How to Cite | Publication History | PlumX Article Matrix
Preventive Effects of Dietaryraisins on Steroid - Induced Bone Changes in Rats
Al-Qtaitat Aiman1* , Al-Dalaien Said2 , Albtoosh Amal1and Fardous Karawya1
, Al-Dalaien Said2 , Albtoosh Amal1and Fardous Karawya1
1Department of Anatomy and Histology, Faculty of Medicine, Mutah University, Jordan
2Department of Pharmacology, Faculty of Medicine, Mutah University, Jordan
Correspondent Author Email: aimanaq@mutah.edu.jo
DOI : http://dx.doi.org/10.13005/bbra/2886
ABSTRACT:
Glucocorticoids is the most common cause of secondary osteoporosis. Osteoporosis is widespread, costly and causes pain, deformity and disability. Several studies report health benefits of raisins. Raisins have a combination of compounds with antibacterial, antioxidant,anticarcinogenic and anti-inflammatory properties. The present study investigated the impact of Raisins onmethylprednisolone-induced osteoporosis in rats. Thirty male albino rats were randomly divided into three main groups, ten rats each. Group I control group; Group II, osteoporotic group, where osteoporosis was induced by injection of methylprednisolone; Group III, protected group, animals were given raisinsconcomitant daily with methylprednisolone. Bone biochemical markers were assisted, hydroxyproline, urinary calcium excretion, serum calcium, alkaline phosphatase and osteocalcin. In addition to histological findings in lumbar vertebrae of the rats.Our findings showed that raisin has a positive osteoprotective effects on methylprednisolone induced bone changes histologically and over the biomarkers examined.This study suggests that dietary raisins may moderate methylprednisolone bone induced changes.
KEYWORDS: Alkaline Phosphatase; Hydroxyproline; Methylprednisolone; Osteoporosis; Osteocalcin; Raisins
Download this article as:| Copy the following to cite this article: Aiman A, Said. A, Amal A, Karawya F. Preventive Effects of Dietaryraisins on Steroid - Induced Bone Changes In Rats. Biosci Biotech Res Asia 2020;17(4). |
| Copy the following to cite this URL: Aiman A, Said. A, Amal A, Karawya F. Preventive Effects of Dietaryraisins on Steroid - Induced Bone Changes In Rats. Biosci Biotech Res Asia 2020;17(4). Available from: https://bit.ly/2KxVqu8 |
Introduction
Corticosteroids are an integral part of many chemotherapy protocols. They are commonly prescribed for different type of diseases in medicine. Steroid-induced osteoporosis is iatrogenic disorder; itis a major disabling complication that require special attention and treatment [1-4].Osteoporosis is an escalating metabolic bone disease associated with both poor bone and muscle mass. Its clinical manifestation is the fragility fracture of which the femoral neck fractures are the most serious. The magnitude of the problem presented by hip fractures is considerable, especially as their frequency is increasing in many countries.The definition of osteoporosis varies among authors.True osteoporosis is when bone strength and mass cannot meet the needs of muscle strength and physical activities so that spontaneous fractures and bone pain occur, mainly in the spine; an intrinsic bone disorder would cause this problem.Osteoporosis can be classified into primary osteoporosis (e.g. postmenopausal and senile osteoporosis) and secondary osteoporosis; some patients may have a combination of primary and secondary causes. Secondary (OP) can occur at any age and is equally common in men and women. The etiology of osteoporosis is multifactorial and involves, for example, genetic factors, life style factors and sex hormone/endocrinology changes with age. Glucocorticoids is the most common cause of secondary osteoporosis,Glucocorticoid-induced osteoporosis (GIO),leading to fracture in patients. For example, methylprednisolonecan induce osteoporosis which causes profound reduction of bone mineral density (BMD), bone quality, bone formation, and bone mechanical properties which lead to fracture. The same pathophysiologic mechanisms responsible for primary osteoporosis could occur in some of the different types of secondary osteoporosis [5-7]. Although, there are histological differences between steroid-induced osteoporosis and primary osteoporosis there are secondary types of the disease that do not have specific histological features [8].
