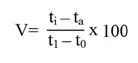How to Cite | Publication History | PlumX Article Matrix
Gousiya Begum and Srinivas Munjam*
and Srinivas Munjam*
Department of Microbiology, Kakatiya University, Warangal -506009, Telangana (TS), India.
Corresponding Author E-mail: munjam17@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2906
ABSTRACT: Pectinases are the commercial enzymes that are abundantly employed in various industries like fruit juice industries for clarification, wine indutsry and paper industry for bleaching up pulp. The present work was done on culture conditions optimization for production of pectinases under submerged fermentation using wheat bran as a substrate. Fungal strains were isolated from vegetable waste dump yard soils of Warangal district of Telangana state and screened for their activity on pectin agar medium. Among 30 isolates, two fungal strains showed good activity and identified them as A. niger and A. flavus. The effects of the different carbon and nitrogen sources on pectinases viz. exo-PG, endo-PG, endo-PL and PME by A. niger with 1% wheat bran was carried out in submerged fermentation. These studies revealed that carbon and nitrogen sources have shown considerable influence on enzyme production. Among all the carbon sources tried, sucrose at 1% was shown to be efficient carbon source for all four types of pectinases under investigation. For endo-PG, endo-PL and PME maximum enzyme production were recorded on 8th day of incubation period but for exo-PG enhanced production was observed on 12th day. A. niger could not produce PME on 12th day from 2.50% to subsequent concentrations. Among nine different nitrogen sources were screened, maximum pectinase production was recorded in sodium nitrate at 0.2 % for A. niger. Endo-PG, endo-PL and PME maximum production were recorded on 8th day of incubation and for exo-PG maximum production was observed on 12th day. No PME production was observed in A. niger on 12th day.
KEYWORDS: A. Niger; Carbon and Nitrogen Sources; Pectinases; Submerged Fermentation; Wheat Bran
Download this article as:| Copy the following to cite this article: Begum G, Munjam S. Carbon and Nitrogen Sources Effect on Pectinase Synthesis by Aspergillus niger Under Submerged Fermentation. Biosci Biotech Res Asia 2021;18(1). |
| Copy the following to cite this URL: Begum G, Munjam S. Carbon and Nitrogen Sources Effect on Pectinase Synthesis by Aspergillus niger Under Submerged Fermentation. Biosci Biotech Res Asia 2021;18(1). Available from: https://bit.ly/3gQICNh |
Introduction
Pectinase enzymes which acts on the glycosidic bonds breakdown in the galacturonic acid chains of the pectin materials17. The enzymes production has developed rapidly and currently, pectinase are the most important products obtained for human needs through microbial sources. Because of their wide applications in food industry29, pectinases accounts for 25% of the global food enzyme market7. Because of wide industrial applications and important cost, there is a need to minimize the production costs. Agricultural wastes, like wheat bran, containing large amount of pectin can be considered as other source of substrate for pectinase production by avoiding the use of expensive chemical components in the media formulation. Agroindustrial by products can be successfully employed for pectinolytic enzyme production and as these residues are low cost raw materials available locally, they can be used for cost effective enzyme production.
Pectinolytic enzymes have been observed in a large variety of bacteria and fungi, mostly commercial preparations of pectic enzymes are acquired from fungal sources. Due to pH optima of enzymes produced by fungal strains are in a range naturally found in materials to be processed. But Aspergillus species are employed in production of pectinases which are used in various applications like extraction, clarification of fruit juices, in maceration of vegetables to produce purees and also in wine making16.
Pectinases are commercially produced using either submerged or solid state fermentation cultures. Semi-solid or submerged fermentation medium is more favorable to fungal growth since in the medium, the moisture content, agro-waste (substrate) and pH are the main factors determining enzyme yield. In solid state fermentation culture, microbial growth and product formation occur at or near the surface of solid substrate particles with low moisture content whereas, in submerged fermentation culture, production of pectinases usually depends on medium compositions such as nitrogen source, pH of the medium, temperature, pectin concentration, and fermentation time.23Inspite of a lot of researchers recommended solid state fermentation for production of microbial enzymes (ex: pectinase), submerged fermentation technique is the suitable system on large-scale5. Therefore, utilization of agricultural residues as carbon sources in enzyme production media has increased enzyme activity with reduction of the production cost30.The aim of the present work is to assess the enhancement of enzyme production by A. nigerin different carbon and nitrogen sources in submerged fermentation using wheat bran as substrate.
Materials and Methods
Sample collection
Soil samples collected from the site where the vegetable wastes were dumped which were obtained from different areas of Warangal, in sterile polythene bags and were immediately transferred to the laboratory for microbial assessment and analysis.
