Manuscript accepted on : 06-04-2020
Published online on: --
Plagiarism Check: Yes
Reviewed by: Hasna Abdul Salam ![]()
Second Review by: Chung Hung Hui ![]()
AMPK-Mediated Hypoglycemic Effect of Banana Stems Juice on Type 2 Diabetes
Nguyen Thi Dong1  and Phung Thanh Huong2*
and Phung Thanh Huong2*
1Haiduong Centre College of Pharmacy, Hai duong Province, Vietnam.
2Hanoi University of Pharmacy, Hanoi, Vietnam.
Corresponding Author E-mail :huongpt@hup.edu.vn
DOI : http://dx.doi.org/10.13005/bbra/2812
ABSTRACT: Musa paradisiaca L. (Musaceae) or banana is a common plant in the tropics. Its stem juice has been long used as a traditional cure for diabetic people in several tropical countries. Its hypoglycemic effect has been reported on some experimental hyperglycemic models. However, there has not been any study revealing the hypoglycemic effect and mechanism of banana stem juice in type 2 diabetes. The current study aimed at discovering its effects by a glucose tolerant test on experimental type 2 diabetic rats and an in vitro test on adenosine monophosphate - activated protein kinase (AMPK), a key enzyme in metabolic regulation. The glucose tolerance test was done after 2 weeks of banana stem juice treatment. The glucose AUC of the treated rats was significantly lower (p<0.05%) compared with that of the controls. At the concentration of 50 µg/ml, the banana sample significantly increased the quantity of phosphorylated AMPK, the active form of AMPK, in C2C12 skeleton cells (p<0.001). In conclusion, the glucose tolerance enhancing effect of the banana stem juice was explained by the activation of AMPK which plays an essential role in metabolic homeostasis.
KEYWORDS: AMPK; Glucose Tolerance; Musa Paradisiaca; Stem Juice; Type 2 Diabetes
Download this article as:| Copy the following to cite this article: Dong N. T, Huong P. T. AMPK-Mediated Hypoglycemic Effect of Banana Stems Juice on Type 2 Diabetes. Biosci Biotech Res Asia 2020;17(1). |
| Copy the following to cite this URL: Dong N. T, Huong P. T. AMPK-Mediated Hypoglycemic Effect of Banana Stems Juice on Type 2 Diabetes. Biosci Biotech Res Asia 2020;17(1). Available from: https://bit.ly/2Vm6o8N |
Introduction
Musa paradisiaca (M. paradisiaca) L. (Musaceae) or banana is a common plant in the tropics. Stem juice of M. paradisiaca has been long used as a traditional cure for diabetic people in several tropical countries, including Vietnam. It was not until recently, in the global trend of using natural antidiabetic products which are safe, effective and at reasonable costs, banana became the focus of scientists1-3. In our preliminary studies, banana had significant hypoglycemic effects on both streptozocine (STZ)-induced hyperglycemic mice and high fat (HF) diet-induced type 2 diabetes (T2D) mice (data not shown). Similarly, in the study of Eleazu et al, STZ-injected rats fed with M. paradisiaca had notably lower fasting blood glucose (FBG) level compared to those fed with standard diet1. Moreover, Ajiboye reported that M. paradisiaca feeding significantly reduced FBG level, with remarkable elevation in insulin and glycogen levels in alloxan-induced hyperglycemic rats2. However, there have not been any study revealing the hypoglycemic effect and mechanism of banana stem juice in type 2 diabetes.
Adenosine monophosphate – activated protein kinase (AMPK) emerges as an important target for type 2 diabetes treatment due to its function as an essential mediator of energy metabolism and a regulating center of mitochondrial dynamics 4. AMPK activation in skeletal muscle enhances glucose disposal and lowered plasma glucose level in diabetic mice 5. A number of antidiabetic agents, including metformin, the most important drug in T2D, have AMPK-dependent mechanisms 6.
The aims of this study is discovering the antidiabetic effects of Musa paradisiaca stem juice by a glucose tolerant test on experimental T2D rats and an in vitro test on AMPK activation.
Methods
Sampling
Stems of Musa paradisiaca L. were collected from Bacninh province, Vietnam after being authenticated by the Department of Botany, Hanoi University of Pharmacy, Hanoi, Vietnam with voucher number HNIP18149/16. The samples were then crushed and pressed to obtain the juice. The filtered juice was evaporated to residue at reduced pressure (yield was 1.6%). The residue was suspended in a 0.5% NaCMC solution to a 50 mg residue/ml suspension for the in vivo test. The residue was suspended in 1% DMSO for the in vitro test.