Dietary calcium, protein phosphorus and vitamin D play active roles in bone metabolism in addition to others which affects bone directly or indirectly (for review see Palacios C, 2006) [9]. Recent research has found that many food substances such as olive oil, soybeans, blueberries, fish oil and dried fruits may a positive effects on bone metabolic diseases [9-12].Boron is a trace element capable of enhancing the development and the growth of bone, vitamin D, and affecting the absorption and elimination its minerals, including calcium, phosphorus and magnesium. It has been shown to be very beneficial for bones and joints, and it is mainly helpful in reducing the complications of post-menopausal osteoporosis.Furthermore, boron deficiency can produce brain changes [7-10]. The top boron-rich food sources are fruits, dried fruits, vegetables, and nuts. Among the dried fruit products, raisins (Vitis vinifera L.) have the highest total phenolic concentrations, antioxidant activity, andis the top nutrient-dense foods with boron[10-16]. Raisins are dried grapes largely consumed for it nutritional value all over the world. Its health beneficial properties has been increasingly reported in literature. This may be contributed to its nutritional composition and its being rich polyphenols. Furthermore, raisins have been used since ancient times for energetic value and nutritional composition. Several studies have been demonstrated the health benefits of raisins, due to their high levels of flavonoids and polyphenols, which have antioxidant properties[17-28].The present study was conducted to evaluate the effects of raisin consumption on steroid – induced bone changes in young male albino rats.
Material and Methods
Chemicals
Methylprednisolone(MP) and all chemicals for sensitive biochemical assays were acquired from Sigma chemical company (St. Louis, MO, USA). Raisin juice was prepared fromdried black grapes (raisin).
Experimental design
The ethical regulations of the Medical Research Ethics Committee (Faculty of Medicine, Mutah University, Jordan) were followed in the management of thirty male albino rats (Sprague Dawley strain), average weight 150-200g. They were fed on basal diet for adaptation and supplied with water ad libitum. The environmental conditions (temperature, humidity and light) were standardized for accommodation of the rats. They were obtained from the animal house of MutahUniversity. The rats were randomly divided into three main groups, 10 rats each.Group I, Normal control group. Animals in this groupwere further subdivided into two subgroup 5 rats each, the first subgroup received no treatment for 4 weeks, while the second subgroup received treated with raisin alone (6 gm per day for each rat orally) for 4 week.Group II, osteoporotic group, where osteoporosis was induced by subcutaneous injection of methylprednisolone 28 mg/kg/week for 4 week. Group III, protected group, animals in this group were given 6 gm of raisin per ratorally, from the first day of experiment,concomitant daily withmethylprednisolone 28 mg/kg/week for 4 week [29, 30].
Collection of urine
24-hour before scarification each rat was kept in a special cage with perforated stand to calculate the urine output for 24 hours.Urine passed to a collecting bottle via a glass funnel fixed under each cage. Urine samples were collected in dry test tubes centrifuged and kept at –20ºC until being analyzed.
Preparation of serum
Blood samples were obtained from the heart of the rat. Blood was then collected into a clean dry non-heparinized Wassermann tubes for separation of serum. The serum was separated by centrifugation at around 3000 rpm for 15 minutes and was stored at –20ºC until assayed.
Biomarkers
the following bone biomarkers were analyzed;Hydroxyproline (mg/L) and Urinary calcium excretion (mmol/L) both demonstrating bone resorption. For bone formation Osteocalcin (ng/ml), Alkaline phosphatase (IU/L) and Serum calcium.
Histological preparation
Followingscarification of the animals on day 28,lumber vertebrae were cleaned from muscle fibers and excised using bone cutter. The bones were placed in 10% formalin solution for 48 hours and then decalcified using EDTA.They were embedded in paraffin blocks. Thin sections of 5 µm thick, were prepared for staining by Hematoxylin and eosin stain and trichrome stain. Sections were then examined under light microscopy.
Statestical analysis
Results were statistically analyzed using SPSS software v.20. The values were expressed as mean±SE. The difference between groups was determined using ANOVA test, p< 0.05 values were considered significant.
RESULTS
Biochemical Results
The present study was carried out to estimate the impact of raisins on methylprednisolone for 4 week induced bone changes in young male albino rats by assessing some biochemical markers of bone turnover,bone formation as well as the histological findings in lumbar vertebrae.Table 1 shows the results of the five biochemical markers of bone turnover expressed as mean±SE in the studied groups.
