How to Cite | Publication History | PlumX Article Matrix
Phytochemical Properties and Pharmacological Role of Plants: Secondary Metabolites
Bhupesh Kaushik, Jatin Sharma, KeshavYadav, Prithik Kumar and Abhilasha Shourie*
Department of Biotechnology, ManavRachna International Institute Research and Studies, Faridabad, India
Corresponding Author E-mail: aashourie@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2894
ABSTRACT:
Over the past decades, there has been increasing attention tothe study of medicinal plants that contain many phytochemicals beneficial for human health. A number of secondary metabolites derived from various plants have been used as drug components to treat several human disorders since ancient times. The traditional therapeutic applications of secondary metabolites have been reported in the whole world. Numerous bioactive phytochemicals constituents have been identified and isolated using many advanced techniques. These bioactive phytochemicals are responsible for many pharmacological activities such as anti-inflammation, anti-cancer, anti-allergic, and antimicrobial infection. These secondary metabolites are not only beneficial for human health but also protect plants themselves from biotic and abiotic stress. These secondary metabolites are classified into many subclasses like terpenoids, alkaloids and phenolics. Each class of secondary metabolites has its pharmacological activities, which is required to be studied thoroughly for better use in pharmaceuticals, cosmetics, food, and other industries. Therefore, this review paper represents many medicinal plants that contain bioactive secondary metabolites and show pharmacological activities, which provides an opportunity to utilize them for improvement of human health and discover new herbal medicines.
KEYWORDS: Alkaloids; Bioactive; Pharmaceutica; Phenolics; Secondary Metabolites
Download this article as:| Copy the following to cite this article: Kaushik B, Sharma J, Yadav K, Kumar P, Shourie A. Phytochemical Properties and Pharmacological Role of Plants: Secondary Metabolites. iosci Biotech Res Asia 2021;18(1). |
| Copy the following to cite this URL: Kaushik B, Sharma J, Yadav K, Kumar P, Shourie A. Phytochemical Properties and Pharmacological Role of Plants: Secondary Metabolites. iosci Biotech Res Asia 2021;18(1). Available from: https://bit.ly/2OzpKpP |
Introduction
Today’s lifestyle is a leading cause to many human diseases. Allopathic medicines often work effectively against the disease but may show extreme side effects in certain cases. Commonly manifested side effects of allopathic medicines are face swelling, rashes on the body, itching, headache, inflammation, and drug resistance. A safer alternative to treat diseases is herbal or plant derived medicines that have been used since the ancient period (Kaberaet.al 2014). India and China provide the best example of the early use of medicinal plants. Both countries enlist countless plant-derived medicines (Tang et.al 1992).The diversity of medicinal plants depends on many factors such as climate, altitude, seasonal fluctuations etc. While many plants are perennial and live for many years contributing as a consistent source of medicinal compounds, other plants have shorter life span ranging from seasonal to annual or biennial. There is a huge variety of seasonal plants that show medicinal properties, some plants grow in summer, some in winters, and some plants occur only in the spring season. Some examples of medicinal plants that grow in different seasonsare Achilleafilipendulina, Santolinachamecyparissus,and Menthalongifolia grows in summer, Cistusmonspeliensi, Ocimumgratissimum grows in the spring season (Soniet.al 2015).
The versatile and vast pharmacological effects of medicinal plants are completely dependent on their phytochemical constituents. Various phytochemicals of plants have been isolated for drug discovery and development.Modern analytical techniques such as electrophoresis, chromatography, enzymology, and isotope techniques have been used to characterize phytochemicals, elucidate their structural formulas and decipher their biosynthetic pathways (Hussein et.al 2018, Okada et.al 2010). To explore the therapeutic use of plants, it is pertinent to have deep understanding of phytochemistry and have detailed knowledge of phytochemical composition of plant extracts which further can be used to develop different medicines.
Generally, the phytochemicals are divided into two categoriesi.e. primary and secondary metabolites based on their role in different metabolic processes.Primary metabolites are involved in primary processes such as respiration, growth, cell division, photosynthesis and food storage. The biomolecules such as carbohydrates, amino acids and lipids are categorized as primary metabolites as they are fundamental reactants and intermediates in carbon metabolism, nitrogen metabolism and associated pathways (Seigleret.al 1995). On the other hand, secondary metabolites are derived from primary metabolites in a very small amount, usually at a certain growth stage or to serve a specific function. Secondary metabolites provide the ability to defend against biotic and abiotic stress in plants. The mechanism of defence in plants varies according to the specific requirements of plants and is affected by physiological conditions, climate variations and environmental factors (Ballhornet.al 2009, Blank et.al 2012).
Plant secondary metabolites are broadly divided into three categories: Terpenoids, Alkaloids, and Phenolics (Savithramma et.al 2011). Each of these classes of secondary metabolites includes a huge array of compounds that have been found effective to treat different diseases, some of these compounds are- atropine, codeine, morphine, and nicotine, coming under alkaloids; linalool comes under terpenoids, while flavonoids, lignans and proanthocyanidins are categorized asphenolics. In the present review, secondary metabolites are studied thoroughly that include properties of secondary metabolites, biosynthetic pathways of secondary metabolites, structures and classification of secondary metabolites, and their pharmacological activities.Pharmacological activities of some secondary metabolites that have been used to treat various diseases are enlisted in the given table (Table 1).