Animals
Healthy adult male Wistar albino rats weighing 80±20g were used. The rats were kept under standard conditions (25 ± 5°C; 12-h light and 12-h dark cycle; 35-60% humidity) and were provided with water ad libitum.
Glucose Tolerant Test
T2D was induced by the method previously described by Srinivasan7 with a combination of HF diet and low dose of streptozocine (STZ). The T2D rats were divided into 4 groups (n= 8). Group 1 included normal rats administered with vehicle; group 2 was T2D rats administered with vehicle; group 3 was T2D rats treated with the banana sample suspension at the dose of 10 ml/kg (equivalent to 500 mg residue/kg), group 4 was T2D rats treated with metformin (120 mg/kg). After 15 days of treatment, an oral glucose tolerance test (OGTT) was done according to the method described by Kwon8. FBG levels was determined using GOD method (Accu-check Active, Roche) at 0; 30; 60; 90 and 120 minutes after the glucose loading. The increment in blood glucose level (FBG) after the glucose loading was expressed by area under the curve (AUC). The in vivo research was complied with all the relevant national and institutional regulations for the care and use of animals and was approved by the IACUC committee of the Haiduong Central College of Pharmacy.
Cell Culture and Differentiation
Mouse C2C12 skeletal myoblasts (Clone CRL-1772) (ATCC) were cultured in DMEM with a supplement of 100 u/ml penicillin; 100 mg/ml streptomycin, 10% fetal bovine serum (Invitrogen) at 37oC in an incubator with humidified atmosphere containing 5% CO2. The cells were reseeded in six-well plates for a further 24h incubation and then the medium was switched to the differentiation medium (5% horse serum added DMEM) for 72h. Before experiments, the samples were added into the wells for 1h incubation.
Western Blot (WB)
The C2C12 cells were washed twice with ice-cold PBS and then lysed with ice-cold ECB (50 mM Tris-Cl, pH 7.4, 120 mM NaCl, 1 mM EDTA, 0.5% Nonidet P – 40, 50 mM NaF) for 30 min. The lysates were then centrifuged at 12,000xg for 15 min at 4oC for supernatants. 30 mg of the total proteins were resolved by 12% SDS-PAGE gel before transferring onto PVDF membranes (Millipore). The membranes were incubated with 5% non-fat dry milk and then were probed overnight with phosphorylated-AMPK (p-AMPKα Thr172) antibody (Cell Signaling Technologies) at 4oC, followed by incubation with horse radish anti-rabbit IgG, HRP-linked secondary antibody (Cell Signaling Technologies) for 2 h. Immunoreactive bands were exposed on X-ray film using the enhanced chemiluminescence WB detection system (GE Healthcare). The band density was quantified using ImageJ software. The level of each protein was normalized to β-actin.
Statistical Analysis
Data were presented as means ±SE. Two-tailed Student’s t-test was used to compare values between two groups using the SPSS 16.0 software. p-values p<0.05 were considered as statistically significant.
Results
Effect of Banana Stem Juice Extract on Glucose Tolerance of Type 2 Diabetes Rats
After the OGTT, the glucose retention in blood, expressed by AUC, of the T2D rats increased significantly compared with that of the normal control (p<0.001). Treatment with metformin (group 3) and the sample (group 4) remarkably lowered the AUC of the T2D rats (p<0.05% and p<0.01%, respectively) (Figure 1).
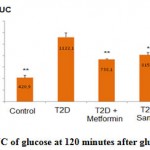 |
Figure 1: AUC of glucose at 120 minutes after glucose loading |
(*): p<0.05; (**) p<0.01
AUC of the rats in group 3 (metformin treated) and group 4 (sample treated) was significantly lower (p<0.01% and p<0.05%, respectively) compared with that of the group 2 (untreated rats).