Isolation of fungi
Potato dextrose agar (PDA) medium consists of (gm/L): Dextrose, 20.0, agar, 15.0 and potato infusion, 200; was chosen as growth medium for preliminary isolation of fungi. All the above mentioned samples were suspended in sterile distilled water. These suspensions were stirred for 20 minutes before making serial dilutions. The dilution-plate method was employed for the isolation purpose12. After serial dilutions, suspensions were spread on potato dextrose agar medium containing 0.08% streptomycin to avoid bacterial contamination. These plates were incubated in an inverted position at 28˚C for 7 days14. Fungi growing on the agar plates were sub-cultured and were preserved on potato dextrose agar slants under refrigeration condition at 4˚C prior to use and maintained for further identification and enzyme studies.
Morphological identification of the fungi
Based on their morphology, mycelia structure and spore formation, the fungal isolates were identified. The identified fungal strains were stained by lacto phenol cotton blue staining.
Primary Screening of Pectinolytic Fungi
Selection of 30 potential pectinolytic fungi were assessed by using 0.1 mL of inoculum from the enriched medium, they were plated on pectin agar media contains 1% pectin, 0.1%, K2HPO4; 0.2%, NaNO3; 0.05%, MgSO4. 7H2O; 0.05%, KCl; 10mg, FeSO4.7H2O; 3%, Sucrose; 0.001%, CuSO4 and 0.001%, ZnSO4 and incubated at 28±1ºC for 4-5 days. After 5 days of incubation, plates were flooded with iodine- potassium iodide solution and observed for zone of hydrolysis around the wells29. Among 30 isolates, two fungal strains showed good activity and identified them as A. niger and A. flavus. Among these two isolates A. niger showed better activity over A. flavus, hence only A. niger presented in this communication.
Pectinases Production Under Submerged Fermentation (Smf)
250 ml Erlenmeyer flask containing 100 ml of culture broth (pH 7.0), contains 1% wheat bran as pectin substrate, 3%, sucrose; 0.2%, NaNO3; 0.1%, MgSO4.7H2O; 0.05%, KCl; 0.01%, K2HPO4; 0.05%, FeSO4.7H2O; 0.001% CuSO4 and 0.001%, ZnSO4; were used for assay of pectinases. After sterilization of the Erlenmeyer flasks containing fermentation medium, young fungal mycelia of 3 day old cultures at the growing edges were inoculated aseptically. Inoculated flasks were incubated in the orbital shaker operating at 120-180 rpm at 28±1ºC for 16 days. 10 ml of incubated broth was withdrawn from the culture flasks at different time intervals. The supernatants obtained from the centrifugations were used as partially purified enzyme sources for assay.
Effect of various carbon sources on the pectinases production was assessed in submerged fermentation (SmF) by culturing Aspergillusniger. The above mentioned production media were supplemented with various carbon sources at 1% (w/v). The carbon sources used in this investigation were fructose, dextrose, lactose, maltose, sucrose, mannitol, arabinose, cellulose. Fermentation medium lacking carbon source was considered as control. Concentration of best carbon source was optimized for pectinase production by using its different concentration (w/v) viz., 0.05%, 1.00%, 1.50%, 2.00%, 2.50%, 3.00% and 3.50%. The inoculated flasks were incubated at temperature 28±1˚C for 16 days enzyme assay was carried from the enzyme source.
To determine the influence of several inorganic and organic nitrogen compounds on pectinase production, ingredients considered as supplying nitrogen in the basal medium were replaced with various nitrogen sources at 0.2% (w/v). The employed nitrogen sources were (casein, peptone, yeast extract, ammonium chloride, urea, ammonium molybdate, sodium dihydrogen phosphate, potassium nitrate, sodium nitrate). Fermentation medium lacking nitrogen source was considered as control. Different concentrations 0.05%, 0.10%, 0.20%, 0.30%, 0.40%, 0.50% w/v of best nitrogen source were added to the production media to study the effect of concentration of nitrogen on enzyme production. After incubation the inoculated flask at temperature 28±1˚for 16 days pectinolytic activity were detected as previously described in SmF
Enzyme recovery
After incubation, to remove mycelium, the culture medium was filtered using filter paper Whatmann No.5, then filtrate was centrifuged at 5000 rpm for 10 min and the clear supernatant was used as the extracellular enzyme source.
Quantitative assay for exo polygalacturonase (Exo-PG)
Exo polygalacturonases, activity was assayed by estimating reducing sugars using DNS method20. The exo-PGase activity was measured using 1% polygalacturonic acid (PGA) as substrate, prepared in sodium acetate buffer (0.1M; pH 4.5). The reaction mixture (2mL) contained equal amounts of enzyme (1.0mL) and substrate (1.0mL) and incubating at 50ºC for 30min in a water bath. By addition of 3ml of 3,5- dinitrosalicilic acid (DNS) reagent, the reaction was stopped and the contents were boiled for 15 minutes. The color developed was read at 540 nm. D-galactouronic acid (1mg/mL) standard curve was prepared to find out the amount of reducing sugars liberated. Enzymatic activity expressed as unit per ml (U/ml), which is defined as the amount of enzyme, which liberates 1μmole of galacturonic acid (reducing sugar) per mL per minute under assay conditions.
