How to Cite | Publication History | PlumX Article Matrix
Antimicrobial and Antioxidant Activity of Five Medicinal Plants Against Different Microbes
Amita Mittal1* , Manpreet1, Sunil1
, Manpreet1, Sunil1 and Geeta Dhania2
and Geeta Dhania2
1Department of Biotechnology, University Institute of Engineering and Technology, Kurukshetra University, Kurukshetra- 136119, India.
2Department of Environmental Sciences, Maharshi Dayanand University, Rohtak, India
Corresponding Author E-mail: agupta2015@kuk.ac.in
DOI : http://dx.doi.org/10.13005/bbra/2960
ABSTRACT:
The present study aims to assess the antimicrobial and antioxidant activity of selected medicinal plants (Achyranthes bidentata, Linum usitatissimum, Pedalium murex, Sphaeranthus indicus and, Terminalia bellirica) extracts against seven different microorganisms Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, Staphylococcus epidermidis, Staphylococcus hominis, and Streptococcus mutants. Leaf, root, and flower extracts of plants were prepared in different solvents like methanol, distilled water, dichloromethane, ethanol, ethyl acetate, chloroform, petroleum ether, propanol, benzene, and hexane. All the prepared extracts showed very good antimicrobial activity except distilled water extract. Most of the extracts were found to have antimicrobial potential against pathogens but Linum usitatissimum and T. bellirica leaf and seed extract prepared in methanol and chloroform solvents show a higher zone of inhibition against E. coli. Only Linum usitatissimum shows activity aganist Candida albicans. Minimum Inhibitory Concentration for Achyranthes bidentata extracts varied from 150µl/ml to 200µl/ml in different solvents. Antioxidant studies were carried out in methanolic extracts of all the plants. The maximum scavenging activity of methanolic leaf extracts was observed between 80 -100 μg/ml concentrations.
KEYWORDS: Antimicrobial Activity; Antioxidant Activity; Leaf extracts; Medicinal plants; Solvents
Download this article as:| Copy the following to cite this article: Mittal A, Manpreet M, Sunil S, Dhania G. Antimicrobial and Antioxidant Activity of Five Medicinal Plants Against Different Microbes. Biosci Biotech Res Asia 2021;18(4). |
| Copy the following to cite this URL: Mittal A, Manpreet M, Sunil S, Dhania G. Antimicrobial and Antioxidant Activity of Five Medicinal Plants Against Different Microbes. Biosci Biotech Res Asia 2021;18(4). Available from: https://bit.ly/3GEKTVw |
Introduction
The Medicinal plants are paving their way into the field of pharmaceuticals, nutraceuticals along cosmetics. Plants used in medicine have a huge range of constituents. These plants have been in use to treat a wide range of diseases for generations now. Their application is not just limited to curing common infectious illness but also play a significant part in healing some chronic diseases as well. Human-being have been using plants for treating common bacterial and fungus infections 1. These plants act as a rich source of micro-organic agents 2. Due to the presence of antimicrobial molecules in abundance, a wide range of herbal plant extracts are used to treat infections worldwide. In Ayurveda, some of these bioactive compounds are used as raw materials after in vitro and in vivo screening 3. Herbs have an upper-hand over synthetic drugs and also have very little or almost no side effects. Some of these benefits have grabbed the attention of experts and has turned it towards phytomedicines 4. However multi-drug resistance property of bacteria and pathogens have diverted researchers and pharmacists into thinking towards the practical application of these restorative plants due to their antimicrobial potential 5. Furthermore these herbs have been a wellspring of bioactive mixtures of pharmacological significance for individuals 6-9. Therefore, scientific testing is important for determining the adequacy of plants and traditional medicines. The biological assays (antimicrobial, antioxidant, and anti-inflammatory activities) related to skin and other diseases and their safety assessment have been affirmed 10. Numerous reports recorded on the natural exercises, phytochemistry, and security of numerous therapeutic plants being utilized in South Africa ordinary medication against skin conditions have not been used to its full extent 11. Recently many researches have been done on numerous botanicals used by Batswana conventional experts for treating skin-related infections in the Ngaka Modiri Molema District Municipality, North West Province, South Africa 12.
Materials and Methodology
Collection of plant material
Five disease-free randomly selected medicinal plants were collected from the Tau Devi Lal Herbal Park near Khizrabad highway at Churpur in district Yamunanagar, Haryana, India. These plants were Achyranthes bidentata, Linum usitatissimum, Pedalium murex, Sphaeranthus indicus, and Terminalia billerica. Leaves roots and flowers of selected plants were shade dried and powdered in a mixer grinder.