Effect of Banana Stem Juice Extract on AMPK of the C2C12 Cells
The activation of AMPK was evaluated by the quantity of its active form (phosphorylated-AMPK). Different concentrations of the banana samples were incubated with the C2C12 cells. Aicar (5-aminoimidazole-4-carboxamide-1-D-ribonucleoside) was used at the concentration of 1mM as positive control. The p-AMPK quantity of the aicar treated cells doubled that of the untreated cells (p<0.001). There was a gradual increment of p-AMPK following the increment of sample concentration. However only at the concentration of 50 µg/ml, the banana sample significantly increased the quantity of p-AMPK (p<0.001) (Figure 2).
 |
Figure 2: Activation of AMPK in C2C12 cells |
(*): p<0.001. The quantity of AMPK active form (phosphorylated-AMPK) significantly increased in the cells treated with either Aicar or the sample at concentration of 50 µg/ml (p<0.001)
Discussions
There have been a number of studies on the effect of various parts of M. paradisiaca on glucose tolerance of STZ-induced hyperglycemic rats9, 10. However, this is the first study on M. paradisiaca effects on glucose tolerance of HF diet–induced T2D rats. This model which was established by a complex of insulin resistance due to obesity and impaired pancreas function due to a low dose of STZ are more stable and more resemble to human type 2 diabetes pathology11, 12. In the current study, there was a significant increase in the glucose AUC after oral glucose loading in the T2D rats compared with the non-diabetic rats. This effect was also reported by various studies with HF diet-induced type 2 diabetes7, 13. On such T2D rats with impaired glucose tolerance, both the banana extract sample and metformin significantly reduced the glucose retention at 120 minutes after the glucose load. The benefits of banana stem juice on glucose tolerance was also reported on STZ-induced hyperglycemic rats10. The amelioration of glucose tolerance may be due to the enhancement of glucose utilization in the body tissues of diabetes rats. In fact, metformin is well known for glucose homeostasis effects mediated by AMPK activation6, 14. That’s why we continued to evaluate the in vitro effect of the banana extract on AMPK.
AMPK plays a central role in metabolic regulation, including the metabolism of protein, lipid glucose and autophagy/ mitochondrial homeostasis6. Once activated, AMPK phosphorylates and activates glucose transporters, thereby increasing glucose uptake into cells and on the other hand, inhibits expression of the gluconeogenic enzymes 4. Therefore, AMPK has emerged as a target for diabetes and other metabolic syndrome15. Notably, AMPK is among the most essential targets in T2D treatment because its function as an energy sensor of the cell and its activation modulate a number of downstream proteins which are also T2D targets such as glucose transporter 4 (GLUT4), Acetyl-coA Carboxylase (ACC), glucose-6-phosphatase (G6Pase) phosphoenol pyruvate carboxykinase (PEPCK) 16…One of the most important mechanism of metformin, the first line T2D medicine is AMPK activation 6. A number of antidiabetic herbal medicines or natural compounds such as resveratrol, epigallocatechin gallate, berberin, curcumin, ginsenosides from Panax ginseng have shown in vitro AMPK activation effect17. AMPK activity is stimulated more than 100 folds by phosphorylation of Thr172 residue18, 19. Therefore, in order to evaluate the effect of the banana extract on AMPK, we used a specific p-AMPK Thr172 antibody for the immunoblotting quantification of AMPK activation in C2C12 muscle cells. Aicar, the firstly discovered pharmacological activator of AMPK which mimics all effects of AMP on the AMPK system20 was chosen as the positive control. Both Aicar and the banana extract sample at the concentration of 50 µg/ml remarkably increased the p-AMPK quantity (p<0.001). The ability of M. paradisiaca to activate AMPK in the muscle cells help to explain its beneficial effect on glucose tolerance of the type 2 diabetes in the current study and its anti-hyperglycemic effects on various experimental models in general.
It should be noted that banana stem which is a by-product of banana fruit harvesting is very easy to be collected and used. Therefore, the revealing of antidiabetic effects and mechanisms of banana stem would open a perspective of utilizing such a cheap and eco-friendly material for diabetes treatment, especially in low and middle income countries.
Conclusion
M. paradisiaca stem juice improved glucose tolerance in HF diet-induced T2D rats. The benefit of M. paradisiaca stem juice was explained partly by the activation of AMPK which plays a key role in metabolic homeostasis in muscle cells. These results explain the benefits of banana stem juice used as a folklore remedy in T2D people. There should be further studies on the active ingredients involved in its hypoglycemic effects.
