniques.” 7th ed., Churchill Livingstone London, UK, 2008; pp. 125-138 and 328-329.
- SPSS “Statistical Package for Social Sciences.” computer software, 2008; ver. 16,London,UK, SPSS company.
- Walton, J. R. in book: “Encyclopedia of Environmental Health” Chapter: Bioavailable aluminum: Its effects on human healthIn: Nriagu, J.O. (ed.).Burlington: Elsevier, 2011;1, pp.331-342.
- Korhonen, H.; and Pihlanto-Leppala, A. “Milk protein derived bioactive peptides: Novel opportunities for health promotion: Dairy nutrition for a healthy future.” Bulletin-International Dairy Federation,2001; 363, 17-26.
- Osman, H.M.; Shayoub, M.E.; Babiker, E.M. et al. Effect of ethanolic leaf extract of moringaoleifera on aluminum-induced anemia in white albino rats. Jordan Journal of Biological Sciences JJBS, 2012;5(4), 255-260.
- Aslinia, F.;Mazza, J. J.; and Yale, S. H. Megaloblastic anemia and other causes of macrocytosis. Clinical Medicine and Research, 2006; 4(3), 236-241.
- Escott-Stump, S.”Nutrition and Diagnosis-related Care.” 6th ed., Lippincott Williams & Wilkins, Wolters Kluwer Health. USA, 2008; p. 382.
- Al-Hashem, F. Camel milk protects against aluminium chloride-induced toxicity in the liver and kidney of white albino rats. American Journal of Biochemistry and Biotechnology, 2009b;5 (3), 98-108.
- Abu Aita, N.A.Hepatoprotectiveeffect of spirulinaplatensis against aluminum chloride induced liver damage in rats. Global Veterinaria, 2014;13 (4), 552-559.
- Akpanyung, E.O.; Nwaokonko, D.U.; Ekong, M.B.; and Ekpo, M.M. Evaluation of the protective effect of moringaoleiferaleaf extract against aluminium induced liver damage in male albino wistar rats. International Journal of Sciences, 2018;7(02), 20-30.
- Belaïd-Nouira, Y.,Bakhta, H., Haouas, Z. et al. Fenugreek seeds reduce aluminum toxicity associated with renal failure in rats. Nutrition Research and Practice, 2013;7(6), 466-474.
- Sallie, R.; Tredger, J.M.; and Williams, R. Drugs and the liver. Part I: Testing liver function. Biopharm. Drug Dispos.,1991;12(4), 251-259.
- Mangood, S.A.; Kamal, A.M.; and Haggag, A.M. Propolis protection from toxicity caused by aluminum chloride in male rats. Isot. Rad. Res.,2012;44(3), 623-633.
- Katyal, R.; Desigan, B.; Sodhi, C.P.; and Ojha, S. Oral aluminum administration and oxidative injury. Biol. Trace Elem. Res.,1997;57(2), 125-130.
- FitzGerald, R. J.; and Meisel, H. Milk protein-derived peptide inhibitors of angiotensin-I converting enzyme. British Journal of Nutrition,2000;84(S1), 33-37.
- Ibrahim, H.R.; Isono, H.; and Miyata, T. Potential antioxidant bioactive peptides from camel milk proteins. Animal Nutrition, 2018;4(3), 273-280.
- Alhaj, O.A.; and Al Kanhal, H.A. Compositional, technological and nutritional aspects of dromedary camel milk. Int. Dairy J.,2010;20, 811–821.
- Barbagallo, M.; Dominguez, L.J.; Tagliamonte, M.R. et al. Effects of vitamin E and glutathione on glucose metabolism: Role of magnesium. Hypertension, 1999;34 (4 part 2), 1002-1006.
- Powell, S.R. Antioxidant properties of zinc. J. Nutr.,2000;130(5S Suppl.), 1447S-1454S.
- Suliman, S.O.; Abdelrahman, S.H.; and Khojali, S.M.E. Effect of heat treatment on some mineral status of camel milk. J. Anim. Sci.,2013;2(1), 4-5.
- Wernery, U.; Hanke, B.; Braun, F.; and Johnson, B. The effect of heat treatment on some camel milk constituents. Preliminary report. Milchwissenschaft,2003;58(5), 277-279.
- Tasturganova, E.; Dikhanbaeva, F.; Prosekov, A. et al. Research of fatty acid composition of samples of bio-drink made of camel milk. Current Research in Nutrition and Food Science Journal, 2018;6(2), 491-499.
- Kirillova, A.V.; Danilushkina, A.A.; Irisov, D.S. et al. Assessment of resistance and bioremediation ability of lactobacillus Strains to lead and cadmium. International Journal of Microbiology,2017;Volume 2017, 1-7.

This work is licensed under a Creative Commons Attribution 4.0 International License.