Table 1: Pharmacological Activities of Secondary Metabolites.
| Name of secondary metabolites | Pharmacological activities | References |
| Linalool | Antibacterial, exert an effect on CNS | Taniguchi et.al 2014,
Zhang et.al 1987 |
| Codeine | Antitussive, antidepressant, analgesic, sedative, and hypnotic properties | Smith et.al 2006,
Vreeet.al 2000 |
| Morphine | Acute pulmonary edam and reduce the shortness of breath | Takitaet.al 2000 |
| Quinine | Antipyretic. Antimalarial, analgesic | EI-Tawilet.al 2010,
Mwitaet.al 2012 |
| Atropine | Anti-cholinergic, ant myopia, effects competitive antagonist of muscarine acetylcholine receptors | Guet.al 2011 |
| Nicotine | Insecticide, anti-inflammatory, antiherbivore | Melton et.al 2006,
Rhoades et.al 1976 |
| Berberine | Antiviral, antibacterial, anticancer, antidiabetic, and anti-inflammatory | Zhaet.al 2010,
Zhang et.al 2010
|
| Gallic acid | antibacterial, antiviral, antifungal, anti-inflammatory, antitumor, ant anaphylactic, antimutagenic, choleretic, and bronchodilator actions and promote muscle relaxation | Harborneet.al 1993 |
| Hydroquinone | Antimicrobial and used as antiseptics | Pelczaret.al 1988 |
| hydrolyzable tannins | Anti-diarrhoeal, antidotes in poisoning by heavy metals, antiangiogenic, also treat urinary tract infections | Jepson et.al 2008 |
| Coumarins | Anti-inflammatory, anticoagulant, anticancer, and anti-Alzheimer’s | Xuet.al 2015 |
Description of classes of secondary metabolites
Secondary metabolites can be classified based on their chemical composition. These phytochemicals are divided into three broad categories-Alkaloids,Phenolics and Terpenoids, as already mentioned above. A brief description of each of these categories is given further.
Alkaloids
Alkaloids are nitrogen-containing compounds which are widely distributed among large number of plant families. These compounds can be found in the whole plant or sometimes in a specific part of the plant. It is a highly diverse and large group consisting of more than 1800 alkaloids, all of which are different from each other and have different chemical structures. Alkaloids contain one or more nitrogen groups in their chemical structures. A number of researches have shown potential pharmacological effects and curative properties of alkaloids against many human diseases and disorders. There isa huge list of alkaloids that are used in pharmacological activities. Some of the alkaloids are enlisted in the Table 2 given below (Egamberdie va et.al 2017).
Table 2: List of plants that contain pharmacologically important alkaloids.
| S.No. | Name of the plant | Alkaloids | References |
| 1 | Liriodendron tulipifera L. | Aporphine, liriodenine, lysicamine, lanuginosine | Ziyaevet.al 1987 |
| 2 | Nitraiaschoberi L. | Schoberine, nitraraine, nitraramine, sibiridine, vasicinone | Tulyaganov and Kozimova 2005 |
| 3 | Convolvulus subhirsutus | Convolvine, convolamine, convolidine, phyllabine, phyllalbine, nortropine, conpropine | Gapparovet.al 2007 |
| 4 | Convolvulus pseudocanthabricaschrenk | Convolvine, convolamine, convolvidine, convolicine | Gapparov and Aripovaet.al 2011 |
| 5 | Arundodonax L. | Deoxyvasicinone, ardine, donine, donaxarine, arundamine | Khuzhaevet.al 2004 |
| 6 | Crambekotschyana | Goitrin and goiridin | Okhunovet.al 2011 |
Phenolic compounds
Phenolic compounds encompass a large number of phytochemicals consisting of one or more phenol groups. Phenols are responsible for the color, flavor, and taste of many herbs that are used in drinks and food. These secondary metabolites are highly valued for their pharmacological activities. Phenols are also used in many drugs due to their important pharmacological properties such as antioxidant, anti-microbial, anti-inflammatory, anti-cancer etc. Phenols are classified on the basis of their different chemical structures, enlisted in Table3 given below, along with their respective pharmacological activities(Puneetet.al 2013, Montanheret.al 2007, Serafiniet.al 2010).
Table 3: Classification of phenolic compounds with their pharmacological activities.
| Types of phenolic compounds | Pharmacological activities |
| Simple phenols | Treat urinary tract infections, antimicrobial, anti-inflammation and used as antiseptic in surgeries |
| Tannins | Used to convert raw animal hides into leather, anti-diarrhoeal, antidotes in poisoning |
| Coumarins | Anticoagulant and anti-Alzheimer |
| Flavonoids | Antithrombotic, anti-allergic, vasoproptective, inhibit tumour to grow and protect gastric mucosa |
| Xanthos | Antifungal |
| Stilbenes | Helps in the production of Estrogen |
| Lignans | Antimicrobial, antifungal activities |
Terpenes
Terpenes also form a diverse group of plant secondary metabolites that mainly consist of a five-carbon isoprene unit. Terpenes are classified according to the number of isoprene units in the molecule,the classes are summarized in Table4 (Hoffmann et.al 2003).
Table 4: Classification of terpenes.
| Name of Terpenes | Name of terpenoids | Location of terpenoids | References |
| Hemiterpene (C5) | Isoprenenol
Isovalenic acid |
Synthesis
Essential oils |
Eadieet.al 2004. Araet.al 2006, Elson et.al 1988 |
| Monoterpene (C10) | Limonene | Essential oil | Espinaet.al 2013 |
| Sesquiterpene ( C15) | ABA (Abscisic acid) | Zhang et.al 1987 | |
| Diterpene
(C20) |
Gibberellin | Gibberellafujikuroi | Hakoshimaet.al 2011 |
| Triterpene
(C30) |
Brassinosteroids | Lychinsviscaria, Brassica napus | Coelho et.al 2013, Krishna et.al 2003 |
| Tetraterpene
(C40) |
Carotenoids | Carrot, chloroplast, and chromoplasts of plants | M.M et.al 2014 |
Some secondary metabolites recognized for their pharmacological activities along with their general chemical structures and examples are enlisted in Table 5.