Table 1: The levels of examined biochemical markers of bone turnover in the studied groups expressed as mean±SE
| Group I
Control group n=10 |
Group II
Osteoporotic group n=10
|
Group III
Protected group n=10 |
|
| Urinary Hydroxyproline (mg/L) | 15.48±0.47 | 23.49±1.01 | 17.09±0.92 |
| Urinary Calcium (mmol/L) | 2.75±0.29 | 4.32±0.45 | 3.01±0.34 |
| Serum Osteocalcin (ng/ml) | 5.12±1.07 | 8.64±0.66 | 5.48±0.44 |
| Serum Alkaline phosphatase (IU/L) | 111.61±1.96 | 125.65±4.97 | 113.60±1.73 |
| Serum Calcium (mg/dl) | 10.14±0.20 | 8.97±0.40 | 9.82±0.23 |
Markers of Bone Resorption
Urinary hydroxyproline was one of the main bone resorption markers available.As illustrated in Table 1 methylprednisolonetreated group (group II) was associated with a significant increase of urinary hydroxyproline excretion when measured 4 week after the experiment compared to control group (group I) (p<0.001). In group III, where methylprednisolonetreated rats concomitant with raisins has been associated with a significant decrease in hydroxyproline level (p<0.001). On the other hand, elevated calcium excretion is generally included among the factors predisposing to osteoporosis. There was an increase of urinary calcium excretion in group IIcompared to a normal control group (group I) (p<0.05). Group III has been associated with a significant decrease ofurinary calcium excretion compared to group II (p<0.05).
Markers of Bone Formation
Alkaline phosphatase and osteocalcin were used clinically as a marker of osteoblast activity.There was a significant increase in serum level of osteocalcin in group II compared to the normal control group (p<0.05).Concomitant treatment with raisinswithmethylprednisolone(group III)was associated with a significant decrease in serum osteocalcin when compared group II (p<0.01). On the other hand, osteoporotic group (group II) showed asignificant increase of serum alkaline phosphatase level as compared to the normal control group (p<0.05). Furthermore, treatment withmethylprednisoloneand raisins (group III) compared with methylprednisolone treatment alone (group II) associated with a significant decrease in serum alkaline phosphatase level (p<0.05). Moreover, group II presented a significant decrease in serum calciumcompared to the normal control group (group I) (p<0.05).However, there was a significant increase of serum calcium in group III compared to group II (p>0.05).
Histological Results
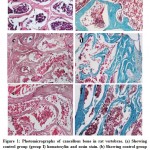
Sections stained with hematoxylin and eosin stain and trichrome stain of the lumber vertebrae were examined of all three groups.Group I;this group revealed typical picture of cancellous bone with regularly thickened, classical regular parallel bone lamellae,multiple branched bony trabeculae delimiting the bone marrow spaces containing red bone marrow. Regularly oriented osteocytes were resident in their lacunae over the bone trabeculae (Figure 1a, b).Group II; this group revealed irregular bone lamellae with variable thickness with increased number of osteoclast.Some of them showed marked thinning and disturbed with anastomosing bone marrow cavities, splitting and separation of bone lamellae, ill-defined immature (osteoid) tissue,and other bone lamellae appeared pale stained with many widened lamellae. The osteocytes are randomly oriented in their lacunae, and some of these lacunae appeared either empty or with peripherally located osteocytes (Figure 1c, d). Group III; this group presented variable degrees of bone affection with widened separated lamellae, trabeculae appeared thinned in some parts with anastomosing bone marrow cavities, many irregular bone lamellae with randomly oriented osteocytes presenting newly formed woven bone were also noticed. The periosteum was thickened and highly cellular (Figure 1e, f).