Quantitative assay for Endo Polygalacturonase (Endo-PG)
Wood’s viscometric method36 was employed to estimate the endo-PG. Polygalacturonic acid (0.5%) was prepared by dissolving 0.5g of polygalacturonic acid in 100ml citrate buffer (pH 5.5). The reaction mixture for the estimation of endo-PG contained polygalacturonic acid (0.5%) substrate, citrate buffer (pH 5.5) and enzyme source in 4:1:2 ratios. The reaction mixture consisting of 12ml of substrate, 4ml of enzyme and 1ml of citrate buffer. The viscosity loss was measured for every 10 minutes over a period of 30 minutes. The reaction mixture with inactivated enzyme (heat killed) and distilled water as control. The viscosity loss percentage was calculated by the formula as given below.
Where,
V = loss of viscosity percentage
ti = flow time of reaction mixture + inactive enzyme.
ta = flow time of reaction mixture + active enzyme
t0= flow time of distilled water + active enzyme at ‘‘O’’ time
The Relative Enzyme Activity (REA) of endo-PG was calculated by dividing 1000 with time required for 50% loss of viscosity (t50) and in relative viscometric units (RVU).
Where tv50 = time required in minutes taken for 50% loss of initial viscosity
Quantitative assay for pectin methyl esterase activity (PME)
Pectin methyl esterase activity was measured by the standard method15. PME activity can be measured either by measuring the amount of methanol released or increase in free carboxyl group by monitoring pH changes.
Pectin methyl esterase activity was estimated by titration method against NaOH with phenolphthalein as a pH indicator. PME activity was assayed by 20ml of 1% pectin (dissolved in 0.15M NaCl, pH-7.0) and 4ml of enzyme extract were taken in a beaker and incubated for 1hr. After incubation, the solution was titrated against the 0.02N NaOH to reach pH 7.0 using the phenolphthalein as indicator (colour change from colourless to pink). The heat killed enzyme extract was used as control.
Pectin esterase activity = Vs – V b (Normality of NaOH) × 100/Vt
Where, Vs-volume of NaOH used to titer the sample (ml), V b-volume of NaOH used to titer the blank (ml), V-volume of incubation mixture (ml), t-Reaction time (min). PME activity was expressed as milliequivalents of NaOH consumed min-1 ml-1 of enzyme extract under the assay conditions.
Quantitative assay for endo pectinlyase (Endo-PL)
Endo-PL activity was assayed viscometric method 36. 1% pectin was used as substrate in this assay. Four ml of culture supernatant and 0.8 ml of tris-HCl buffer pH (8.0) were added to 12ml of pectin solution. Viscosity changes of reaction mixture were determined by using Ostwald viscometer. Initial reading time and the reading after 30 minutes of incubation were determined. The loss of viscosity was estimated for every 10 minutes over a period of 30 minutes. The reaction mixture with inactivated enzyme (heat killed) and distilled water served as control. Enzyme activity was expressed in RVU units (relative viscometric units).
Statistical analysis
The enzyme activities are presented as Mean±SE of all values. Results found in this study were subjected to analysis of variance using oneway ANOVA and difference between means were separated by Duncan Multiple Range Test using SPSS software 17.0 version33. The results are presented in tables (1-2) and figures (1-8)
Results and discussion
Influence of carbon sources on pectinases
The influence of carbon source on exo-PG was recorded minimum on 8th day and maximum on 12th day of incubation period. The highest exo-PG (0.690U/ml) was obtained in the medium containing sucrose on 12th day of incubation followed by dextrose (0.610U/ml) and fructose (0.520 U/ml). Cellulose (0.310 U/ml) and lactose (0.300 U/ml) showed intermediate production (fig. 1). Rest of the carbon sources showed meager activity. Very less activity was obtained in arabinose (0.024U/ml). Similar results were expressed by Banuet al.6 in sucrose (29.1U/ml) and by Mojsov21 in galactose (140.0UL-1) by A. niger.
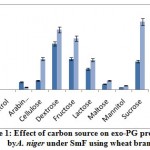 |
Figure 1: Effect of carbon source on exo-PG production by A. niger under SmF using wheat bran |
The influence of carbon source on endo-PG was recorded maximum on 8th day comparatively 12th day of incubation period. The maximum endo-PG activity was found in the medium containing sucrose (105.0RVU) on 8th day of incubation (fig 2). Very less activity was obtained in dextrose (27.76RVU). In another study Patil and Dayanand25 reported that 4-6% glucose increased the production of pectinase in submerged fermentation.