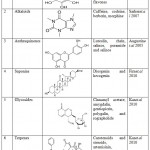 |
Table 5: Secondary metabolites and their examples. |
Properties of phytochemicals and Pharmacological activities of plants
Plants survived on the planet for more than 400 million years. Plants cannot move from one place to another so they have to face lots of biotic and abiotic stress that are represented in Figure1. Plants neither have any active weapon to attack plant-eating animals or herbivores and microbes,nor do they have any shield to protect themselves from environmental stress. Secondary metabolites serve as the defense system of plants as they protect them from all the biotic and abiotic stresses (Asif 2015). Owing to their bio activity, secondary metabolites have been historically used not only in Indian medicines (Ayurveda) but also used traditionally in Kampo medicines, European medicines, American, Australian, and traditional medicine system of Africa.There is extensive research that has been carried out in search of novel and safe plant derived medicine. For example, Alorkpaet.al 2016 extracted out bio active compounds from Carica papaya leaves and investigated their antimicrobial activity. They identified the presence of many secondary metabolites such as alkaloids, flavonoids, saponins and glycosides and found that the extracts showed antimicrobial activity against human pathogenic bacteria and fungi. Plant derived extracts and compounds have many beneficial uses due to their biochemical, pharmaceutical and therapeutic properties. Some of the uses and beneficial properties of phytochemicals are enlisted in Table 6 given below.
 |
Figure 1: Representation of plant stresses. |
Table 6: Example of plant molecules that used for human health.
| Phytochemicals | Properties | References |
| Menthol, benzyl acetate, linalool, limonene, 2-phenylthel alcohol, vanillin | Flavors | Altemimiet.al 2017 |
| Vitamins, Taxol, quinine, minerals, amino-acids, enzymes, morphine, polysaccharides | Health | Fridlenderet.al 2015 |
| Stevioside, rebaudioside | Sweeteners | Soejartoet.al 2019 |
| Vitamins, non-dairy milk, genistein, daidzein, lycopene, genistein, daidzein, resveratrol | food and nutrition | Rahalet.al 2014 |
Secondary metabolites work alone or in combination with other compounds/ metabolites to cure diseases. Such combinations can enhance the efficacy of treatment of a disease which have been proven in many studies (Wink et.al 2015). Many phytochemicals have shown great success to defeat the dreadful disease like cancers (Secaet.al 2018, Rainaet.al 2014).The medicinal plant Hypericumper for atum is used for it anti-depressant, anti-inflammatory, antiviral, anticancer, and antibacterial properties. This plant contains fluoxetine and sertraline that cures depression, and other metabolites like hypericin, hyperforin, flavonoids and xanthones,which further enhance its medicinal value (Shakyaet.al 2017).Badgujaret.al in 2014 studied the use of Ficuscarica to treat many disorders that are related to the digestive, endocrine, reproductive, and respiratory system. Ficuscaricabelongs to angiosperm genera and consists of more than 800 different species. Phaleriamacrocarpa belongs to Thymelaeaceae family and has been traditionally used in Malaysia and Indonesia.Many diseases such as rheumatism, high blood pressure, diabetes mellitus, cancer, skin diseases, allergies, stroke, migraine, and hemorrhoids have been treated using this plant(Or et.al 2016). Echinacea purpurea, a medicinal herb with many secondary metabolites, has been used to cure anxiety, depression, cytotoxicity, and mutagenic disorders. However the use of this plant has been controversial as apart from its beneficial effects,it has potential side effects that are revealed by many studies, such as abdominal pain, nausea, angioedema, rash, and pruritus were reported in many patients after treatment (Manayiet.al 2015).Ziziphora species comprises a large number of flowering plants that belong to Lamiaceae family and further have been classified into 236 genera and 6900-7200 species. These plants are rich in essential oils or many secondary metabolites used in the field of pharmaceutical, medicinal, traditional, and folk medicines. This species is used to treat cold, fever, inflammation, intestinal disorders, insomnia, and cardiovascular malfunction for centuries (Mohammad hosseini et.al 2017). Secondary metabolites investigation on Thymus alternates showed that this species contains terpenoids, pentacyclic, and betulinic acid. Thephytochemicals of this plant have been used as flavoring agents while for medicinal purpose it has been found effective against cancer cell lines (Acquaet.al 2017). PhyllanthusUrinarica L. genus belonging to Phyllantaceae family has been investigated asa rich source of lignans, tannins, flavonoids, phenolics, terpenoids, and other secondary metabolites. These secondary metabolites cure jaundice, diabetes, malaria, and liver disease. This plant also shows activity against cancer, microbial infections, and cardiovascular effects (Geethangili et.al 2018).
Some more investigations are there that represent the pharmacological activity of medicinal plants. Ipomoea batata L., commonly known as sweet potato, is widely consumed all over the world. It has many beneficial effects on human health as it contains many vitamins and phytochemicals. These phytochemicals also reveal activity against cancer, diabetes, inflammation, and antioxidants. Sweet potato also contains beta-carotene and a precursor of vitamin A that helps to cure night blindness and overcome the deficiency of vitamin A (Ghasemzadeh et.al 2016). South Indian grass that belongs to Cyperaceae species possesses large number of secondary metabolites that belong to classes alkaloids, flavonoids, steroids, phenols, and quinones. Out of all the phytochemicals, this grass contains alkaloids in a large amount and also shows many pharmaceutical activities that cure microbial infections and inflammation (Babuet.al2014). Capparisspinosa has lots of secondary metabolites that help to improve biomarkers of cardiovascular diseases and diabetes (Zhang et.al 2018). Glycyrrhizaglabra root revealed the presence of many phytochemicals. These phytochemicals are very beneficial for human health in the enhancement of memory, cures depression, helps to maintain the glucose level in the body, and shows many other pharmacological effects (Ali Esmail AL-Snafi 2018).Ocimum sanctum L. commonly known as Holy basil or Tulsi, is used in India as medicine sinceancient times as it helps to improve stress, inflammation, and cancer (Sing et.al 2018, Siva et.al 2016).
