How to Cite | Publication History | PlumX Article Matrix
Faruk Arodiya , Chirag Makvana
, Chirag Makvana and Kokila Parmar*
and Kokila Parmar*
Department of Chemistry, Hemchandracharya North Gujarat University, Patan, Gujarat, India.
Corresponding Author E-mail: drkap_chem@yahoo.com
DOI : http://dx.doi.org/10.13005/bbra/2952
ABSTRACT:
Generally, synthesis and encapsulation process improve therapeutic value of nano encapsulated drugs. The silver nanoparticles (AgNPs) biosynthesized from Ziziphus nummularia leaves and encapsulated with polyvinyl pyrrolidone (PVP) polymer as antibacterial agents, due to its high bioavailability, better encapsulation and less toxic properties. The nanoparticles (AgNPs) biosynthesized from Ziziphus nummularia leaves and capped with polyvinyl pyrrolidone (PVP) polymer, The acquired AgNPs and polymeric functionalized AgNPs were fully characterised by the UV- Visible spectroscopy , Transmission electron microscopy (TEM), X-Ray diffraction pattern (XRD) and Fourier transform infrared spectroscopy (FTIR).The crystalline Ag NPs and Polymer Functionalized AgNPs have a face-centered cubic structure with an average size of 9.20 nm, according to X-ray Diffraction spectroscopy. Fourier Transform Infrared spectroscopy revealed that biomolecules such as proteins are incapable of reducing metal ions and the formation of an encapsulating layer in terms of metal ions. High-Resolution transmission electron microscopy revealed that Polymer functionalized AgNPs ranged in size of 10 nm. AgNPs and Polymer functionalized AgNPs showed effective antimicrobial and antioxidant activity. The biosynthesized monodisperse silver nanoparticles and encapsulated silver nanoparticles demonstrated better antimicrobial and antioxidant activity which can be used in various biomedical applications.
KEYWORDS: Antibacterial and Antioxidant; Characterization; Green synthesis; Polyvinyl pyrrolidone (PVP); Ziziphus nummularia
Download this article as:| Copy the following to cite this article: Arodiya F, Makvana C, Parmar K. Polymer Capped Silver Nanoparticles from Ziziphus Nummularia Leaves Extract: Potent Antibacterial and Antioxidant Activity. Biosci Biotech Res Asia 2021;18(4). |
| Copy the following to cite this URL: Arodiya F, Makvana C, Parmar K. Polymer Capped Silver Nanoparticles from Ziziphus Nummularia Leaves Extract: Potent Antibacterial and Antioxidant Activity. Biosci Biotech Res Asia 2021;18(4). Available from: https://bit.ly/3pyb6zq |
Introduction
Silver nanoparticles are among one of the most extensively studied nanomaterials. Which metal ions have been used for the treatment of various diseases and biomedical applications [1]. Silver and Gold were used in the form of “Bhasma (Swarna and Rajat) for asthma, anemia, chronic fever, cough, sleeplessness, muscle weakness, and weak digestion[2,3]. Concern for environmental issues led to the development of eco-friendly methods in chemistry and chemical technology[4]. Silver nanoparticles are highly commercialised materials [5]. As a result, a biological technique for the manufacture of silver nanoparticles that is simple, cheap, and ecofriendly is needed [6]. Recent advances in chemistry methodologies for the manufacture of silver nanoparticles have demonstrated their potential in all biomedical applications. Silver nanoparticle production from biosources has always been an attractive task for researchers due to its numerous applications. For the manufacture of silver nanoparticles, some scientists have used plant sources (extract of leaves, roots, flowers, seeds, stems, and fruits) and microbiological sources (such as bacteria, fungus, and their culture media [7].The reduction and stability of nanoparticles have been linked to a number of medicinally important biomolecules, including alkaloids, proteins, phenols, saponins, tannins, enzymes, and terpenoids[8].Diseases such as cancer, bacterial infections, cardiovascular disease, and neurological disease can be detected and treated using metal nanoparticles [9]. There are numerous uses for Phyto nanoparticles, including antibacterial, anticancer, image contrast agents, fluorescence probes, and drug delivery systems due to their superior biocompatibility as well as their medical potential[10].Various analytes related to agriculture, diagnostics and environmental sector used sensors which are developed by the phytosynthesized silver nanoparticles[11]. An extract of Ziziphus nummularia has been found to contain the DPPH radical. This is important. Using plants to synthesise