Discussion
Synthetic glucocorticoids are indicated in various conditions. Their use have been accompanied with many side effects in many systems within the human body, particularly those over the skeletal system,consequential producing osteoporosis.Treatment of osteoporosis emphasis on preventing bone resorption and affecting the remodeling process.Prophylactically management of osteoporosis are limited to calcium and vitamin D[31-34].Diet and health have been linked since ancient times. Recently, remedies of specific food and diets are recommended for certain illnesses for their preventive values. Chronic diseases, like osteoporosis, have been recently treated with herbal plants due to the recent advancement in phytotherapy.Few herbal plants have been investigated clinically to assess their therapeutic effect on bone [35]. Dried fruits provide healthy bone, e.g. prunes, because it contains vitamin K, manganese, boron, copper and potassium [36]. Raisins are rich in potassium, magnesium, and iron; and they have low sodium compared to other fruits.Additionally, raisins offer high fiber content, essential nutrients and protective components [37]. There are few studies concerning human health and raisins. Most of these studies are related to cardiovascular diseases, diabetes and oral health[19, 24].The bone biochemical and histological data presented above confirm that raisins have the ability to reduce the side effects of glucocorticoids over bone in GIO rats. Raisins are extremely rich in boron. Boron is supposedto be necessary for bones and joints development [37, 38]. As an estimate of bone resorption, the fasting urinary hydroxyproline excretion and urinary calcium excretion were measured. Hydroxyproline and urine calcium levels were found to increase in the methyl prednisolone (MP ) treated group. These results are in agreement with other studies [39], which showed that glucocorticoids increase the urine calcium level and decrease serum calcium levels in GIO. Additionally, in postmenopausal women, there were a significant reduction in both magnesium and calcium excretion after daily administration of 3 mg of boron [37].Furthermore, this prebiotic compound, not only reduce calcium excretion but also increases the levels of ionized calcium in plasma [40]. Most of human studies have shown that boron may be useful in preventing osteoporosis, by decreasing the loss of calcium and magnesium, which are essential for bone health [41, 42]. The present study demonstrated that methyl prednisolone (MP) induced bone changes is clearly associated with increased bone turnover, evidenced by the biochemical as well as the histological results
Our results showed that Methyl prednisolone (MP )decreased serum calcium levels as compared to serum calcium of the control group. Those findings are supported by other studies where, a similar pattern of fall in serum calcium and phosphate in adrenalectomized rats [31, 43]. However, at the end of treatment, raisins treated group showed a significant increase in serum calcium levels were observed as compared to methyl prednisolone (MP) treated group. Similarly, raisins demonstrated a significant decrease in the urine calcium levels as compared to the methyl prednisolone (MP) treated group. Probiotics in raisins may encouragethe augmentation of bone mineral content, by increasingcalcium and magnesium absorption to maintain the quality of bone [44]. Further studies have shown that oligosaccharides present in raisins can improvethe absorption and retention of mineral consequently supporting the bone mineralization [44].
In terms of the direct impact on bone, endogenous glucocorticoids at physiologic concentrations may have a role in promoting osteogenesis, while excess glucocorticoids increase osteoclastogenesis and suppress osteblastogenesis in cell culture, murine, and human models [45]. In our study, there was an increase in the number of osteoclasts in the bones of animals treated with MP as compared to the control group. Bones of rats treated with raisins showed increased trabecular bone thickness and decreased a number of osteoclasts as compared to the bones of methyl prednisolone (MP) treated group. Glucocorticoids also inhibit the formation of osteoblast and encourage osteoblasts and osteocytes apoptosis, causinga decline inbone formation [46]. These finding were evident in the present study by decrease in the number of osteocytes inside lacunae or its dark and eccentric nuclei. MP usually target trabecular bone loss in lumbar spine leading to osteoporosis, which was successfully demonstrated by H and E staining and trichrome stains. MP reduced the trabecular thickness as compared to the control group. At the end of the experiment, there was a distinct difference in the trabecular thickness of both the MP treated group and raisins treated. Those findings agree with the results obtained by others where, dried plum, apple, apricot, and grape suppressed the formation of osteoclasts in osteopenic overectomized mice [47].Furthermore, the same study showed that bone densitometry analysisapproved that dried plum, apricot and grape have protective effects for bone tissue. These biomechanical improvements, including bone strength and stiffness,influencethe bone quality.
