How to Cite | Publication History | PlumX Article Matrix
Department of Microbiology, Faculty of Life Sciences, Guru Nanak College Of Science, Ballarpur, (Gondwana University), Dist.- Chandrapur (Maharashtra State), India 442 701.
Corresponding Author E-mail: knsahare@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2926
ABSTRACT: In the present work antifilarial active fraction was isolated from the leaves Chloroform extract of Aegle marmelos Corr. evaluated in vitro for antifilarial activity and studied the possible oxidative role against Setaria cervi parasite. Antifilarial study was carried out with isolated fractions by worm motility and MTT assays. Complete parasite motility inhibition was observed at 0.002 to 0.08 mg/mL in motility assay and in MTT assay plant fraction gave > 50% reduction 58.9, 74.6 and 97.2% at concentrations 0.02, 0.04 and 0.08 mg/mL at 10, 6 and 2 hours incubation period respectively (p< 0.05). Inhibitory concentration (IC50) was found to be 0.015 mg/mL. Oxidative parameters levels for MDA, Carbonyl content and Nitric oxide were identified as antifilarial activity achieved. The level of oxidative parameters was calculated in dose dependent manners as compared to the control level. The antifilarial activity of isolated fraction is associated with the oxidative mechanism in this study.
KEYWORDS: Antifilarial; Aegle marmelos; leaves; Oxidation
Download this article as:| Copy the following to cite this article: Sahare K. N. Antifilarial Screening and Oxidative Role of Isolated Fraction from Aegle Marmelos Corr. Leaves Extract. Biosci Biotech Res Asia 2021;18(2). |
| Copy the following to cite this URL: Sahare K. N. Antifilarial Screening and Oxidative Role of Isolated Fraction from Aegle Marmelos Corr. Leaves Extract. Biosci Biotech Res Asia 2021;18(2). Available from: https://bit.ly/2XJmwEN |
Introduction
Filariasis appears in the tropical and subtropical areas of the world. Filariasis is mainly infected by filarial nematodes Wuchereria bancrofti and Brugia malayi parasite. It is spread by mosquito vectors1. It is an important health problem, which affects more than 100 million populations throughout the world. In lymphatic filariasis major side effects are swelling in lower legs and disfigure in prevalent sites, which lead to considerable social, economical and psychological effects. In India, 48 million populations are infected from filariasis and approximately 45% of its 1 – billion persons are lives in filariasis known areas2 and calculated for 40% of global disease burden3. Yearly loss cause to a billion dollars by this disease, as per the various social and economic studies4.
World health organization has documented as a main community health crisis in filariasis endemic areas. It is specifically documented in its TDR mandate and initiated a world agenda for filaria disease eradication (GPELF)5. Antifilarial known drugs Albendazole, DEC and Ivermectin are recently giving to a population for this disease1. The drugs can not to kill adult worms. Adult worms survive many years in infected peoples6. So, it needs to formulate a potent and safe drug to treat and remove the filarial parasite. Herbal resources contain a variety of plant active molecules which is utilized the most in herbal therapeutics. WHO has recommended traditional medicine as a key substitute resource for potent filarial disease in his TDR plan7, but the lack of scientific study is the most important lacuna to use conventional therapeutics.
Aegle marmelos Corr. belongs to Rutaceae family. A middle sized slender aromatic armed plant. It is spread all over the India, from sub – Himalayan forest, Bengal, Central and SoutInern part of India and Burma8. This plant has anti-inflammatory, antipyretic and analgesic properties9, antithyroid, antioxidative and antihyperglycemic activity10, hypoglycemic and anti-hyperglycemic activity11, antihyperglycemic and antidyslipidemic12, analgesic activity13, acute and sub acute toxicity studies14, anti fertility15, hepatoprotective effect16, Insecticidal activity17, Immunomodulatory activity18 and protective effect19.
Significant antifilarial activity20 and oxidative status identified against microfilaia21. Current data has proved about polyphenols are the main molecules found in various flavonoids and alkaloids compounds, which might be worked as pro-oxidants22. In apoptosis, oxidative event is crucial for parasite death23.