Preparation of the plant extracts
The powder was submerged in various organic solvents such as methanol, chloroform, dichloromethane, distilled water, benzene, n-hexane, petroleum ether, and ethanol in the ratio of 1:10 (20gm in 200ml solvent) for 72 hours at room temperature. The extracts were filtered using Whatman filter paper no.1 after the incubation period of 72 hours and then total evaporation of the solvent was observed in the water bath at the boiling temperature of the respective solvent.
Study of the antimicrobial activity
Culture collection
Microbial cultures of pathogens were obtained from the Institute of Microbiology and Technology (IMTECH) Chandigarh. Candida albicans (MTCC NO. 3017), Escherichia coli (43), Pseudomonas aeruginosa (2295), Staphylococcus aureus (3160), Staphylococcus epidermidis (9041), Staphylococcus hominins (4435), and Streptococcus mutans (1943) were used in this study. The highly pure cultures of bacteria were transferred and maintained on nutrient agar plates for better vegetative growth.
Preparation of extract dilutions
A small quantity (5 g of leaf/ flower/root) of powder was weighed out. It was soaked in 20 ml of solvents for 3 days, filtered in the new flask and the residue was discarded. The filtrate was then put into a water bath at 45-50ºC, after which all the solvent was evaporated from the filtrate. Then solvent got extracted, and DMSO was used to dissolve residual powder and left it in the refrigerator at 4oC for further analysis. Agar well diffusion method was used for the evaluation of antimicrobial potential on Mueller Hinton Agar medium [13]. The antimicrobial potency of the extract was assessed in the form of the diameter of inhibition zones (mm). The mean value of inhibition zones of the triplicates was taken as the final result.
The Minimum inhibitory concentration of plants crude extract
The Minimum inhibitory concentration (MIC) of plant crude extracts in various solvents was carried out using the standard MIC analysis method.
Antioxidant activity of selected plants extracts
DPPH radical scavenging activity
The IC50 value was determined. DPPH assays done in all the plant samples in methanolic extracts and ascorbic acid were taken and used as standard. The antioxidant activity of the extracts was measured. A similar amount of DPPH and methanol was used as control and methanol was taken as blank. Different concentrations of the ascorbic acid standard (0.1 ml) (10 to 100ug/ml) were prepared and 3ml of DPPH was added in whole concentration. The test solution was also prepared in different concentrations (10 to 100ug/ml). Both standard and samples were incubated in dark conditions for half-hour. Absorbance was recorded at 517nm using a spectrophotometer. From this absorbance, the percentage of inhibition activity was calculated. The IC50 value of extract was expressed as the free radical scavenging activity. This IC50 defines the inhibition of DPPH radical by 50% through different concentrations of the plant extract.
% Scavenging activity = [(A0–A1)/A0] ×100
A0 = Absorbance of control A1= Absorbance in the presence of the extract
Results and Discussion
The antimicrobial activity of plant extracts against microbes
A.bidentata root extract showed the maximum antimicrobial activity within dichloromethane and chloroform extracts. The chloroform extract of roots showed maximum inhibition zone diameters (22mm) against S. mutans while there was no activity against the S. hominis and C. albicans [Table 1]. The maximum zone of inhibition of Achyranthes bidentata methanol extract was 21. 2mm, when tested against S. aureus, observed in some other researches 14.
Table 1: Zone of inhibition diameter (in mm) of A. bidentata root extract in different solvent extracts against various pathogenic strains.
| SOLVENTS | MICROBES | ||||||
| E.coli | P.aeruginosa | S.mutans | S.aureus | S.epidermidis | S.hominis | C.albicans | |
| Methanol | 13 | 15 | 22 | 14 | 15 | – | – |
| Dichloromethane | 12 | 16 | 21 | 13 | 16 | 17 | – |
| Distilled water | – | – | – | – | – | – | – |
| Ethanol | 13 | 16 | 21 | 12 | 14 | 16 | – |
| Ethyl acetate | 13 | 15 | – | 14 | 14 | 12 | – |
| Petroleum ether | 11 | – | 20 | 14 | 15 | – | – |
| Propanol | 14 | 12 | 22 | 18 | 16 | – | – |
| Chloroform | 14 | 13 | 25 | 21 | 16 | – | – |
| Benzene | 11 | 19 | 19 | 19 | 14 | – | – |
| Hexane | 13 | 11 | 22 | 16 | 15 | – | – |
L. usitatissimum flower extracts contain antimicrobial activity in all the solvents except distilled water. The activity was better in chloroform extract of leaves against strains S. hominis, Escherichia coli, and Pseudomonas aeruginosa, and no activity was seen against S. mutans and C. albicans. Maximum inhibition zone diameters of 26 mm against S. hominis were recorded while no activity was observed against S. mutans and C. albicans [Table 2].