Genus Macaranga Thou.Belongs to Euphorbiaceae that comprises 300 species of plants. These species are mainly found in the tropics of Africa, Australia, Pacific regions, and Asia. This genus is traditionally used to treat cuts, sore, bruises, boils, and swelling (Magadulaet.al 2014). Pleurotussajorcajuis commonly known as mushrooms, are great source of primary and secondary metabolites and contain about 40-49% of protein. Apart from this mushrooms have anticancer, antidiabetic, antibacterial, and anti-inflammation activities. Mushrooms also play an important role in healing (Devi et.al 2015). Cymbopogancitratusstapf, Eugenia unifloraleaves and Citrullus vulgaris schard also contain many primary and secondary metabolites that are reported by Geethaet.al 2014, Daniel et.al 2014 and Hannah et.al 2015. Calophyllum Inophyllum belongs to clusiaceae family and it occurs above the high tide mark along the sea coast of Northern Australia and expanding throughout South India and S south-East Asia. This plant species contains lots of secondary metabolites in their root, stem, and leaves that help to fight against microbial infections, inflammation, and used in cosmetics (Sunduret.al 2014).Morusalba belongs to Moraceae family and contains many medicinal plants, and has numerous applications in various fields such as agriculture, food, cosmetic and pharmaceutical industries. Pharmacological activities of these plants help in the treatment of an inflammatory condition, gastrointestinal disorder, cancer, and microbial infections with the help of many secondary metabolites (Hussainet.al 2017). Another study was performed by B. J. Divya and other scientists in 2017 that worked on Allium sativum. Allium sativum belongs to the family Amaryllidaceae and commonly known as garlic. In this study, to extract secondary metabolites from garlic cloves different chemicals and techniques were used. Hexane, ethyl acetate, methanol and water revealed steroids, alkaloids, flavonoids and other bioactive compounds. These phytochemicals of garlic cloves have been used to treat several infections from ancient period.
Momordicadiocca commonly known as Kakrol or spiny gourd is mainly found in India and Bangladesh, and is not only used as medicinal plant but also consumed as vegetable on a large scale. This plant consist of many minerals compositions, preventive, protective and curative agents in their root, stem and fruit. This plant includes many pharmacological activities such as anti-oxidant, analgesic, nephron protective, neuro protective, antiallergic, antimalarial, hepato protective and antihe patotoxic activity (Talukdar and Hossain 2014). Genitinais an important genus of Gentianaceae family that comprises 400 species and distributed all over the world. Based on investigation, this plant is used traditionally in Iran. This plant species consist lots of phytochemicals such as gentipicroside, xanthones, monoterpenes, alkaloids and flavonoids. This plant species has lots of promising bioactive agents are present that cure menstrual over bleeding, animal venom poisoning, infected wounds, injuries, vitiligo and swelling of liver, spleen, stomach and sprain of muscles (Mirzaeeet.al 2017). Some more medicinal plants with their pharmacological activities are summarized in Table 7 given below.
Table 7: Pharmalogical activities of medicinal plants with their common names.
| S. No. | Name of plant species | Common name of plant | Phytochemical name | Pharmacological activity of plant | References |
| 1 | Curcuma longa | Haldi | Flavonoid | Anti-inflammatory, anticancer, hepato-protective | Sharma et.al 2013 |
| 2 | Withaniasomnifera | Ashwagandha | Withanolides, steroidal lactones | Helps to treat Alzheimer’s and Parkinson’s disorders, helps to enhance memory and immunomodulatory, anti-cancerous and chemo preventive | Rathinamoorthyet.al 2014 |
| 3 | Catharanthusroseus | Sadabahar | Alkaloids | Anticancer | Priyadarshiniet.al 2012 |
| 4 | AzadirachtaIndica | Neem | Di and Tri terpenoids, limonoids | Blood purifier that prevents skin disease, anti-diabetic, inhibit colon cancer, anti-allergic | Gupta et.al 2014 |
| 5 | Piper nigrum | Kali mirch | Dehydro-pipernonaline, piperidine | Helps to remove cough, purify lungs, used in weight loss with turmeric, epilepsy, anti-carcinogenic, anti-hyperlipidaemic | Kaushiket.al 2002 |
| 6 | Tinosporacordifolia | Geloy | Tinosporin, isoquinoline alkaloids | Cardioprotective, anti-diabetic, immunomodulator, chemo preventive | Nisaret.al 2012 |
| 7 | Aloe vera | GhritKumari | ß-sitosterol, compesterol, emodin and aloin | Helps to nourish skin and hairs, anti-diabetic, has healing properties, shows antiseptic effects, anti-viral and antitumor | Mittal et.al 2014 |
| 8 | Phyllanthusemblica | Amla | Emblicanin B, punigluconin and pedunculagin | Good for skin, eyes and hairs, antiviral, anticancer, antidiabetic, anticancer and hepatoprotective | Paarakhet.al 2010 |
| 9 | Cinchona robusta | Quina | Quinine | Antiparasitic and helps to treat malaria | Paarakhet.al 2010 |
| 10 | Swertiachirata | Chirayita | Amarogenitine, ophellic acid, sawertiamarine and mangeferin | Antiviral, hepato-renal protective and shows anti-diabetic effect | Krishnaaet.al 2004 |
| 11 | Allium sativum | Lahsun | Allicin | Anti-inflammatory, cardioprotective (helps to maintain hypertension) | Joshi et.al 2005 |
| 12 | Bergenia ciliate | Pakhenbhed | IS-01246 | Anti-arthritis (helps to treat Rheumatoid) | Seyyedet.al 2012 |
By using advance research technologies, scientists are working hard to produce rich variety of phytochemicals under laboratory conditionsusing plant cell cultures (Yueet.al 2014). Guerrieroet.al 2018,culturedArtemisia, Coffeaarabica L. and Urticadioica L. to produce large amount of secondary metabolites terpenoids, alkaloids and phenolic compounds respectively. Many trans genes such as rol ABC genes are also used by Kianiet. al. 2015, to increase the production of phytochemicals. Secondary metabolites are extracted from many plant species and used to make many drugs that cures different disorders. There are number of drugs that are composed of heterogenous phytochemicals and are available in market. Some of the drugs are enlisted in Table 8 (Garnatjee.al 2017). These drugs not only cure the diseases but also solve the problem of drug resistance and provide a new path for scientist to discover more drugs to fight against dreadful diseases (Anandet.al 2019).