Looking at these viewpoints, in the current investigation, evaluated antifilarial study of extracted fraction from Aegle marmelos Corr. leaves and screened the probable mechanism of the herbal compound to identify the probable relation of oxidative stress rationale in this study.
Materials and Methods
Procurement of plant materials
Plant leaves ofAegle marmelos Corr. were collected from the natural field of local areas of Bhopal. It is taxonomically identified by Botany Department, Safia Science College, Bhopal. The voucher specimen no. 418/Bot./Safia/2012 was given by the department.
Extraction
Aegle marmelos Corr. leaves were extracted in petroleum ether (60ºC – 80ºC), CHCl3 and Methanol respectively23, 24.
Fractionation
To know the number of phytochemical compounds present in chloroform extract, thin layer chromatography was performed by using pure Ethyl acetate as a mobile phase and detected with Anisaldehyde and Sulphuric acid spraying solution. Further, the extract was passed through column chromatography. It was performed by using pure Ethyl acetate as a mobile phase and the fraction was isolated23, 24, 26.
Parasite
Parasite Setaria cervi were collected from slaughtered buffalo. The Parasite was washed in 0.85% saline27.
In-vitro motility inhibition assay
Parasites were transferred to DMEM media with 0.01% Strepto-penicillin and 10% heat- fetal calf serum. Aegle marmelos leaves fraction concentration 0.002 – 0.08 mg/mL was used for testing. In each petri plate two parasites (Female & Male) were taken. Plates were incubated in a CO2 incubator (5%) at 37ºC for 24 hrs and after 2 to 24 hrs interval motility was observed. Each concentration was tested thrice28, 20.
MTT assay
MTT assay was used to check the activity of plant fraction against parasites by the methodology given by Strote G., 1998. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) is yellow coloured dye, reduced by certain cellular enzymes to the blue colour formazan product. Only female parasites have been taken for this assay. The worms were incubated in 0.5 mL PBS containing 0.25 mg/mL MTT upto 30 minutes. Further worms were incubated in DMSO for 1 hour in shaking conditions to extract formazan. OD was taken at 492 nm in ELISA reader. DMSO exposed female parasites was established as a positive control. At 56ºC heat killed negative control was taken and treated with MTT dye. Worms viability was estimated29 by the formula:-
% inhibition parameters = 100 – [(T – H) / (C – H)] ×100
Thus T, C, and H are O.D. values of formazan developed with test, control and heat killed parasites.
MDA estimation
To MDA standards (2.5 – 40 nM/mL), 0.5 ml of parasite culture supernatants of extract fraction and 20% TCA (2.5 mL) + 0.67% TBA (1 mL) were taken and vortex mixed. Mixtures heated in a hot water bath till 30 min. After cooling, chromogen was extracted in n – butanol and centrifuged machine at 3,000 rpm for 10 minutes for organic phase was separation. Absorbance was taken at 530 nm. Concentrations of MDA culture supernatant were calculated (nmol MDA/mL)30.
Protein Carbonylation Assay
For protein carbonylation estimation31 Culture supernatants were reacting with TCA (10%) to react with 10 mM (0.5 mL) of DNPH in 2M HCl for 1 hr at room temperature. Centrifuge the precipitated ice-cold 10% TCA, at 5000 rpm till 5 minutes and washed three times with ethylacetate – ethanol mixture. Washed pellets are dissolved in protein dissolving solution (1.5 ml) and incubated at 370C for 10 minutes. O.D. were taken at 370 nm against HCl (2M).
Nitric Oxide Assay
Nitric oxide levels were estimated in parasite culture supernatants32. Griess reagent (100μL) was added in culture supernatant (100 μL) wells of ELISA plats and incubated for10 min. Optical density was taken at 542 nm. Standard graph (0.005 to 0.08 µM/mL) was plotted and Nitric oxide levels in culture were calculated.