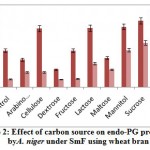 |
Figure 2: Effect of carbon source on endo-PG production by A. niger under SmF using wheat bran. |
Results from the figure 3 reveal highest endo-PL activity recorded in the medium containing sucrose (58.0RVU) followed by maltose (57.0RVU) on 8th day of incubation. Very less activity recorded in arabinose (28.20 RVU) and no activity was in fructose on 8th day. Maximum production was recorded on 8th day of incubation comparatively 12th day. Control failed to show the enzyme activity. Several workers reported that fructose influenced the maximal production of pectinase out of all carbon sources used2. The reason could be that the fructose is a simple sugar and pectinolytic microorganism utilizes simple sugars more efficiently as compared to complex polysaccharide such as starch and produced maximum, galacturonic acid from their substrates.
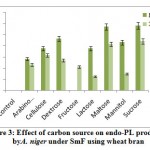 |
Figure 3: Effect of carbon source on endo-PL production by A. niger under SmF using wheat bran. |
The highest PME activity was obtained in the medium containing sucrose (0.051 meq. of NaOH consumed/min/ml) followed by maltose (0.049 meq. of NaOH consumed/min/ml) on 8th day of incubation (fig 4). Rest of the carbon sources showed minimum production. Very less activity obtained in arabinose (0.004 meq. of NaOH consumed/ min/ml) on 12th day of incubation. Solis-Pereira et al.32 was proved that there was a catabolite repression of pectic enzymes in presence of glucose and other sugars.
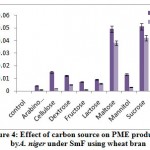 |
Figure 4: Effect of carbon source on PME production by A. niger under SmF using wheat bran. |
Enzyme production started at 0.50% concentration, reached optimum at 1.0% and gradually decreased in subsequent concentrations (Table. 1.). Sucrose at a concentration of 1% was observed to be the best carbon source for all types of pectinases by A. niger. Among different concentration of sucrose a significant exo-PG activity (1.529U/ml) was observed on 12th day of incubation. While increased endo-PG (83.0RVU) and endo-PL (86.7RVU) activity observed on 8th day of incubation. Similarly increased PME activity also observed (0.060 meq. of NaOH consumed/min/ml) on 8th day of incubation. Twelfth day of incubation favoured more exo-PG activity and rest of the pectinases shows maximum production on 8th day.
The present results are on par with the findings of Palaniyappan and his associates who reported an enhanced activity of pectinases in glucose as carbon source by A. fumigatus24. Similar findings were observed earlier in which low enzyme production with other carbon sources is maybe due to catabolite repression3. Previous studies reported the same results that glucose followed by lactose observed with the highest production of pectinase24. Jayaniet al.11 revealed different results about glucose. Phutelaet al.26 mention the positive effect of sucrose on the production of pectinolytic enzymes, produced by Aspergillus fumigatus, which leads to the enhanced production of the enzymes.
Table 1: Effect of sucrose concentration on pectinases production by A. niger under SmF using wheat bran.