Table 8: Commercially available plant derived medicines.
| Plant Name | Name of the drug |
| Colchicum autumnale L. | Colchicine |
| Filipendulaulmaria (L.) Maxim | Aspirin |
| Artemisia annua L. | Artemisinin |
| Camptotheca acuminate Decne | Camptothecin |
| Taxusbrevifolia Nutt. | Paclitaxel |
| Artemisia annua L. | Artemisinin |
| Catharanthusroseus (L.) G. Don | Vinblastine and vincristine |
| Papaversomniferum L. | Codeine |
| Papaversomniferum L. | Papaverine |
| Cannabis sativa L. | Cannabidiol |
Conclusion
Plants are a valuable resource that yields numerous phytochemicals which can be used as potential drugs to treat and prevent many human ailments and diseases. These drugs also provide a safer alternative to allopathic medicines overcoming the problems of drug resistance, toxicity and side effects. The bioactivity of plant extracts and their component phytochemicals have been studied extensively and put to use since ancient times. Novel approaches are now being explored for enhanced production and efficient yielding of secondary metabolites through cell and tissue cultures. Advances in cell line culture allowing in-vitro bioactivity testing also opens avenues for faster drug development.
Acknowledgment
We sincerely acknowledge the contribution of Dr. Shilpa S. Chapadgaonkar, Associate Professor, Department of Biotechnology, MRIIRS, Faridabad, India, in form of her valuable inputs to the co-authors in preparing the manuscript.
Conflicts of Interest
The authors declare that there is no conflicts of interest.
Funding Source
There is no funding source
References
- Ahmad, N., Fazal, H., Abbasi, B.H., Farooq, S., Ali, M. and Khan, M.A., 2012. Biological role of Piper nigrum L.(Black pepper): A review. Asian Pacific Journal of Tropical Biomedicine, 2(3), pp.S1945-S1953.
CrossRef - Alara, O.R., Alara, J.A. and Olalere, O.A., 2016. Review on Phaleriamacrocarpa pharmacological and phytochemical properties. Drug Des, 5(134), pp.2169-0138.
- Alorkpa, E.J., Boadi, N.O., Badu, M. and Saah, S.A., 2016. Phytochemical screening, antimicrobial and antioxidant properties of assorted Carica papaya leaves in Ghana.
- Al-Snafi, A.E., 2018. Glycyrrhizaglabra: A phytochemical and pharmacological review. IOSR Journal of Pharmacy, 8(6), pp.1-17.
- Altemimi, A., Lakhssassi, N., Baharlouei, A., Watson, D.G. and Lightfoot, D.A., 2017. Phytochemicals: Extraction, isolation, and identification of bioactive compounds from plant extracts. Plants, 6(4), p.42.
CrossRef - Anand, U., Jacobo-Herrera, N., Altemimi, A. and Lakhssassi, N., 2019. A comprehensive review on medicinal plants as antimicrobial therapeutics: potential avenues of biocompatible drug discovery. Metabolites, 9(11), p.258.
CrossRef - Ara, K., Hama, M., Akiba, S., Koike, K., Okisaka, K., Hagura, T., Kamiya, T. and Tomita, F., 2006. Foot odor due to microbial metabolism and its control. Canadian journal of microbiology, 52(4), pp.357-364.
CrossRef - Asif, M., 2015. Pharmacological activities and phytochemistry of various plant containing coumarin derivatives. Current Science Perspectives, 1(3), pp.77-90.
- Aziz, R.K., Breitbart, M. and Edwards, R.A., 2010. Transposases are the most abundant, most ubiquitous genes in nature. Nucleic acids research, 38(13), pp.4207-4217.
CrossRef - Babu, H.R. and Savithramma, N., 2014. Screening of secondary metabolites of underutilized species of Cyperaceae. Int J Pharm Sci Rev Res, 24, pp.182-187.
- Badgujar, S.B., Patel, V.V., Bandivdekar, A.H. and Mahajan, R.T., 2014. Traditional uses, phytochemistry and pharmacology of Ficuscarica: A review. Pharmaceutical biology, 52(11), pp.1487-1503.
CrossRef - Ballhorn, D.J., Kautz, S., Heil, M. and Hegeman, A.D., 2009. Analyzing plant defenses in nature. Plant signaling&behavior, 4(8), pp.743-745.
CrossRef - Biswas, K., Chattopadhyay, I., Banerjee, R.K. and Bandyopadhyay, U., 2002. Biological activities and medicinal properties of neem (Azadirachtaindica). CURRENT SCIENCE-BANGALORE-, 82(11), pp.1336-1345.
- Clifford, M., Leah, M. and Charles, N., 2012. Antiepileptic properties of Quinine: A systematic review. Annals of neurosciences, 19(1), p.14.
CrossRef - Coelho, V., Mazzardo-Martins, L., Martins, D.F., Santos, A.R.S., da Silva Brum, L.F., Picada, J.N. and Pereira, P., 2013. Neurobehavioral and genotoxic evaluation of (−)-linalool in mice. Journal of natural medicines, 67(4), pp.876-880.
CrossRef - Dall’Acqua, S., Peron, G., Ferrari, S., Gandin, V., Bramucci, M., Quassinti, L., Mártonfi, P. and Maggi, F., 2017. Phytochemical investigations and antiproliferative secondary metabolites from Thymus alternans growing in Slovakia. Pharmaceutical biology, 55(1), pp.1162-1170.
CrossRef - Daniel, G. and Krishnakumari, S., 2015. Quantitative analysis of primary and secondary metabolites in aqueous hot extract of Eugenia uniflora (L) leaves. Asian Journal of Pharmaceutical and Clinical Research, 8(1), pp.334-338.