Statistical analysis
Analysis was carried out to compare the results of test and controls. For this student’s t test was used. P < 0.05 was measured as a significant value.
Result
Fraction isolation
Extract fraction was dried at reduced pressure.
In vitro motility inhibition assay
Extract fraction was tested against Setaria cervi for antifilarial activity. Concentrations 0.002 to 0.08 mg/mL inhibits the motility of parasite at 2 to 24hrs incubation respectively but all parasites were active in control (Table 1). The results exhibited that, concentrations of extract fraction, inhibits the motility very fast at concentration dependant manners.
Table 1: Antifilarial activity of fraction against filarial worm in vitro motility inhibition.
| Test concentration of compound (mg/mL) | Incubation time
(end point) in hrs |
Worm motility inhibition (Test) | Worm motility inhibition (Control) |
| 0.002
0.005 0.01 0.02 0.04 0.08 |
24
20 14 10 6 2 |
#
# # # # # |
†
† † † † † |
#Completely Immotile worm †Completely motile worms.
MTT – Formazan colorimetric assay
Antifilarial activity of extract fraction was confirmed by MTT assay. The formazan was extracted in DMSO. 0.327 value obtained for the heat – killed parasite because very less amount of formazan was produced in killed parasite. The, percentage inhibition .(>50%) was 58.9, 74.6 and 97.2% at 0.02, 0.04 and 0.08 mg/mL at 10, 6 and 2hrs incubation, considered significant activity of extract fraction (Table 2). Inhibitory concentration (50%) was calculated to be 0.015 mg/mL.
Table 2: Antifilarial activity of fraction against filarial worms in term of MTT assay.
| Sample | Incubation time
(In hrs.) |
Test concentrations
(mg/mL) |
Absorbance at 492 nm (mean ± SEM) | % reduction to solvent controlC, heat killedH & treated parasiteT | IC50
(mg/mL) |
| CControl
HHeat killed T Treated
|
24
0.5 24 20 14 10 6 2 |
–
– 0.002 0.005 0.01 0.02 0.04 0.08 |
0.996±0.008
0.327±0.023 0.898±0.004* 0.811±0.008* 0.723±0.006* 0.613±0.006* 0.497±0.005* 0.346±0.006* |
–
– 14.7 27.7 40.9 58.9 74.6 97.2 |
0.015 |
C Positive controls, HNegative controls, TTreated parasite with extract fraction.
*P values correspond to the levels of significance, P < 0.05 when compared to the mean value of O.D. observed for the formazan formed for treated and control parasites.
MDA Estimation
The lipid peroxidation was checked by measuring the MDA levels in parasite culture. It is carried out by TBA test which was modified by Satoh K in1978. The concentrations of MDA were calculated (Table- 3). The absorbance values were plotted on a standard graph The MDA levels for test 0.7, 1.1, 1.7, 2, 2.3 and 2.9 for control 0.2 nM/mL were calculated. MDA values were obtained as antifilarial activity obtained (Figure 1).
Protein carbonilation
The protein carbonilation content in parasite culture after 24 hrs expressed in nM/mg. Carbonyl content 0.09, 0.14, 0.23, 0.39, 0.51, 0.86 and 0.05 for control 0.4 nM/mg (Table- 3) were obtained. Carbonyl content calculated as antifilarial activity obtained (Figure 2).
Nitric oxide Assay
The Nitric oxide levels in culture supernatants were checked. The O.D. values were plotted on standard graph and the values of Nitric oxide levels were measured (Table- 3). It was represented in µM/mL. The Nitric oxide values 0.009, 0.015, 0.027, 0.054, 0.076 and 0.095, for control it was 0.006µM/mL were calculated. Nitric oxide levels were estimated as antifilarial activity obtained. (Figure 3).