| Sucrose (%) | Exo -PG
(U/ml) |
Endo-PG
(RVU) |
Endo-PL
(RVU) |
PME (meq. of NaOH
consumed/min/ml) |
||||
| 8th day | 12th day | 8th day | 12th day | 8th day | 12th day | 8th day | 12th day | |
| 0.50% | 0.807bc±0.001 | 1.480b±0.005 | 65.0b±0.001 | 28.50cd±0.001 | 55.0cd±0.003 | 17.5b±0.001 | 0.0020d±0.001 | 0.001f±0.001 |
| 1.00% | 1.099a±0.001 | 1.529a±0.005 | 83.0a±0.001 | 46.0a±0.001 | 86.7a±0.003 | 20.0a±0.001 | 0.060a±0.001 | 0.030a±0.001 |
| 1.50% | 0.893b±0.001 | 1.391cd±0.005 | 57.0cd±0.001 | 30.0bc±0.001 | 57.8b±0.003 | 10.1c±0.001 | 0.0046b±0.001 | 0.020b±0.001 |
| 2.00% | 0.893b±0.001 | 1.305cd±0.005 | 57.0cd±0.001 | 30.0bc±0.001 | 46.7e±0.003 | 8.6d±0.001 | 0.0031c±0.001 | 0.020b±0.001 |
| 2.50% | 0.893b±0.001 | 0.960ef±0.005 | 40.5e±0.001 | 28.5cd±0.001 | 40.0f±0.003 | 6.5e±0.001 | 0.0031e±0.001 | — |
| 3.00% | 0.893b±0.001 | 0.807g±0.005 | 24.0f±0.001 | 24.0d±0.001 | 35.5g±0.003 | 4.2f±0.001 | 0.0020d±0.001 | — |
| 3.50% | 0.893b±0.001 | 0.910ef±0.005 | 12.5g±0.001 | 10.5e±0.001 | 28.0h±0.003 | 2.1g±0.001 | 0.0020d±0.001 | — |
Values are significant at P< 0.005
— No activity
Effect of nitrogen source on pectinases
The effect of nitrogen sources on exo-PG production by A. nigerunder SmF shown in figure 5. It was observed that sodium nitrate (0.613U/ml) followed by urea (0.550U/ml), peptone (0.506U/ml), ammonium chloride (0.480U/ ml), potassium nitrate (0.430U/ml) supported maximum for the production of exo-PG on 12th day of incubation. Casein (0.255U/ml), sodium dihydrogen phosphate (0.235U/ml), yeast extract (0.210U/ml), control (0.200U/ml) caused poor induction of exo-PG on both the incubation periods. Fawole and Odunfa9 who found that ammonium sulphate and ammonium nitrate were best nitrogen sources for A. niger while glycine and tryptophane were not supported for enzyme production. El Garhyet al,8 observed that among five nitrogen sources tested for screening their effect on pectinase production, yeast extract was reported to be the good nitrogen source producing the optimum level of pectinase activity by P. chrysogenum.
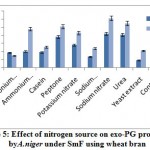 |
Figure 5: Effect of nitrogen source on exo-PG production by A. niger under SmF using wheat bran. |
Results from the figure 6. reveal the maximum endo-PG activity in sodium nitrate (163RVU) followed by urea (70.66RVU) whereas, sodium dihydrogen phosphate (57.6RVU), ammonium molybdate (54.33RVU), potassium nitrate (53.66RVU), ammonium chloride (48.66RVU), peptone (48.66RVU), casein (46.0RVU) and yeast extract (42.5RVU) showed moderate levels while, control (23.0RVU) caused poor production of enzyme on 8th day of incubation. Mrudula and Anitharaj22 they were found highest pectinase activity of 223.3Ug-1 in yeast extract + (NH4)2SO4 by A. niger. The current result is on par with the result of Rajmane and Korekar28. They showed maximum pectinase activity of 89.0U/ml by Botryodiplodia theobromae.
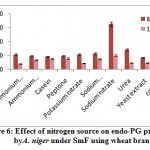 |
Figure 6: Effect of nitrogen source on endo-PG production by A. niger under SmF using wheat bran. |
The highest endo-PL activity recorded in sodium nitrate (43.66RVU) followed by potassium nitrate (42.0RVU), whereas peptone (32.40RVU), urea (26.2RVU) showed moderate level while, ammonium chloride (18.0RVU), casein (16.63RVU), ammonium molybdate (14.66RVU), sodium dihydrogen phosphate (13.0RVU) yeast extract (12.50RVU) and control (10.0RVU) showed poor production on 8th day of incubation. (fig 7). Patil and Dayanand25 who found greater values of endo- and exo-pectinases when the medium was supplemented with ammonium sulphate, (0.3%) as nitrogen source in SmF and SSF by A. nigerDMF 27 and DMF 45. It has been demonstrated that yeast extract in combination with (NH4)2SO4 was observed to support maximum production of pectinase (831U/g) by A.niger IM09 10.
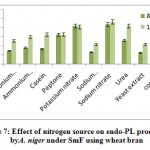 |
Figure 7: Effect of nitrogen source on endo-PL production by A. niger under SmF using wheat bran. |
Figure 8. reveal highestPME activity obtained in sodium nitrate (0.043 meq. of NaOH consumed/min/ml) followed by urea (0.034 meq. of NaOH consumed/min/ml), and potassium nitrate (0.030 meq. of NaOH consumed/min/ml) on 8th day of incubation whereas, yeast extract 0.022 meq. of NaOH consumed/min/ml), casein (0.020 meq. of NaOH consumed/min/ml) and control (0.020 meq. of NaOH consumed/min/ml) showed moderate level of production, while ammonium chloride (0.017 meq. of NaOH consumed/min/ml), sodium dihydrogen phosphate (0.012 meq. of NaOH consumed/min/ml), ammonium molybdate (0.011 meq. of NaOH consumed/min/ml) and peptone (0.008 meq. of NaOH consumed/min/ml) showed very less production of PME on 8th day of incubation. Abbasiet al.1 also found finding of highest exo and endopectinase activity in ammonium sulphate (1.6Uml-1 and 0.0013Uml-1) than sodium nitrate (1.2Uml-1 and 0.0011Uml-1) as nitrogen source by A. niger.