Table 2: Inhibitory zone diameters (in mm) of L. usitatissimum of flower extract in different solvents against various microbes.
| SOLVENTS | MICROBES | ||||||
| E.coli | P.aeruginosa | S.
mutans |
S.
aurues |
S.
epidermidis |
S.
hominis |
C.
albicans |
|
| Methanol | 26 | 23 | 24 | 20 | – | 21 | 20 |
| Dichloromethane | 18 | 19 | 20 | 16 | 20 | 12 | 18 |
| Distilled water | – | – | – | – | – | – | – |
| Ethanol | 24 | 23 | 16 | 18 | 19 | 14 | 12 |
| Ethyl acetate | 19 | 18 | 17 | 16 | 15 | 11 | 14 |
| Petroleum ether | 23 | 18 | 13 | 20 | 21 | 20 | 13 |
| Propanol | 20 | 19 | 11 | 21 | 16 | 18 | 14 |
| Chloroform | 28 | 26 | 24 | 23 | 18 | 16 | 12 |
| Benzene | 10 | 12 | 15 | 14 | 11 | 14 | 11 |
| Hexane | 19 | 14 | 13 | 13 | 12 | 12 | 12 |
Different zone of inhibition was observed in same plants in different studies which revealed that L. usitatissimum plant shows significant activity against S. aureus and least activity against K. pneumonia. It was observed that the plant extract showed antimicrobial activity against gram-positive bacteria and less activity against gram-negative bacteria 15, whereas the present study result indicates the activity of ethanol and chloroform extract against both gram-positive and gram-negative bacteria 16-18.
murex leaf exhibited maximum antimicrobial activity in methanol and chloroform solvents. The extract showed antimicrobial activity against almost all pathogenic microbes while P. murex did not affect the growth of S. hominis, S. mutans, and C. albicans [Table 3]. According to previous research the Pedalium murex, ethyl acetate and petroleum ether extracts showed significant results against Staphylococcus aureus, Escherichia coli, Salmonella typhi 19
Table 3: inhibition zone diameter (in mm) of P. murex leaf crude extract in different solvents against various pathogenic strains.
| SOLVENTS | MICROBES | ||||||
| E.coli | P.aeruginosa | S.mutans | S.aureus | S.epidermids | S.hominis | C.albicans | |
| Methanol | 16 | 16 | – | 14 | 16 | – | – |
| Dichloromethane | 12 | 13 | – | 11 | 11 | – | – |
| Distilled water | – | – | – | – | 12 | – | – |
| Ethanol | 15 | 12 | – | – | 12 | – | – |
| Ethyl acetate | 12 | – | – | 12 | 12 | – | – |
| Petroleum ether | 13 | – | – | – | 13 | – | – |
| Propanol | – | 13 | – | 13 | 13 | – | 14 |
| Chloroform | 15 | 12 | – | 15 | 16 | – | – |
| Benzene | 12 | 12 | – | 12 | 14 | – | – |
| Hexane | 13 | – | – | 13 | 12 | – | – |
S. indicus leaf extract in methanol showed major inhibition zone against P. aeruginosa (26 mm). The dichloromethane and aqueous extracts were found less active against S. hominis, S. mutans, and C. albicans [Table 4]. Whereas some studies evaluated that the pathogenic bacterial growth was inhibited by S. indicus hexane extract of the stem. The extract prepared from methanol showed higher activity against S. aureus(25.67mm) but showed least activity against P. aeruginosa (9.83) which is opposite to our results.20 Some past researches also reveals that the root extract inhibits a smaller number of bacterial isolates 21
Table 4: Inhibition zone diameters (in mm) of S. indicus leaf crude extract in different solvents against various pathogenic strains.