Alkaline phosphatase (ALP) is an enzyme formed by osteoblasts;it facilitates the mineralization of bone.The earlier spreading of alkaline phosphatase at the calcification front assist the preparation function of the enzyme [48]. Our results indicate that alkaline phosphatase has been increased initially in ostoporotic group and then decreased to almost normal control group levels. Those results supported the results obtained by others [49], where the same was appeared by observing the increase levels of ALT, AST, ALP and GGT in negative control group, while doses of raisins extracts significantly decreased the levels of these four enzymes indicating protective effects of the extract. Alkaline phosphatase is produced by several cells in various tissues, such as liver, bone and kidney. Clinically, bone alkaline phosphatase has been generally accepted diagnostic enzyme for bone disease. On the other hand, serum alkaline phosphatase increases in severalnon-bone diseases and conditions, such as liver diseases[50]. However, in the presence of liver disease, the specificity of serum alkaline phosphatase measurements is improved by measuring bone alkaline phosphatase. In most other clinical situations, serum alkaline phosphatase appears to provide sufficient clinical information [51].
Osteocalcin is a protein synthesized by osteoblast; it is the most available noncollagenous protein in bone, forming about 20% of the non-collagen proteins. Like alkaline phosphatase, osteocalcin is examined clinically as anindicator of osteoblast activity; also, serum osteocalcin is a well-accepted serum marker of bone formation measured by radioimmunoassay [48]. To monitor the effect of each respective treatment on bone formation, serum osteocalcin, was measured. As expected, MP led to a significant increase in serum osteocalcin level. Also as expected, with raisins treatment reduced bone turnover (resorption as well as formation) in osteoporotic rats, as indicated by the marked and significant decrease in serum osteocalcin to almost normal control group levels. Under normal physiological conditions, bone resorption is coupled to bone formation. Consequently, our results harmonized with the results obtained by others where, an inhibition of bone resorption, estradiol and boron treatment in ovariectomized animals, is frequently followed by a decrease in bone formation [52]. Furthermore, those results are consistent with these in human; serum osteocalcin levels in postmenopausal women supplemented with boron were significantly higher than that of control group [53]. Therefore, daily raisins intake may affect osteocalcin levels in induced osteoporosis positively.
Noteworthy, antioxidants have a great potential in preventing and reducing degenerative diseases. Therefore, consumption food rich in antioxidants may help reduce the risk of suffering from these diseases. Raisins contain many antioxidant bioactive components [see for review, 54]. Phenolic compounds in raisins provide antibacterial and antioxidant action. Several in vivo and in vitro studies have shown the association of phenol compounds in raisins and their antioxidant properties. Our results do exclude that raisin intake may cause changes in the antioxidant defensive system,which show positive effects observed on bone.However, these conclusions needs further investigation in this context.
In conclusions:This study suggests that daily raisins intake may affect bone metabolism in methylprednisolone-induced osteoporosis positively. Raisins may contain specialbioactive compounds that have positive effects on bone. However, further investigation of the bioactive components is needed and this will consolidate this specific conclusion at this time.
References
- Silverman S, Curtis J, Saag K, Flahive J, Adachi J, Anderson F,Chapurlat R, Cooper C, Diez-Perez A, Greenspan S, Hooven F, Le Croix A, March L, Netelenbos JC, Nieves J, Pfeilschifter J, Rossini M, Roux C, Siris E, Watts N, Compston J, International management of bone health in glucocorticoid-exposed individuals in the observational GLOW study. Osteoporos. Int. 2015; 26(1): 419–420.
CrossRef - Balasubramanian A, Wade SW, Adler RA, Lin CJF, Maricic M, O’Malley CD, Saag K, Curtis JR. Glucocorticoid exposure and fracture risk in patients with new-onset rheumatoid arthritis. Osteoporos. Int. 2016; 27(11): 3239–3249.
CrossRef - Overman RA, Gourlay ML, Deal CL et al. Fracture rate associated with quality metric-based anti-osteoporosis treatment in glucocorticoid-induced osteoporosis. Osteoporos Int2015; 26(5): 1515–1524.
CrossRef - Overman RA, Yeh JY, Deal CL. Prevalence of oral glucocortidoid usage in the United States: a general population perspective. Arthr Care Res.2013; 65:294–8.
CrossRef - Majumdar SR, Lix LM, Morin SN et al. The disconnect between better quality of glucocorticoids-induced osteoporosis preventive care and better outcomes: a population-based cohort study. J Rheumatol2013; 40:1736–41.