- Devi, M.R. and Krishnakumari, S., 2015. Quantitative estimation of primary and secondary metabolites in hot aqueous extract of Pleurotussajorcaju. Journal of Pharmacognosy and Phytochemistry, 4(3), p.198.
- Eadie, M.J., 2004. Could valerian have been the first anticonvulsant?. Epilepsia, 45(11), pp.1338-1343.
CrossRef - Egamberdieva, D., Mamedov, N., Ovidi, E., Tiezzi, A. and Craker, L., 2017. Phytochemical and pharmacological properties of medicinal plants from Uzbekistan: A review. Journal of Medicinally Active Plants, 5(2), pp.59-75.
- Elson, C.E., Maltzman, T.H., Boston, J.L., Tanner, M.A. and Gould, M.N., 1988. Anti-carcinogenic activity of d-limonene during the initiation and promotion/progression stages of DMBA-induced rat mammary carcinogenesis. Carcinogenesis, 9(2), pp.331-332.
CrossRef - ElTawil, S., Al Musa, T., Valli, H., Lunn, M.P., Brassington, R., ElTawil, T. and Weber, M., 2015. Quinine for muscle cramps. Cochrane database of systematic reviews, (4).
CrossRef - Espina, L., Gelaw, T.K., de Lamo-Castellví, S., Pagán, R. and García-Gonzalo, D., 2013. Mechanism of bacterial inactivation by (+)-limonene and its potential use in food preservation combined processes. PloS one, 8(2), p.e56769.
CrossRef - Fridlender, M., Kapulnik, Y. and Koltai, H., 2015. Plant derived substances with anti-cancer activity: from folklore to practice. Frontiers in plant science, 6, p.799.
CrossRef - Gapparov, A.M. and Aripova, S.F., 2011. Alkaloids from the aerial part and roots of Convolvulus pseudocanthabrica indigenous to Uzbekistan. Chemistry of Natural Compounds, 47(4), p.673.
CrossRef - Gapparov, A.M., Razzakov, N.A. and Aripova, S.F., 2007. Alkaloids of Convolvulus subhirsutus from Uzbekistan. Chemistry of Natural Compounds, 43(3), pp.291-292.
CrossRef - Garnatje, T., Peñuelas, J. and Vallès, J., 2017. Ethnobotany, phylogeny, and ‘omics’ for human health and food security. Trends in plant science, 22(3), pp.187-191.
CrossRef - Geetha, T.S. and Geetha, N., 2014. Phytochemical screening, quantitative analysis of primary and secondary metabolites of Cymbopogancitratus (DC) Stapf. leaves from Kodaikanal hills, Tamilnadu. International Journal of pharmtech research, 6(2), pp.521-529.
- Ghasemzadeh, A., Talei, D., Jaafar, H.Z., Juraimi, A.S., Mohamed, M.T.M., Puteh, A. and Halim, M.R.A., 2016. Plant-growth regulators alter phytochemical constituents and pharmaceutical quality in Sweet potato (Ipomoea batatas L.). BMC complementary and alternative medicine, 16(1), p.152.
CrossRef - Gu, L., Li, N., Gong, J., Li, Q., Zhu, W. and Li, J., 2011. Berberine ameliorates intestinal epithelial tight-junction damage and down-regulates myosin light chain kinase pathways in a mouse model of endotoxinemia. Journal of Infectious Diseases, 203(11), pp.1602-1612.
CrossRef - Guerriero, G., Berni, R., Muñoz-Sanchez, J.A., Apone, F., Abdel-Salam, E.M., Qahtan, A.A., Alatar, A.A., Cantini, C., Cai, G., Hausman, J.F. and Siddiqui, K.S., 2018. Production of plant secondary metabolites: Examples, tips and suggestions for biotechnologists. Genes, 9(6), p.309.
CrossRef - Hakoshima, T., Murase, K., Hirano, Y. and Sun, T.P., 2011. Gibberellin Perception by the Gibberellin Receptor and its Effector Recognition. NKG, 52(1), pp.37-41.
- Hannah, M.A.C. and Krishnakumari, S., 2015. Quantitative estimation of plant metabolites in the hot aqueous seed extract of watermelon (Citrullus vulgaris Schrad.). Journal of Medicinal Plants, 3(5), pp.107-111.
- Harborne, J.B., Baxter, H. and Moss, G.P.A., 1993. A handbook of bioactive compounds from plants. Phytochemical dictionary.
- Hoffmann, D., 2003. Medical herbalism: the science and practice of herbal medicine. Simon and Schuster.
- Hussain, F., Rana, Z., Shafique, H., Malik, A. and Hussain, Z., 2017. Phytopharmacological potential of different species of Morusalba and their bioactive phytochemicals: A review. Asian Pacific journal of tropical biomedicine, 7(10), pp.950-956.A study on phytochemicals, functions, groups and mineral composition of Allium sativum (Garlic) cloves, 20 march 2017, B. J. Divya, B. suman, M venkataswamy, K. Thyagaraju.
CrossRef - Hussein, R.A. and El-Anssary, A.A., 2018. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal Medicine.
CrossRef - Hussein, R.A. and El-Anssary, A.A., 2018. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal Medicine.
CrossRef - Hussein, R.A. and El-Anssary, A.A., 2018. Plants secondary metabolites: the key drivers of the pharmacological actions of medicinal plants. Herbal Medicine.
CrossRef - Javadzadeh, S.M. and Fallah, S.R., 2012. Therapeutic application of different parts Berberis vulgaris.
- Jepson, R.G., Williams, G. and Craig, J.C., 2012. Cranberries for preventing urinary tract infections. Cochrane database of systematic reviews, (10).
CrossRef - Joshi, P. and Dhawan, V., 2005. Swertiachirayita–an overview. Current science, pp.635-640.
- Kabera, J.N., Semana, E., Mussa, A.R. and He, X., 2014. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J Pharm Pharmacol, 2, pp.377-392.