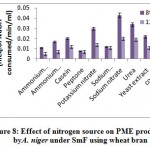 |
Figure 8: Effect of nitrogen source on PME production by A. niger under SmF using wheat bran. |
Influence of different concentrations of sodium nitrate on pectinases production discussed in table 1.1. Sodium nitrate at 0.2% concentration was proved to be the best nitrogen source for pectinases production. An increasing trend in enzyme activity was observed in different concentrations (0.05%, 0.10%, 0.20%, 0.30%, 0.40% and 0.50%) up to 12 days and decreased in subsequent incubations. Exo-PG activity showed a significant level of 0.320U/ml in 0.2% sodium nitrate on 12th day of incubation and decreased subsequently. Similarly an increased endo-PG activity of 51.8U/ml was also observed in 0.2% sodium nitrate but on 8th day of incubation. Similar results of endo-PL activity (71.5U/ml) and PME activity (0.068U/ml) were also observed in 0.2% sodium nitrate on 8th day of incubation. Overall, highest pectinases production was recorded on 8th day incubation for all pectinases except exo-PG; it showed its maximum activity on 12th day. There was no PME enzyme activity on 12th day of incubation becausethe longer an enzyme is incubated with its substrate, the higher the amount of product that will be formed. As a result, the rate of formation of product slows down as along as the incubation proceeds, and if the incubation time is too long, then there is no activity of the enzyme.
It was also studied that ammonium sulphate had great effect on the production of pectinase (15.74IU/ml) by A. niger19. Previous studies reported that increased pectinase activity (85.0U/mg) in ammonium persulphate by Pencillium chrysogenum6. Similar view was expressed by other workers34 and reported increased pectinase activity of 65.0U/ml in ammonium sulphate by Pencillium chrysogenum. The current view is on par with the results of Lahaet al.18 and reported increased activity in ammonium sulphate (0.936mg/ml) by Pencillium chrysogenum.
Surprisingly, previous studies reported ammonium dihydrogen phosphate as the good nitrogen source for the growth and production of pectinase by A. niger13. Sethiet al.31 found regarding three-fold increase in pectinase activity with ammonium persulfate. Previous studies revealed as ammonium sulphate (1.69%) as a good nitrogen source for pectinase production in Penicillium chrysogenum4.
Table 2: Effect of sodium nitrate concentration on pectinases production by A. niger under SmF using wheat bran.
| NaNO3
(%) |
Exo -PG
(U/ml) |
Endo-PG
(RVU) |
Endo-PL
(RVU) |
PME (meq. of NaOH
consumed/min/ml) |
||||
| 8th day | 12th day | 8th day | 12th day | 8th day | 12th day | 8th day | 12th day | |
| 0.05% | 0.061c±0.001 | 0.140cd±0.001 | 42.8c±0.005 | 20.5c±0.001 | 49.0de±0.005 | 25.5d±0.002 | 0.031d±0.001 | — |
| 0.1% | 0.070b±0.001 | 0.175b±0.001 | 47.6b±0.005 | 22.7b±0.001 | 56.5c±0.005 | 32.0b±0.002 | 0.052b±0.001 | — |
| 0.2% | 0.110a±0.001 | 0.320a±0.001 | 51.8a±0.005 | 30.5a±0.001 | 71.5a±0.005 | 41.5a±0.002 | 0.068a±0.001 | — |
| 0.3% | 0.066c±0.001 | 0.160cd±0.001 | 41.3d±0.005 | 22.0b±0.001 | 66.6b±0.005 | 28.0c±0.002 | 0.036c±0.001 | — |
| 0.4% | 0.047e±0.001 | 0.055e±0.001 | 33.3e±0.005 | 15.5d±0.001 | 53.6de±0.005 | 23.6e±0.002 | 0.028e±0.001 | — |
| 0.5% | 0.0241f±0.001 | 0.020f±0.001 | 22.8f±0.005 | 11.0e±0.001 | 21.6f±0.005 | 20.6f±0.002 | 0.018f±0.001 | — |
Values are significant at P < 0.005
— No activity
Conclusion
In conclusion, A. niger was identified as the best strain for pectinase enzyme production by submerged fermentation. The effect of added carbon sources such as arabinose, cellulose, dextrose, fructose, lactose, maltose, mannitol and sucrose was also investigated. Out of all the different carbon added sources sucrose found to be best for optimum production of pectinase. Sucrose acts as an inducer and stimulate the production of enzyme. Various concentration of sucrose (0.50% to 3.50%) for maximal productivity of enzyme was screened. Highest enzyme activity was observed at 1.0 %. Above or below the optimal concentration of carbon source leads to decline in the productivity of enzyme.