| SOLVENTS | MICROBES | ||||||
| E.coli | P.aeruginosa | S.mutans | S.aureus | S.epidermids | S.hominis | C.albicans | |
| Methanol | 19 | 21 | 20 | 24 | 26 | 24 | – |
| Dichloromethane | 14 | 13 | – | 11 | 23 | – | – |
| Distilled water | – | – | – | – | – | – | – |
| Ethanol | 24 | 23 | – | 23 | 20 | 23 | – |
| Ethyl acetate | 18 | 16 | – | 19 | 18 | – | – |
| Petroleum ether | 16 | 14 | – | 18 | 26 | – | – |
| Propanol | 15 | 13 | – | 16 | 21` | – | – |
| Chloroform | 20 | 23 | – | 23 | 24 | – | – |
| Benzene | 14 | 16 | – | – | 16 | – | – |
| Hexane | 12 | 14 | – | – | 19 | – | – |
T. billerica seed methanol extract contains maximum antimicrobial activity against all the pathogens except S. mutans and C. albicans. Their methanol extract showed a very high activity, observed against P. aeruginosa (28mm). These extracts prepared in various solvents showed a maximum inhibition zone against P.aeruginosa E. coli, S. aureus, and S. epidermidis as shown in [Table 5]. While work done by researchers revealed that the ethyl acetate extracts of this plant show significant activity towards microorganisms and aqueous extract of Terminalia bellirica maximum zone of inhibition of 6mm were observed which is very lowest as compared to our study 22.
Table 5: Inhibitory zone diameter (in mm) of T. bellirica seed crude extract in different solvents against different pathogenic strains.
| SOLVENTS | MICROBES | ||||||
| E.coli | P.aeruginosa | S.mutans | S.aureus | S.epidermidis | S.hominis | C.albicans | |
| Methanol | 25 | 28 | – | 24 | 23 | 20 | – |
| Dichloromethane | 20 | 23 | – | 20 | 18 | 19 | – |
| Distilled water | – | – | – | – | – | – | – |
| Ethanol | 23 | 24 | – | 16 | 26 | 12 | – |
| Ethyl acetate | 19 | 12 | – | 11 | 12 | 16 | – |
| Petroleum ether | 18 | 16 | – | 18 | 12 | 14 | – |
| Propanol | 16 | 14 | – | 17 | 14 | 13 | – |
| Chloroform | 24 | 23 | – | 20 | 21 | 23 | – |
| Benzene | 12 | 12 | – | 18 | 19 | – | – |
| Hexane | 12 | 11 | – | 16 | 15 | – | – |
The methanolic and chloroform extract of S. indicus leaves showed multiple resistance towards P. aeruginosa, S. aureus, and S. epidermidis while it exhibited no activity against C. albicans. The extract of L. usitatissimum showed the maximum antimicrobial activity against S. aureus, E. coli, S. hominis and, P. aeruginosa while no activity was observed against S. epidermidis, Streptococcus mutans, and C. albicans. DPPH (1,1-diphenyl, -2- picryldihydrazyl) radical scavenging activity of plant extract is shown in [Fig:1]. The IC50 value of all plants A. bidentata, L. usitatissimun, P.murex, S. indicus and, T. billerica was found to be 57ug/ml, 16.26ug/ml, 78.24ug/ml, 75.67ug/ml, 94.67ug/ml respectively whereas IC50 value of ascorbic acid is 38.28ug/ml. All medicinal plant crude extracts give outstanding antioxidant activities over DPPH radical as shown in [Table 6].
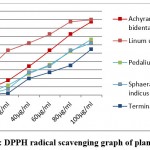 |
Figure 1: DPPH radical scavenging graph of plant extract. |
Table 6: IC 50 Value of different plants against DPPH radicals.