AgNPs is based on the fact that the process is faster, less hazardous to the environment, more efficient, and cost-effective than traditional approaches. The formation of silver nanoparticles and also functionalized with polymers to further enhance their biocompatibility for the desired application. Many plants have been recently used in the synthesis of AgNPs, such as Crateva Religiosa[12], Bauhinia Variegata[13], Cleistanthus collinus[14], Morinda citrifolia[15], Iris germanica[16], Ceropegia thwaitesii[17], Sauropus androgynous[18], Rhizophora stylosa[19], Ganoderma lucidum[20]etc. There are so many medicinal plants used to synthesize metal nanoparticles [21-24]. Ziziphus nummularia possesses various pharmacological activities like antioxidant, analgesic and anti-inflammatory, antinociceptive, antipyretic activity. The leaves are used to treat coughs, colds, and typhoid, as well as to heal cuts and skin illness [25].Significantly, DPPH radical scavenging activity in Ziziphus nummularia extracts has been demonstrated [26]. In consideration of these findings, we studied the green production of polymer functionalized AgNPs from Ziziphus nummularia leaves aqueous extract and examined their antibacterial and antioxidant properties.
Material and Method
Material
Salt of AgNO3 was received from Sigma Aldrich, fresh leaf of Ziziphus nummularia was collected from farm of North Gujarat region. Bacterial culture was purchased from MTCC Chandigarh. DPPH was purchased from ACS, sigma Aldrich product of polyvinyl pyrrolidone (PVP mw 40,000) in its purity was procured commercially and used without further purification.
Plant extract preparation
Fresh Ziziphus nummularia leaves were thrice cleaned in double-distilled water to remove any impurities. The leaves were rinsed, dried, and then finely cut into fine pieces. 10 gram of plant material (fine pieces) was cooked in 100 ml of double-distilled water for 10 minutes at 40-500C and then cooled. The obtained extract was filtered by Whatman filter paper no.1 and kept in a freezer at 4°C for later use in the synthesis.
Green synthesis of silver nanoparticles
90 ml of 1mM AgNO3 solution was added to 10 ml leaf extract and this reaction mixture was placed on a hot plate at 600C with constant stirring with a magnetic stirrer for 2 hours. Silver nanoparticle generation was preliminarily confirmed by a change in colour from yellowish to dark brown at the beginning of the reaction. The reduction of Ag+1 to Ag0 in the reaction mixture was due to the biomolecules present in the plant which acts as a reducing agent. UV visible analysis was used to demonstrate the production of silver nanoparticles. For isolation of AgNPs from the reaction mixture centrifugation process is used. The reaction mixture was centrifuged at 10,000 rpm for 20 minutes. In the bottom of the centrifuge tube, nanoparticles were found, which had been twice purified with double distilled water, then collected and dried at 70-750C in an oven for 2 hrs. Dry crystalline powder of AgNPs was kept in an airtight bottle for biological activity and characterization (FT-IR, XRD, and HR-TEM).
Preparation of PVP formulated silver nanoparticles
0.2 gm PVP were dissolved in 100 ml of distilled water and stirred for 1 hr at 800C. Subsequently, the solution was progressively added to the homogenous solution of AgNPs that had been generated by leaf extraction. Final confirmation of formation of PVP functionalized silver nanoparticles was studied by UV visible Analysis. Using centrifugation, silver nanoparticles encapsulated with PVP were isolated. For 15 minutes, the reaction mixture was rotated at 6000 rpm. Nanoparticles were observed purified twice by double distilled water then collected and dried at 80-85 0C.
 |
Figure 1: Colour change from yellowish to dark brown [A] Initial Time, [B] After 2 hr and [C]After adding PVP Ag NPs. |
Characterization of Green synthesized Silver nanoparticles
A UV-visible spectrophotometer was used to measure the absorption spectra of produced AgNPs in the range of 200 nm to 800 nm (Shimadzu UV-1800 UV-visible spectrophotometer). The shape and size of the generated AgNPs were determined using high-resolution transmission electron microscopy (HR-TEM). Nanoparticles were investigated using Fourier transform Infrared spectroscopy (FTIR) to determine their surface chemistry. The Rigaku D/max 40 kV diffractometer equipped with the graphite chromator was used to conduct an XRD investigation to purify crystalline structure with an average particle size.