The effect of various inorganic and organic nitrogen sources (ammonium chloride, ammonium molybdate, casein, peptone, urea, potassium nitrate, sodium dihydrogen phosphate, sodium nitrate, and yeast extract were studied. The nitrogen sources enhanced the fungal growth and provoked the secretion of enzyme. Several additional nitrogen sources added, out of all nitrogen sources, sodium nitrate proved to be maximum pectinase production. Various concentrations of sodium nitrate (0.05% to 0.50%) for maximum productivity of enzyme was screened. Maximal enzyme activity was recorded at 0.2%. Because inorganic nitrogen source like sodium nitrate maintains the fungal growth better than that of organic nitrogen sources. Fungal strain hydrolyzes the sodium nitrate easily so, various nutrient components and growth factors which were released assimilated into fungal metabolism that eventually increases their growth. But, further studies must be carried out to identify the strain in genetic levels and to satisfy its commercial application in large-scale food formulation and processing.
Acknowledgement
We are thankful to the Head, Department of Microbiology, Kakatiya University, Warangal for facilities and encouragement.
Conflict of Interest
All authors declare that there is no conflict of interest in this work.
Funding Source
There is no funding source
References
- Abbasi H., Mortazavipour R. and Setudeh M., Polygalacturonase (PG) production by fungal strains using agro-industrial bioproduct in solid state fermentation. Chemical Engineering Research Bulletin, 15(1), 1-5 (2011).
CrossRef - Abdullah R., Jafer A., Nisar K., Kaleem A., Iqtedar M., Iftikhar T. and Naz S., Process optimization for pectinase production by locally isolated fungal strain using submerged fermentation. Bioscience Journal, 34(4),(2018).
CrossRef - Ahlawat S., Dhiman S. S., Battan B., Mandhan R. P. and Sharma J., Pectinase production by Bacillus subtilis and its potential application in biopreparation of cotton and micropoly fabric. Process Biochemistry, 44(5), 521-526 (2009).
CrossRef - Akhter N., Morshed M. A., Uddin A., Begum F., Sultan T. and Azad A. K., Production of pectinase by Aspergillusniger cultured in solid state media. Int J Biosci, 1(1), 33-42 (2011)
- Azzaz H. H., Aziz H. A., Alzahar H. and Murad H. A., Yeast and Trichodermaviride don’t synergistically work to improve olive trees by products digestibility and lactating Barki ewe’s productivity. Biol. Sci, 18, 270-279 (2018)
CrossRef - Banu A. R., Devi,M. K., Gnanaprabhal G. R., Pradeep B. V. and Palaniswamy M., Production and characterization of pectinase enzyme from Penicilliumchrysogenum. Indian Journal of Science and Technology, 3(4), 377-381 (2010)
CrossRef - Chauhan S, Vohra A, Lakhanpal A. and Gupta R. Immobilization of commercial pectinase (Polygalacturonase) on celite and its application in juice clarification. Journal of Food Processing and Preservation. 9(6), 2135-41 (2015)
CrossRef - El Garhy G. M., Azzaz H. H., Abd El Mola A. M. and Mousa, G.A., Fungal Pectinase Production Optimization and its Application in Buffaloe’s Diets Degradation. (2020)
- Fawole O. B. and Odunfa S. A., Some factors affecting production of pectic enzymes by Aspergillusniger. International Biodeterioration& Biodegradation, 52(4), 223-227 (2003)
CrossRef - Islam S., Feroza B., Alam A. K. M. R. and Begum S., Pectinase production by Aspergillusniger isolated from decomposed apple skin. Bangladesh Journal of Scientific and Industrial Research, 48(1), 25-32 (2013).
CrossRef - Jayani R. S., Saxena S. and Gupta R., Microbial pectinolytic enzymes: a review. Process Biochemistry, 40(9), 2931-2944 (2005).
CrossRef - Johnson, L. E. and Curl, E. A., Methods for Research on the Ecology of Soil-borne Plant Pathogens. Burgess Publ. Co. Minneapolis (1972).
- Joshi V. K., Parmar M., and Rana N. S., Pectin Esterase Production from Apple Pomace in Solid-State and Submerged Fermentations. Food Technology & Biotechnology, 44(2), (2006).
- Kaur, S., Kaur, H. P., Prasad, B. and Bharti, T., Production and optimization of pectinase by Bacillus sp. isolated from vegetable waste soil. Indo American Journal of Pharmaceutical Research, 6(1), 4185-41906 (2016).
- Kertesz Z. I. and Mccollocch R. J., Enzymes acting on pectic substances. In Advances in carbohydrate chemistryAcademic Press 5, 79-102 (1950).