Funding
The study was funded by Haiduong Department of Science & Technology (Grant No YD.23.CĐD.16-17)
Conflict of Interest
Authors state no conflict of interest
References
- Eleazu CO, Okafor P. Use of unripe plantain (Musa paradisiaca) in the management of diabetes and hepatic dysfunction in streptozotocin induced diabetes in rats. Interventional medicine & applied science. 2015;7: 9-16.
CrossRef - Ajiboye BO, Oloyede HOB, Salawu MO. Antihyperglycemic and antidyslipidemic activity of Musa paradisiaca-based diet in alloxan-induced diabetic rats. Food science & nutrition. 2017;6: 137-145.
CrossRef - Nguyen D, Novakova A, Spurna K. Antidiabetic Compounds in Stem Juice from Banana2017.
- Herzig S, Shaw RJ. AMPK: guardian of metabolism and mitochondrial homeostasis. Nature reviews. Molecular cell biology. 2018;19: 121-135.
CrossRef - Cokorinos EC, Delmore J, Reyes AR, et al. Activation of Skeletal Muscle AMPK Promotes Glucose Disposal and Glucose Lowering in Non-human Primates and Mice. Cell Metab. 2017;25: 1147-1159.e1110.
CrossRef - Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60: 1577-1585.
CrossRef - Srinivasan K, Viswanad B, Asrat L, Kaul CL, Ramarao P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol Res. 2005;52: 313-320.
CrossRef - Kwon S, Kim YJ, Kim MK. Effect of fructose or sucrose feeding with different levels on oral glucose tolerance test in normal and type 2 diabetic rats. Nutrition research and practice. 2008;2: 252-258.
CrossRef - Abdel Aziz SM, Ahmed OM. Antihyperglycemic Effects and Mode of Actions of Musa paradisiaca Leaf and Fruit Peel Hydroethanolic Extracts in Nicotinamide/Streptozotocin-Induced Diabetic Rats2020;2020: 9276343.
CrossRef - Singh SK, Kesari AN, Rai PK, Watal G. Assessment of glycemic potential ofMusa paradisiaca stem juice. Indian J Clin Biochem. 2007;22: 48-52.
CrossRef - Karasawa H, Nagata-Goto S, Takaishi K, Kumagae Y. A novel model of type 2 diabetes mellitus based on obesity induced by high-fat diet in BDF1 mice. Metabolism. 2009;58: 296-303.
CrossRef - Guo X-X, Wang Y, Wang K, Ji B-P, Zhou F. Stability of a type 2 diabetes rat model induced by high-fat diet feeding with low-dose streptozotocin injection. Journal of Zhejiang University. Science. B. 2018;19: 559-569.
CrossRef - Leng YP, Qiu N, Fang WJ, Zhang M, He ZM, Xiong Y. Involvement of increased endogenous asymmetric dimethylarginine in the hepatic endoplasmic reticulum stress of type 2 diabetic rats. PLoS One. 2014;9: e97125.
CrossRef - Song R. Mechanism of Metformin: A Tale of Two Sites. Diabetes Care. 2016;39: 187-189.
CrossRef - Zhang BB, Zhou G, Li C. AMPK: An Emerging Drug Target for Diabetes and the Metabolic Syndrome. Cell Metabolism. 2009;9: 407-416.
CrossRef - Cantó C, Auwerx J. AMP-activated protein kinase and its downstream transcriptional pathways. Cellular and molecular life sciences : CMLS. 2010;67: 3407-3423.
CrossRef - Hardie DG. AMPK: a target for drugs and natural products with effects on both diabetes and cancer. Diabetes. 2013;62: 2164-2172.
CrossRef - Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116: 1776-1783.
CrossRef - Willows R, Sanders MJ, Xiao B, et al. Phosphorylation of AMPK by upstream kinases is required for activity in mammalian cells. Biochem J. 2017;474: 3059-3073.
CrossRef - Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229: 558-565.
CrossRef
List of Abbreviations
AMPK, AMP-activated protein kinase; AUC, area under the curve; DMEM, Dulbecco’s modified eagle medium; DMSO, Dimethyl sulfoxide; FBG, fasting blood glucose; HF, high fat; M. paradisiaca, Musa paradisiaca; NaCMC, Sodium Carboxymethyl Cellulose; STZ, Streptozocine; T2M, type 2 diabetes.
(Visited 1,249 times, 1 visits today)
This work is licensed under a Creative Commons Attribution 4.0 International License.