Table 3: MDA, Carbonyl content and Nitric oxide levels estimated (nM/mL). Result expressed are Mean + SEM.
| Concentrations of extract fraction (mg/mL) | MDA level (nM/mL) | Carbonyl contents (nM/mg) | Nitric oxide level (µM/mL) |
| 0.002 | 0.7 + 0.005 | 0.09 + 0.012* | 0.009 + 0.001 |
| 0.005 | 1.1 + 0.152* | 0.14 + 0.019 | 0.015 + 0.001* |
| 0.01 | 1.7 + 0.173* | 0.23 + 0.045* | 0.027 + 0.004* |
| 0.02 | 2 + 0.152* | 0.39 + 0.017* | 0.054 + 0.002* |
| 0.04 | 2.3 + 0.115* | 0.51 + 0.035* | 0.076 + 0.001* |
| 0.08 | 2.9 + 0.205* | 0.86 + 0.049* | 0.095 + 0.001* |
| Control | 0.2 + 0.057 | 0.05 + 0.024 | 0.006 + 0.001 |
*P value represent the levels of significance, P < 0.05 considered significant as compared to control values.
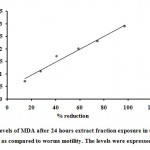 |
Figure 1: Levels of MDA after 24 hours extract fraction exposure in the in vitro experiments as compared to worms motility. The levels were expressed in nM/mL. |
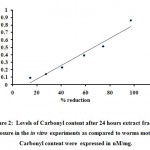 |
Figure 2: Levels of Carbonyl content after 24 hours extract fraction exposure in the in vitro experiments as compared to worms motility. Carbonyl content were expressed in nM/mg. |
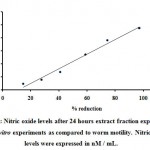 |
Figure 3: Nitric oxide levels after 24 hours extract fraction exposure in the in vitro experiments as compared to worm motility. Nitric oxide levels were expressed in nM / mL. |
Discussion and Conclusion
Due to the huge social and economic encumber of filariasis in the endemic countries, identification of potent therapeutic novel medicine is necessary, as per WHO direction. Herbal drug are being used by most part of global population in the developing countries. These drugs are safe, compatible and suitable for human being with lesser side effects33.
Aegle marmelos Corr. is a traditional medicinal plant used in many Ayurvedic drugs. Leaves are very useful in the treatment of filariasis. Taking three Bael leaves every day helps both in the prevention and cure of filariasis34. In the present investigation fraction isolated from Aegle marmelos Corr. leaves Chloroform extract, showed significant anti-filarial activity at their respective concentration also found a direct effect of this compound on the adult parasite in dose dependent manner. The oxidative parameters MDA, Carbonyl content and Nitric oxide content values were obtained increasingly as a reduction in parasite motility were found. The result observed for oxidative stress parameters indicate the close connection of oxidative / nitrosative basis in anti filarial activity. The considerable connection between each parameter and reduction in parasite motility at the concentration range indicate a primary effect of such oxidative damage in the parasite by extract fraction. In a similar study high anti filarial activity at less concentration revealed a considerable relationship with oxidative stress parameters in a respective drug content manner for filarial worms35, 21. The close association of Nitric oxide found in host defence and intracellular pathogens in various studies36, 37. The results of the present study showed the effect of plant fraction might be as a nitrosative or oxidative stress mediated mechanism.
In conclusion, extract fraction isolated from Aegle marmelos Corr. leaves has shown significant antifilarial activity against Setaria cervi parasite and probable mechanism identified as oxidative. Some active phytoceutical content, might be responsible for filaricidal activity. These findings indicate the significance of identification of active molecule present in Aegle marmelos Corr. leaves to formulate the cost effective potential anti filarial drug candidate to fight filariasis.
Acknowledgement
Authors are thankful to Dr. Zia-Ul Hasan, Department of Botany, Safia Science College, Bhopal (M.P.) for taxonomical identification of the plant species.
Conflict of interest
Author declares that, there is not conflict of interest.
Funding source
This work was supported by Indian Council of Medical Research (ICMR), Govt. of India, New Delhi.
References
- Liu L.X., Weller P.F. Drug therapy: Antiparasitic drugs. Engl. J. Med. 1996; 334: 1178-84.