CrossRef - Khan F. and Latif Z., Molecular characterization of polygalacturonase producing bacterial strains collected from different sources. Anim. Plant Sci, 26(3), 612-618 (2016).
- Khattab M. S., Azzaz H. H., El Tawab A. M. A. and Murad H. A., Production optimization of fungal cellulase and its impact on ruminal degradability and fermentation of diet. J. Dairy Sci, 14, 61-68 (2019).
CrossRef - Laha S., Sarkar D. and Chaki S., Optimization of production and molecular characterization of pectinase enzyme produced from penicilliumchrysogenum. Scholars Academic Journal of Biosciences, 2(5), 326-335 (2014).
- Meenakshisundaram V., Optimization of pectinase enzyme production by using sour orange peel as substrate in solid state fermentation. Asian J Biochem Pharm Res, 2(1), 16-26 (2012).
- Miller G. L., Use of dinitrosalicylic acid reagent for determination of reducing sugar. Analytical chemistry, 31(3), 426-428 (1959).
CrossRef - Mojsov K., Experimental investigations of submerged fermentation and synthesis of pectinolytic enzymes by Aspergillusniger: effect of inoculums size and age of spores. Applied Technologies & Innovations, 2(2), 40-46 (2010).
CrossRef - Mrudula S. and Anitharaj R., by Aspergillusniger Using Orange Peel as Substrate. Global journal of Biotechnology and Biochemistry, 6(2), 64-71(2011).
- Oluwayemisi O. A., Effect of Blanching, Ripening and Other Treatments on the Production Characteristics of Pectinolytic Enzymes from Banana Peels by Aspergillusniger. Glob J Sci Front Res Chem, 12(2), 37-46 (2012).
- Palaniyappan M., Vijayagopal V., Viswanathan R. and Viruthagiri T., Screening of natural substrates and optimization of operating variables on the production of pectinase by submerged fermentation using Aspergillusniger MTCC 281. African journal of Biotechnology, 8(4),(2009).
- Patil S. R. and Dayanand A., Production of pectinase from deseeded sunflower head by Aspergillusniger in submerged and solid-state conditions. Bioresource technology, 97(16), 2054-2058 (2006).
CrossRef - Phutela, U., Dhuna, V., Sandhu., S., and Chadha, B., “Pectinase and polygalacturonase production by a thermophilic Aspergillus fumigatus isolated from decomposting orange peels, , J. Microbiol.36 (1), 63-69 (2005).
CrossRef - Prakash D., Nawani N., Prakash M., Bodas M., Mandal A., Khetmalas M. and Kapadnis B., Actinomycetes: a repertory of green catalysts with a potential revenue resource. BioMed research international, (2013).
CrossRef - Rajmane S. D. and Korekar S. L., Impact of carbon and nitrogen sources on pectinase production of post-harvest fungi. Current Botany. (2012).
- Reddy, P. L. and Sreeramulu, A., Isolation, identification and screening of pectinolytic fungi from different soil samples of Chittoor district. International Journal of Life Sciences Biotechnology and Pharma Research, 1(3), 1-10 (2012).
- Sabah M.H. Al-Shatty, Alaa Kareem Niamah, Bayan Y Abdullah., The ability of Trichodermaharzianum on cleavage of cellulose of date palm leaves. Journal of Tikrit University for Agricultural Sciences, 11(4), 2011.
- Sethi B. K., Nand, P. K. and Sahoo S., Enhanced production of pectinase by Aspergillusterreus NCFT 4269.10 using banana peels as substrate. 3 Biotech, 6(1), 36 (2016)
CrossRef - Solís-Pereira S., Favela-Torres E., Viniegra-González G. and Gutiérrez-Rojas M., Effects of different carbon sources on the synthesis of pectinase by Aspergillusniger in submerged and solid state fermentations. Applied Microbiology and Biotechnology, 39(1), 36-41 (1993).
CrossRef - SPSS Inc. Released 2008. SPSS Statistics for Windows, Version 17.0. Chicago: SPSS Inc; 2008.
- Tariq A. L. and Reyaz A. L., The influence of carbon and nitrogen sources on pectinase productivity of Penicillium chrysogenum in solid state fermentation. Res. J. Microbiol., 3, 202-207 (2012).
- Teixeira, Maria F. S., Lima Filho, José L. and Durán, Nelson., Carbon sources effect on pectinase production from Aspergillusjaponicus 586. Brazilian Journal of Microbiology,31(4), 286-290 (2000).
CrossRef - Wood R. K. S., Studies in the Physiology of Parasitism: XVIII. Pectic Enzymes secreted by Bacterium aroideae. Annals of Botany, 19(1), 1-27 (1955).
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.