- Kar, A., 2007. Pharmaocgnosy and Pharmacobiotechnology (Revised-Expanded Second Edition). New Age International LimtedPublishres New Delhi, pp.332-600.
- Kar, A., 2007. Pharmaocgnosy and Pharmacobiotechnology (Revised-Expanded Second Edition). New Age International LimtedPublishres New Delhi, pp.332-600.
- Khuzhaev, V.U., 2004. Alkaloids of Arundodonax. XVIII. Nitrogenous bases in flowers of cultivars. Chemistry of natural compounds, 5(40), pp.516-517.
CrossRef - Kiani, B.H., Ullah, N., Haq, I.U. and Mirza, B., 2019. Transgenic Artemisia dubia WALL showed altered phytochemistry and pharmacology. Arabian Journal of Chemistry, 12(8), pp.2644-2654.
CrossRef - Krishna, P., 2003. Brassinosteroid-mediated stress responses. Journal of Plant Growth Regulation, 22(4), pp.289-297.
CrossRef - Krishna, S., Uhlemann, A.C. and Haynes, R.K., 2004. Artemisinins: mechanisms of action and potential for resistance. Drug Resistance Updates, 7(4-5), pp.233-244.
CrossRef - Kumar Gupta, S. and Sharma, A., 2014. Medicinal properties of Zingiberofficinale Roscoe-A review. Pharm. Biol. Sci, 9, pp.124-129.
CrossRef - Magadula, J.J., 2014. Phytochemistry and pharmacology of the genus Macaranga: a review. Journal of Medicinal Plants Research, 8(12), pp.489-503.
CrossRef - Manayi, A., Vazirian, M. and Saeidnia, S., 2015. Echinacea purpurea: Pharmacology, phytochemistry and analysis methods. Pharmacognosy reviews, 9(17), p.63.
CrossRef - Melton, L., 2006. Body blazes. Scientific American, 294(6), pp.24-24.
CrossRef - Mirzaee, F., Hosseini, A., Jouybari, H.B., Davoodi, A. and Azadbakht, M., 2017. Medicinal, biological and phytochemical properties of Gentiana species. Journal of Traditional and Complementary Medicine, 7(4), pp.400-408.
CrossRef - Mittal, J., Sharma, M.M. and Batra, A., 2014. Tinosporacordifolia: a multipurpose medicinal plant-A. Journal of Medicinal Plants, 2(2).
- Mohammadhosseini, M., 2017. The ethnobotanical, phytochemical and pharmacological properties and medicinal applications of essential oils and extracts of different Ziziphora species. Industrial crops and products, 105, pp.164-192.
CrossRef - Montanher, A.B., Zucolotto, S.M., Schenkel, E.P. and Fröde, T.S., 2007. Evidence of anti-inflammatory effects of Passifloraedulis in an inflammation model. Journal of Ethnopharmacology, 109(2), pp.281-288.
CrossRef - Okada, T., MochamadAfendi, F., Altaf-Ul-Amin, M., Takahashi, H., Nakamura, K. and Kanaya, S., 2010. Metabolomics of medicinal plants: the importance of multivariate analysis of analytical chemistry data. Current computer-aided drug design, 6(3), pp.179-196.
CrossRef - Okhunov, I.I., Levkovich, M.G., Abdullaev, N.D., Khuzhaev, V.U. and Aripova, S.F., 2011. Alkaloids from Crambekotschyana endemic to Uzbekistan. Chemistry of Natural Compounds, 47(3), p.487.
CrossRef - Paarakh, P.M., 2010. Terminaliaarjuna (Roxb.) Wt. and Arn.: a review. IJP-International Journal of Pharmacology, 6(5), pp.515-534.
CrossRef - Pelczar, M.J., Chan, E.C.S. and Krieg, N.R., 1988. Control of microorganisms, the control of microorganisms by physical agents. Microbiology, 469, p.509.
- Priyadarshini, K. and Keerthi, A.U., 2012. Paclitaxel against cancer: a short review. Med chem, 2(7), pp.139-141.
- Raina, H., Soni, G., Jauhari, N., Sharma, N. and Bharadvaja, N., 2014. Phytochemical importance of medicinal plants as potential sources of anticancer agents. Turkish Journal of Botany, 38(6), pp.1027-1035.
CrossRef - Rathinamoorthy, R. and Thilagavathi, G., 2014. Terminaliachebula-review on pharmacological and biochemical studies. Int J PharmTech Res, 6(1), pp.97-116.
- Rhoades, D.F. and Cates, R.G., 1976. Toward a general theory of plant antiherbivore chemistry. In Biochemical interaction between plants and insects(pp. 168-213). Springer, Boston, MA.
CrossRef - Samuni-Blank, M., Izhaki, I., Dearing, M.D., Gerchman, Y., Trabelcy, B., Lotan, A., Karasov, W.H. and Arad, Z., 2012. Intraspecific directed deterrence by the mustard oil bomb in a desert plant. Current biology, 22(13), pp.1218-1220.
CrossRef - Sarker, S.D. and Nahar, L., 2007. Chemistry for pharmacy students. John Willey & Sons Ltd. UK, pp.322-4.
CrossRef - Savithramma, N., Rao, M.L. and Ankanna, S., 2011. Screening of traditional medicinal plants for secondary metabolites. International Journal of Research in Pharmaceutical Sciences, 2(4), pp.643-647.
- Scalbert, A., Johnson, I.T. and Saltmarsh, M., 2005. Polyphenols: antioxidants and beyond. The American journal of clinical nutrition, 81(1), pp.215S-217S.
CrossRef - Seca, A.M. and Pinto, D.C., 2018. Plant secondary metabolites as anticancer agents: successes in clinical trials and therapeutic application. International journal of molecular sciences, 19(1), p.263.
CrossRef - Seigler, D.S., 2012. Plant secondary metabolism. Springer Science & Business Media.