CrossRef - Thomas T, Horlait S, Ringe JD et al. Oral bisphosphonates reduce the risk of clinical fractures in glucocorticoid-induced osteoporosis in clinical practice. Osteoporos Int.2013; 24:263–9.
CrossRef - Vanderoost J, Soe K, Merrild DM, Delaisse JM, van Lenthe GH. Glucocorticoid-induced changes in the geometry of osteoclast resorption cavities affect trabecular bone stiffness. Calcif. Tissue Int. 2013; 92(3): 240–250.
CrossRef - Aaron JE, Makins NB and Sagreiya K. The microanatomy of trabecular bone loss in normal aging men and women. Clinical Orthopedic and Related Research 1987; 215: 260-271
CrossRef - Palacios c. The Role of Nutrients in Bone Health, from A to Z. Critical Reviews in Food Science and Nutrition 2006; 46: 621–628.
CrossRef - Rizzoli R, Adachi JD, Cooper C et al. . . . Management of glucocorticoid-induced osteoporosis. Calcif Tissue Int. 2012; 91:225–43.
CrossRef - Kucukkurt I, Akbel E, Karabag F, Ince S. The effects of dietary boron compounds in supplemented diet on hormonal activity and some biochemical parameters in rats.Toxicol Ind. Health 2015;31:255–260
CrossRef - Ince S, Kucukkurt I, Demirel HH, Acar oz DA, Akbel E, Cigerci H. Protective effects of boron on cyclophosphamide induced lipid peroxidation and genotoxicity in rats. Chemosphere 2014;108:197–204
CrossRef - Ince S, Keles H, Erdogan M, Hazman O, Kucukkurt I. Protective effect of boric acid against carbon tetrachloride- ınduced hepatotoxicity in mice. Drug Chem Toxicol 2012; 35:285–292.
CrossRef - Ince S, Kucukkurt I, Cigerci IH, Fidan AF, Eryavuz A. The effects of dietary boric acid and borax supplementation on lipid peroxidation, antioxidant activity, and DNA damage in rats. J Trace Elem Med Biol. 2010; 24:161–164.
CrossRef - Devirian TA, Volpe SL. The physiological effects of dietary boron. Crit Rev Food Sci Nutr. 2003; 43: 219-31.
CrossRef - Pawa S, Ali S. Boron ameliorates fulminant hepatic failure by counteracting the changes associated with the oxidative stress. Chem Biol Interact. 2006; 160: 89-98.
CrossRef - Davoud Ghorbanian, Mohammed Gol, Mohsen Pourghasem, Jamshid Faraji, Kaveh Pourghasem, and Nabiollah Soltanpour. Spatial Memory and Antioxidant Protective Effects of Raisin (Currant) in Aged Rats. Prev Nutr Food Sci. 2018; 23(3): 196–205.
CrossRef - O’Grady J, O’Connor EM, Shanahan F. Review article: Dietary fibre in the era of microbiome science. Aliment. Pharmacol. Ther. 2019; 49: 506–515.
CrossRef - Jeszka-Skowron M, Zgoła-Grze´skowiak A, Stanisz E, Wa´skiewicz A. Potential health benefits and quality of dried fruits: Goji fruits, cranberries and raisins. Food Chem. 2017; 221: 228–236.
CrossRef - Fanelli, F, Cozzi G, Raiola A, Dini I, Mulè G, Logrieco AF, Ritieni A. Raisins and currants as conventional nutraceuticals in Italian market: Natural occurrence of ochratoxin A. J. Food Sci. 2017; 82: 2306–2312.
CrossRef - Vallverdú-Queralt, A.; Regueiro, J.; Alvarenga, J.F.R.; Martinez-Huelamo, M.; Leal, L.N.; Lamuela-Raventos, R.M. Characterization of the phenolic and antioxidant profiles of selected culinary herbs and spices: Caraway, turmeric, dill, marjoram and nutmeg. Food Sci. Technol. 2015, 35, 189–195.
CrossRef - Kelebek, H, Jourdes M, Selli S, Teissedre PL. Comparative evaluation of the phenolic content and antioxidant capacity of sun-dried raisins. J. Sci. Food Agric. 2013; 93: 2963–2972.