| IC50 value Mean+SD | ||||
| A.bidentata | L. usitatissimum | P.murex | S. indicus | T. bellirica |
| 57±2.5 | 16.26±3.0 | 78.24±1.6 | 75.67±2.3 | 94.67±0.6 |
This outcome confirms that the crude extracts of the plants (Achyranthes bidentata, Linum usitatissimum, Pedalium murex, Sphaeranthus indicus, and Terminalia billerica) used in the study are the proficient and best solution for hinder the development of dental caries microbes with its overflowing wellspring of auxiliary metabolites. This examination leads to the improvement of better treatment with natural herbs-based medicine to conquer the incidental effects brought about by anti-toxins/antibiotics. The outcome shows the strength of methanol and chloroform crude extracts of plants as antimicrobial agents. The current investigation gives a qualitative ratio of some potential plant species used for antimicrobial activity. For future preclinical and clinical investigations, the fundamental information on the harmfulness profile of ethanol and aqueous extract of this therapeutically significant plant was gathered from past examinations and results which is valuable in clinical work for treating microbial illness. Through this study, it was seen that in-vitro antimicrobial profiling of methanol extract of roots of Achyranthes bidentata was discovered to be very effective against the bacterial strains, Staphylococcus aureus, and Streptococcus mutants. A subjective phytochemical examination was done for the identification of carbohydrates, saponins, flavonoids, alkaloids, tannins, reducing sugars, steroids, gums. The photochemical analysis of alkaloids, flavonoids, tannins shows antimicrobial movement and saponin might be credited against tainting specialists where flavonoids showed antimicrobial action by complexing with the cell wall and also binds to adhesion. The current investigation was done to investigate the antibacterial adequacy of five therapeutic plants against a few bacterial strains which could be additionally utilized for characterization of the novel phytochemicals in the treatment of contagious diseases, particularly which works against drug-resistant microorganisms and lead to the development of more effective antimicrobial compounds 23. Therapeutic plants have been known for synthesis of active metabolites with established potential antimicrobial and antibacterial activities, which undoubtedly have framed the reason for their applications in drugs, alternative medicines, and normal treatments 24. Achyranthes bidentata showed a maximum zone of inhibition and for two pathogenic strains. Because of solvents’ polarity that confirms the type of reaction and solubility of compounds, the zonation differences are produced in each extract. Most all distillates have a better capacity which may be allocated to the ability to extract the natural antimicrobial chemicals such as flavonoids, alkaloids, terpenoids, and phenolic compounds 25. Thus, the examination guarantees the plants’ worth utilized in Ayurveda, which could be very significant for the improvement of new medications.
Conclusion
The result of the present study has addressed several new ways and different research problems in the present scenario. All the plant showed the specific antimicrobial activity against the pathogen used in the different solvents. Among the solvents used methanol and ethanol extracts showed the highest activity with regard to the inhibition of microbial growth while distilled water shows the minimum antimicrobial activity against different plant extracts. Very large inhibition zone is shown by A. bidentata, P. murex, Terminalia bellirica rest shows the satisfactory activity in various solvents against different pathogens. All plant exhibited antimicrobial activity but the highest activity is observed in the. L. usitatissimum. The purified phytonutrients fractions can be further used to isolate the lead compound from the fractions and then 3D examination studies of structure can be done in the future. So that active ingredients can be further subjected to clinical research to provide a new drug. Some more studies and researches may be done on these plants to identify and isolate their bioactive compound causing antimicrobial and antioxidant activity. More studies need to be conducted using in vivo/in vitro models to find the precise molecular mechanisms and targets for cell growth inhibition which will allow the rationale design for more effective chemicals for the eventual chemo preventive and/or therapeutic agent.
Acknowledgement
The authors are grateful to Honorable Vice-Chancellor, Kurukshetra University, Kurukshetra for providing necessary infrastructural facilities to carry out the research work.
Conflict of Interest
The authors declare that there is no conflict of interest.
Funding Sources
The authors declare that there is no funding source.
References
- Ernest D and Kuppan E. A review on the antimicrobial efficacy of some traditional medicinal plants in Tamilnadu. Journal of Acute Disease., 2013; 2:99-105.
CrossRef - Jindal A and Vashist H. Antimicrobial Activities of Medicinal Plants-Review. International Journal of Research in Pharmaceutical and Biomedical Sciences., 2013; 3(1): 222- 230.
- Renisheya J, Johnson M, Mary U. M, Arthy. A. Antibacterial activities of ethanolic extracts of selected medicinal plants against the human pathogen. Asian Pac J Trop Biomed., 2011; 1: S76-S78.
CrossRef - B, Fozia, Abdul. W, Ali. R, Rehman A, et al. Antimicrobial activity of Malva neglecta and Nasturtium microphyllum. Int. J. Res., Ayurveda Pharm., 2011; 3: 808-810.
CrossRef - Dangi A.S, Sharma M, Aparna, Yadav J.P, Arora D.R, et al. Antimicrobial activity of some medicinal plants. International Journal of Environmental Sciences., 2012;3: 904-909.
- Otang W. M, Grierson D. S and Ndip R. N. Phytochemical Studies and Antioxidant Activity of Two South African Medicinal Plants Traditionally Used for the Management of Opportunistic Fungal Infections in HIV/AIDS Patients. BMC Complement. Altern. Med., 2012; 12:43. [CrossRef].