CrossRef - WHO, The disease Filariasis: in TDR Eighth Programme Report, 1987; 63-3
- Das P.K., Ramaiah K.D., Augustin D.J., Kumar A. Towards elimination of lymphatic filariasis in India. Parasitol. 2001; 17: 457-60.
CrossRef - Ramaiah K.D., Kumar K.N., Ramu K., Pani S.P., Das P.K. Functional impairment caused by lymphatic filariasis in rural areas of south India. Med. Int. Health. 1997; 2(9): 832-38.
CrossRef - Sahare K.N., Singh V., Antifilarial Activity of Ethyl Acetate Extract of Vitex negundo Leaves In – vitro. Asian Peci. J. of Tropi. Medi. September 2013; 689-692.
CrossRef - Vanamail, P., Subramanian S., Das P.K., Pani S.P. Rajagopalan P.K. Estimation of fecundic life span of Wuchereria bancrofti from longitudinal study of human infection in an endemic area of Pondicherry (South India). Indian J. Med. 1990; 9: 293-97.
- Das P.K., Ramaiah K.D., Augustin D.J., Kumar A. Towards elimination of lymphatic filariasis in India. Trends in Parasito. 2001; 17: 457-460.
CrossRef - Joshi S.G.: Medicinal plant, 1st edn. Calcutta: Oxford and IBH Publishing Co. Pvt. Ltd. 2000: pg 188-91
- Arul V., Miyazaki S., Dhananjayan R. Studies on the anti-inflammatory, antipyretic and analgesic properties of the leaves of Aegle marmelos Corr. of Ethnopharmaco. 2005; 96: 159-63.
CrossRef - Panda S., Kar A. Evaluation of the antithyroid, antioxidative and antihyperglycemic activity of scopoletin from Aegle marmelos leaves in hyperthyroid rats. Res. 2006; 20 (12): 1103-05.
CrossRef - Narayan A., Kumar R., Kumar S. Hypoglycemic and anti-hyperglycemic activity of Aegle marmelos seed extract in normal and diabetic rats. of Ethno. 2006; 107: 374–79.
CrossRef - Narender T., Shweta S., Tiwari P., Reddy K.P., Khaliq T., Prathipati P. (2007). Antihyperglycemic and antidyslipidemic agent from Aegle marmelos. Bioorganic & Medicinal Chemistry Letters. 2007; 17: 1808-11.
CrossRef - Shankarananth V., Balakrishnan N., Suresh D., Sureshpandian G., Edwin E., Sheeja E. Analgesic activity of methanol extract of Aegle marmelos Biological Trace Element Research. 2007; 78: 258-59.
CrossRef - Veerappan A., Miyazaki S., Kadarkaraisamy M., Ranganathan D. Acute and subacute toxicity studies of Aegle marmelos an Indian medicinal plant Phytomedicine. International Journal of Phytotherapy and Phytopharmaco, 2007; 14(2-3): 209-15.
CrossRef - Chauhan A., Agarwal M., Kushwaha S., Mutreja A. Suppression of fertility in male albino rats following the administration of 50 % ethanolic extract of Aegle marmelos. Contraception. 2007; 76: 474-81.
CrossRef - Singanan V., Singanan M., Begum H. The hepatoprotective effect of bael leaves (Aegle marmelos) in alcohol induced liver injury in albino rats. J. of Scien. & Tech. 2007; 2(2): 83-2.
- Kumar R., Kumar A., Prasa, C.S. Dubey N.K. Insecticidal Activity Aegle marmelos (L ) Correa Essential Oil against Four Stored Grain Insect Pests. J. of Food Safety. 2008; 10: 39-9.
- Patel P., Mohammed S., Asdaq B. Immunomodulatory activity of methanolic fruit extract of Aegle marmelos in experimental animals. Saudi Pharmaceutical Journal. 2010; 18(3): 161-65.