CrossRef - Di Lorenzo C, Frigerio G, Colombo F, de Sousa LP, Altindisli A, Dell’Agli M, Restani P. Phenolic profile and antioxidant activity of different raisin (Vitis vinifera L.) samples. BIO Web Conf. 2016; 7: 1–7
CrossRef - Parker TL, Wang XH, Pazmiño J, Engeseth NJ. Antioxidant capacity and phenolic content of grapes, sun-dried raisins, and golden raisins and their effect on ex vivo serum antioxidant capacity. J. Agric. Food Chem. 2007; 55: 8472–8477.
CrossRef - Camire ME, Dougherty MP. Raisin dietary fiber composition and in vitro bile acid binding. J. Agric. Food Chem. 2003; 51: 834–837.
CrossRef - Li YO, Komarek AR. Dietary fibre basics: Health, nutrition, analysis, and applications. Food Qual. Saf. 2017; 1: 47–59.
CrossRef - Uchida K, Nakajima H, Miyazaki T, Yayama T, Kawahara H, Kobayashi S, et al. Effects of alendronate on bone metabolism in glucocorticoid-induced osteoporosis measured by18F-fluoride PET: A prospective study. J Nucl Med. 2009; 50:1808-14.
CrossRef - Nansie A, McHugh NA, Vercesi HM, Egan RW, Hey JA.In vivo rat assay: Bone remodeling and steroid effects on juvenile bone by pQCT quantification in 7 days. Am J Physiol Endocrinol Metab. 2003; 284:E70-5.
CrossRef - Wang Y, Ohtsuka-Isoya M, Shao P, Sakamoto S, Shinoda H. Effects of methylprednisolone on bone formation and resorption in rats. Jpn J Pharmacol. 2002; 90:236-46.
CrossRef - Hayman AR, Bune AJ, Bradley JR, Rashbass J, Cox TM. Osteoclastic tartrate-resistant acid phosphatase (Acp 5): Its localization to dendritic cells and diverse murine tissues. J Histochem Cytochem. 2000; 48:219-28.
CrossRef - Mahgoub A, Hirsch PF, Munson PL. Calcium-lowering action of glucocorticoids in adrenalectomized-parathyroidectomized rats. Specificity and relative potency of natural and synthetic glucocorticoids. Endocrine. 1997; 6:279-83.
CrossRef - Akhtar B, Mahto RR, Dave AR, Shukla VD. Clinical study on Sandhigata Vata w.s.r. to oste Caplan, A., Fett, N., Rosenbach, M., Werth, V. P. & Micheletti, R. G. Prevention and management of glucocorticoid-induced side effects: a comprehensive review: a review of glucocorticoid pharmacology and bone health. J. Am. Acad. Dermatol. 2017; 76: 1–9.
- Vandewalle J, Luypaert A, De Bosscher K and Libert C. Therapeutic mechanisms of glucocorticoids. Trends Endocrinol. Metab. 2018; 29: 42–54.
CrossRef - Mazziotti G, Angeli A, Bilezikian JP, Canalis E & Giustina A. Glucocorticoid-induced osteoporosis: an update. Trends Endocrinol. Metab. 2006; 17: 144–149.
CrossRef - James Jam Jolly, Kok-Yong Chin, Ekram Alias, Kien Hui Chua and Ima Nirwana Soelaiman. Protective Effects of Selected Botanical Agents on Bone Int. J. Environ. Res. Public Health. 2018; 15: 963
CrossRef - Jennette Higgs, Emma Derbyshire, Kathryn Styles. Nutrition and osteoporosis prevention for the orthopaedic surgeon: a wholefoods approach EFORT Open Rev. 2017; 2(6): 300–308.
CrossRef - Arianna Carughi, Thea Lamkin and Dalia Perelman, RD. Health Benefits of Sun-Dried Raisins Review of the Scientific Literature Health Research & Studies Center July 2008. http://www.raisins.net /Raisins_and_ Health_200810.pdf
- Nora M Al-aboud. Effect of Red Raisins (Vitis Vinifera L.) Intake on the Level of Some Hematological Tests in a Group of Female Volunteers . Biomed J Sci & Tech Res. 2018; 2 (3): 2659-2665
CrossRef - Rubin MR & Bilezikian JP. Clinical review 151: the role of parathyroid hormone in the pathogenesis of glucocorticoid-induced osteoporosis: a re-examination of the evidence. J. Clin. Endocrinol. Metab. 2002; 87: 4033–4041.