CrossRef - Sharma Sk, Singh L, and Singh S. A Review on Medicinal Plants Having Antioxidant Potentia. Ind. J. Res. Phar. Biotechnol., 2013; 1: 404–409.
- Nigamm V and Sodhi J. S. Some Medicinal Plants with Antioxidant Activity—A Review. Int. J. Pharm. Biol. Sci., 2014; 4: 73–178.
- AG, Waltenberger. B, Lindear. T et al. Discovery and Resupply of Pharmacologically Active Plant-Derived Natural Products- A Review. Biotechnol. Adv., 2012; 33: 1582–1614. [CrossRef] [PubMed].
- N and Kishore. N. Are Plants Used for Skin Care in South Africa Fully Explored. J.Ethnopharmacol., 2014; 153,6184.[CrossRef] [PubMed] CrossRef
- U, Van Vuuren. S and Viljoen.A. Southern African Medicinal Plants Used to Treat Skin Diseases. S.Afr.J.Bot., 87: 175-193,2013. [CrossRef].
CrossRef - J.A, Ndhlovu. P. T, Khosana. N. S. et al. Medicinal Plants Used for Skin-Related Diseases among the Batswanas in Ngaka Modiri Molema District Municipality, South Africa. South Africa journal of Botony., 2019; Vol 126.
CrossRef - A, Grein. E, Antibacterial activity of plant extracts used externally in traditional medicine J Ethnopharmacol., 1994; 44(1):35-40.
CrossRef - S, Sharif. M, Rahman. M.M, et al. Potential antimicrobial activity of Achyranthes bidentata methanol extract against both gram (+) ve and gram (-ve) bacteria.Pharmacologyonline., 2018; 3:223-228.
- F D.S and Farnsworth. N.R. The value of plants used in traditional medicine for drug discovery. Environmental Health Perspective., 2001; 09: 69-75.
CrossRef - Thomas Natural ways to health, in Alternative medicine. An Illustrated encyclopedia of Natural Healing. Times-Life Books, Alexandria, VA., 1977; 432-456.
- M, Sadiki. M, Ibnsouda.S.K. Methods for in vitro evaluating antimicrobial activity. A review. Journal of Pharmaceutical Analysis, 2016; 6: 71–79
CrossRef - M, Iqbala. M. J, Hassanb. W et al. Evaluation of methanolic crude extract of Linum usitatissimum for the removal of biofilm in diabetic foot isolates Brazilian Journal of Biology, 2021; 83: e245807
CrossRef - Fadzir, U.A, Darnis, D.S, Mustafa. B.E. and Mokhtar. K.I. Linum usitatissimum as an antimicrobial agent and a potential natural healer: a review. Archives of Orofacial Science, 2018; 13[2]: 55-62.
- Gaafar, A.A., Salama, Z.A., Askar, M.S., EL-Hariri, D.M. and Bakry, B.A. In vitro antioxidant and antimicrobial activities of Lignan flax seed extract (Linum usitatissimum L.). International Journal of Pharmaceutical Sciences Review and Research,2013; 23 [2]; 291-297
- M 2018. Evaluation of antimicrobial activity of linseed (Linum usitatisimum) on different types of bacteria. Iraq: University of Basrah.
- Kaleeswaran .B and Ramadevi .S. Phytochemical analysis and pathogenic inhibition activity of Pedalium murex against urinary tract infection bacteria. International journal of current research,2016; 8[9]: 38546-38551
- D and Gupta. A.K. Comparative assessment of antimicrobial and antioxidant activity between whole plant and parts of Sphaeranthus indicus Linn. (Asteraceae). Clinical Phytoscience,2020;6:23.
CrossRef - Krishna MT, Thota SP, Jadhav M, Kumar K, Venuganti A, Devi M, Vadiari S, Mittapelli G. Studies on in vitro antioxidant and antimicrobial activities of Sphaeranthus indicus (Linn). Int J Pharmaceutical Res Biomedical Analysis 2013;2:1–9.
- Sharma A.R, Verma and Padmini, Ramteke. Antibacterial Activity of Some Medicinal Plants Used by Tribals against UTI Causing Pathogens. World Appl Sci J, 2009 7 (3): 332-339.

This work is licensed under a Creative Commons Attribution 4.0 International License.