CrossRef - Vanphawng Lalremruta, Gurunath S. Prasanna. Evaluation of protective effect of Aegle marmelos in an animal model of chronic fatigue syndrome, Indian J. Pharmaco. 2012; 44(3): 351–56.
CrossRef - Sahare K.N., Anandharaman V., Meshram V.G, Meshram S.U., Reddy M.V.R. and Tumane P.M. Anti microfilarial activity of methanolic extract of Vitex negundo and Aegle marmelos and their phytochemical analysis. Indian J. Exp. Biol. 2008; 46: 128-31.
- Sharma R.D., Veerpathran A.R., Dakshinamoorthy G., Sahare K.N., Goswami K., Reddy M.V.R. Possible implication of oxidative stress in anti filarial effect of certain traditionally used medicinal plants in vitro against malayi microfilariae. Pharmacogn. Res. 2010; 2(6): 350-54.
CrossRef - Fujisawa S., Atsumi T., Kadoma Y., Sakagami H. Anti-oxidant and pro-oxidant action of eugenol-related compounds and their Cytotoxicity. 2002; 177: 39-5.
CrossRef - Buttke T.M., Sandstrom P.A. Oxidative stress as mediator of apoptosis. Immunol Today. 1994; 1: 7-10.
CrossRef - Kokate C.K.: Practical Pharmacognosy, 4th edn. New Delhi: Vallabh Publication. 1993; 252-82
- Khandelwal K.R.: Textbook of Practical Pharmacognosy, 7th edn. Pune: Nirali publication. 2000; pp 152-01
- Angelo Talamon: Laboratory Chromatography Guide, 15th Switzerland: AG Büchi Labortechnik, CH 9230 Flawil.2005: pp 320-325.
- Comley J.C.W., Rees M.J., Turner C.H., Jenkins D.C. Colorimetric quantification of filarial viability. J. Parasit. 1989; 19: 77-3.
CrossRef - Murthy P.K. Evaluation of two in vitro systems employing Brugia malayi parasite for prescreening of potential antifilarials. Sci. 1999; 77(8): 1084-89.
- Strote G., Bonow I., Kromer M., Tatjana Rubio de, Kromer, Attah S., Opoku N. Chemotherapy for Onchocerciasis: results of in vitro experiments with promising new compounds. Med. Int. Health. 1998; 3: 397-07.
- Satoh K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Chem. Acta. 1978; 90: 37-3.
CrossRef - Chakraborty H., Ray S.N., Chakrabarti S. Lipid peroxidation associated protein damage in rat brain crude synaptosomal fraction mediated by iron and ascorbate. Neurochem Int. 2001; 39: 311-7.
CrossRef - Waitumbi J., Warburg A. Phlebotomus papatasi saliva inhibits protein phosphatase activity and Nitric oxide production by Murine macrophages. Immun. 1998; 66(4): 1534–37.
CrossRef - Martins Ekor. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Pharmacol.2013; 4: 177.
CrossRef - Bhagwandas Dr.: Ayurvedic remedies for common diseases, 7th edn. New Delhi: Hind Pocket Book. 2000; pp 36-7
- Rachna Sabharwal Mahaja, Anandharaman Veerpathran, Gajalakshmi Dakshinamoorthy, Richa Dwarkaprasad Sharma, Kalyan Goswami and Maryada Venkatarami Reddy. Effect of Certain Antibiotics Against Filarial Parasite Brugia malayi in vitro: Possible Role of Oxidative Stress. J. Clin. Biochem. 2010; 25(4): 362–66.
CrossRef - Rajan T.V., Porte P., Yates J.A, Keefer L., Shultz L.D., Role of nitric oxide in host defense against an extracellular metazoan parasite Brugia malayi. Infect. Immun. 1996; 64: 3351-53.
CrossRef - Thomas G.R, McCrossan M. Selkirk M.E. Cytostatic and cytotoxic effects of activated macrophages and nitric oxide donors on Brugia malayi. Infect. Immun., 1997; 65(7): 2732–39.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.