CrossRef - Forrest H, NielsenCurtiss D, HuntLoanne M, Mullen Janet R. Hunt Effect of dietary boron on mineral, estrogen, and testosterone metabolism in postmenopausal women.FASEB Journal. 1987;1 (5): 394-39
CrossRef - Cristina Palacios, The Role of Nutrients in Bone Health, from A to Z Critical Reviews in Food Science and Nutrition. 2006; 46:621–62
CrossRef - Tara A Devirian & Stella L Volpe. The Physiological Effects of Dietary Boron.Critical Reviews in Food Science and Nutrition. 2003;43(2): 219-23
CrossRef - Claudia D’Alessandro, Pietro Manuel Ferraro, Caterina Cianchi, Massimiliano Barsotti, Giovanni Gambaro and Adamasco Cupisti. Which Diet for Calcium Stone Patients: A Real-World Approach to Preventive Care. Nutrients . 2019; 11: 118
CrossRef - Corrie M. Whisner, Luisa F. Castillo Prebiotics, Bone and Mineral Metabolism. Calcif Tissue Int. 2018; 102:443–47
CrossRef - Emory Hsu and Mark Nanes . Advances in Treatment of Glucocorticoid-Induced Osteoporosis. Curr Opin Endocrinol Diabetes Obes. 2017; 24(6): 411–41
CrossRef - Charles A O’brien, Dan Jia, Lilian I Plotkin, Teresita Bellido, Cara C Powers, Scott A Stewart, Stavros C Manolagas and Robert S Weinstein. Glucocorticoids Act Directly on Osteoblasts and Osteocytes to Induce Their Apoptosis and Reduce Bone Formation and Strength. Endocrinology. 2004; 145(4):1835–184
CrossRef - Elizabeth Rendina, Kelsey D Hembree, McKale R Davis, Denver Marlow, Stephen L Clarke, Bernard P Halloran , Edralin A Lucas , Brenda J Smith. Dried Plum’s Unique Capacity to Reverse Bone Loss and Alter Bone Metabolism in Postmenopausal Osteoporosis Model. PLOS ONE. 2013; 8 (3): 1-
CrossRef - Aiman I Al-Qtaitat, Saed M Aldalaen. A review of non-collagenous proteins; their role in bone. American Journal of Life Sciences. 2014; 2(6): 351-355
CrossRef - Shamim A Qureshi, Uzma Imran, Tooba Lateef , Muhammad Bilal Azmi, Sumera Rais, Hina Akram Mudassir, Musarrat Jahan , Syed Muhammad Shabib Zaidi, Rabia Badar and Dildar Ahmed. Raisins: A kitchen cabinet item can restores the liver function and structure. Pak. J. Pharm. Sci. 2020;33(4):1787-1794
- Brommage R, Allison C, Stavisky R, Kaplan J. Measurement of serum bone-specific alkaline phosphatase activity in cynomolgus macaques. J Med Primatol 1999; 28:329-333
CrossRef - Henning W, Woitge, Markus J. Seibel, and Reinhard. Comparison of total and bone-specific alkaline phosphatase in patients with nonskeletal disorders or metabolic bone diseases. Clinical Chemistry. 1996; 42(11): 1796-1804
CrossRef - Matilda Hc. Sheng L, Janette Taper, Hugo Veit, Elizabeth A. Thomas, Sanford J Ritchey, and Kh William Lau. Dietary Boron Supplementation Enhances the Effects of Estrogen on Bone Mineral Balance in Ovariectomized Rats Biological Trace Element Research. 2001;81P: 29-4
CrossRef - Boyacioglu O, Orenay-Boyacioglu S, Yildirim H, Korkmaz M. Boron intake, osteocalcin polymorphism and serum level in postmenopausal osteoporosis. J Trace Elem Med Biol. 2018;48:52–
CrossRef - Olmo-Cunillera, Danilo Escobar-Avello, Andy J Pérez, María Marhuenda-Muñoz, Rosa M Lamuela-Raventós and Anna Vallverdú-Queralt. Is Eating Raisins Healthy? Alexandra Nutrients. 2020; 12 (1): 54.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.