- Serafini, M., Peluso, I. and Raguzzini, A., 2010. Flavonoids as anti-inflammatory agents. Proceedings of the Nutrition Society, 69(3), pp.273-278.
CrossRef - Shakya, A.K., 2016. Medicinal plants: future source of new drugs. International Journal of Herbal Medicine, 4(4), pp.59-64.
- Shakya, A.K., 2016. Medicinal plants: future source of new drugs. International Journal of Herbal Medicine, 4(4), pp.59-64.
- Shakya, P., Marslin, G., Siram, K., Beerhues, L. and Franklin, G., 2019. Elicitation as a tool to improve the profiles of high‐value secondary metabolites and pharmacological properties of Hypericumperforatum. Journal of Pharmacy and Pharmacology, 71(1), pp.70-82.
CrossRef - Sharma, V., 2013. Part based HPLC-PDA quantification of podophyllotoxin in populations of PodophyllumhexandrumRoyle “Indian Mayapple” from higher altitude Himalayas. Journal of Medicinal Plants Studies, 1(3), pp.176-183.
CrossRef - Singh, D. and Chaudhuri, P.K., 2018. A review on phytochemical and pharmacological properties of Holy basil (Ocimum sanctum L.). Industrial Crops and Products, 118, pp.367-382.
CrossRef - Siva, M., Shanmugam, K.R., Shanmugam, B., Venkata, S.G., Ravi, S., Sathyavelu, R.K. and Mallikarjuna, K., 2016. Ocimum sanctum: a review on the pharmacological properties. International Journal of Basic & Clinical Pharmacology, 5(3), pp.558-565.
CrossRef - Smith, J., Owen, E., Earis, J. and Woodcock, A., 2006. Effect of codeine on objective measurement of cough in chronic obstructive pulmonary disease. Journal of Allergy and Clinical Immunology, 117(4), pp.831-835.
CrossRef - Soejarto, D.D., Addo, E.M. and Kinghorn, A.D., 2019. Highly sweet compounds of plant origin: From ethnobotanical observations to wide utilization. Journal of ethnopharmacology, 243, p.112056.
CrossRef - Soni, U., Brar, S. and Gauttam, V.K., 2015. Effect of seasonal variation on secondary metabolites of medicinal plants. Int J Pharm Sci Res, 6(9), pp.3654-62.
- Sundur, S., Shrivastava, B., Sharma, P., Raj, S.S. and Jayasekhar, V.L., 2014. A review article of pharmacological activities and biological importance of Calophylluminophyllum. International Journal of Advanced Research, 2(12), pp.599-603.
- Takita, K., Herlenius, E., Yamamoto, Y. and Lindahl, S.G., 2000. Effects of neuroactive substances on the morphine-induced respiratory depression; an in vitro study. Brain research, 884(1-2), pp.201-205.
CrossRef - Talukdar, S.N. and Hossain, M.N., 2014. Phytochemical, phytotherapeutical and pharmacological study of Momordicadioica. Evidence-Based Complementary and Alternative Medicine, 2014.
CrossRef - Tang, W. and Eisenbrand, G., 2013. Chinese drugs of plant origin: chemistry, pharmacology, and use in traditional and modern medicine. Springer Science & Business Media.
- Taniguchi, S., Hosokawa Shinonaga, Y.U.M.I., Tamaoki, D., Yamada, S., Akimitsu, K. and Gomi, K., 2014. Jasmonate induction of the monoterpene linalool confers resistance to rice bacterial blight and its biosynthesis is regulated by JAZ protein in rice. Plant, cell & environment, 37(2), pp.451-461..
CrossRef - Tulyaganov, T.S. and Kozimova, N.M., 2005. Alkaloids from Nitrariaschoberi. O-acetylnitraraine. Chemistry of natural compounds, 41(5).
CrossRef - Vree, T.B., Van Dongen, R.T. and Koopman-Kimenai, P.M., 2000. Codeine analgesia is due to codeine-6-glucuronide, not morphine. International Journal of Clinical Practice, 54(6), p.395.
- Wink, M., 2015. Modes of action of herbal medicines and plant secondary metabolites. Medicines, 2(3), pp.251-286.
CrossRef - Yue, W., Ming, Q.L., Lin, B., Rahman, K., Zheng, C.J., Han, T. and Qin, L.P., 2016. Medicinal plant cell suspension cultures: pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Critical reviews in biotechnology, 36(2), pp.215-232.
CrossRef - Zha, W., Liang, G., Xiao, J., Studer, E.J., Hylemon, P.B., PandakJr, W.M., Wang, G., Li, X. and Zhou, H., 2010. Berberine inhibits HIV protease inhibitor-induced inflammatory response by modulating ER stress signaling pathways in murine macrophages. PLoS One, 5(2), p.e9069.
CrossRef - Zhang, H. and Ma, Z.F., 2018. Phytochemical and pharmacological properties of Capparisspinosa as a medicinal plant. Nutrients, 10(2), p.116.
CrossRef - Zhang, J., Schurr, U. and Davies, W.J., 1987. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. Journal of experimental botany, 38(7), pp.1174-1181.
CrossRef - Zhang, J., Schurr, U. and Davies, W.J., 1987. Control of stomatal behaviour by abscisic acid which apparently originates in the roots. Journal of experimental botany, 38(7), pp.1174-1181.
CrossRef - Zhang, Q., Cai, L., Zhong, G. and Luo, W., 2010. Simultaneous determination of jatrorrhizine, palmatine, berberine, and obacunone in PhellodendriAmurensis Cortex by RP-HPLC. ZhongguoZhongyaozazhi= Zhongguozhongyaozazhi= China journal of Chinese materiamedica, 35(16), pp.2061-2064.
- Ziyaev, R., Abdusamatov, A. and Yunusov, S.Y., 1987. Alkaloids ofLiriodendron tulipifera. Chemistry of Natural Compounds, 23(5), pp.521-528.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