Antibacterial activity of silver nanoparticles
Antibacterial activity of synthesized AgNPs and polymer functionalized AgNPs was carried out by Harsh Mistry et.al.(2020) with some modifications27. All of the test bacterial strains were cultured in nutrient broth overnight at 37°C and adjusted to 0.5 according to McFarland standards. 100 μL of gram-positive (Staphylococcus aureus) and gram-negative (Escherichia coli) strains were dispersed on each nutrient agar plate under sterile conditions. Using a cork borer, a 10 mm diameter well was punched in the agar plate, and the synthesised AgNPs, polymer functionalized AgNPs, and AgNO3 were injected in each well. Plates were incubated for 24 hours at 37°C, and the inhibitory zone’s diameter was measured using a zone scale to determine bacterial activity (HiMedia).
Antioxidant activity by 2,2-diphenyl -1-picrylhydrazyl (DPPH) method
Antioxidant capacity of synthesized AgNPs and polymer capped AgNPs was performed according to Harsh et.al. 27 with slight modification. Ascorbic acid was taken as standard due to its high antioxidant properties. The radical scavenging activity of AgNPs, polymer functionalized AgNPs, and vitamin C was determined using the DPPH. 1 mL of various concentration(10,50,80,100 μg/ml) AgNPs and PVP functionalized AgNPs and standard ascorbic acid solution were mixed separately with 1ml of DPPH solution and incubated for 30 min. The absorbance was measured by UV- Visible Spectrophotometer at 517nm. The free radicals scavenging activity was calculated as a percentage of inhibition. The following formula is used to calculate.
% of scavenging = [(Pcontrolled-Psample)/Pcontrolled] × 100
Where Pcontrolled is the absorbance of the control and Psample is the absorption of AgNPs/polymer capped AgNPs/vitamin C.
Result and Discussion
UV-visible spectroscopic analysis.
A UV-visible spectrophotometer was used to confirm the creation of AgNPs. The reduction of Ag+1 to Ag0, as indicated by a colour change from light brownish to dark brownish, was due to the excitation surface plasmon resonance (SPR) of AgNPs 28.The observed results are substantially similar with those of a recent research 29.The successful formation of AgNPs and polymer functionalized AgNPs absorbance peak at 431 and 443 nm show in figure 2 30. This result is consistent with earlier research 31–33. According to studies, the SPR of the majority of metallic compounds is shape and size dependent 34-36.
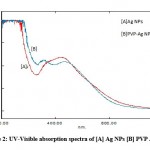 |
Figure 2: UV-Visible absorption spectra of [A] Ag NPs [B] PVP Ag NPs. |
Fourier transform infrared spectroscopy (FTIR) analysis
The FTIR spectrum of synthesized AgNPs is shown in figure 3. This result in absorption peaks positioned between the area about 4000 cm-1 and 500 cm-1. FTIR spectrum of plant extract and AgNPs displays peak at 3300, 3050, 2890,1597,1350,1050 cm-1 for silver nanoparticles. The obtained results are congenial with the previous report 29. The AgNPs show several peak at 3200,613, 532cm-1 region. The peak at 1421 cm-1is common to both the extract and AgNPs and is characteristic of the C-H bending vibration. Vibration stretching at 3300 cm-1 peak correspond to O-H stretching of water and phenolic compounds. The peak at 1350 and 1050 cm-1 is evidence of the C-H stretching for respective amines. The existence of a peak at 420 cm-1 shows metal oxide bonding. The peak at 1640 cm-1 corresponds to metal carbonyl stretching polymer mediated samples have prominent peaks where the stretching vibration associated with O-H and C-H/ CH2 groups are located at 3350 cm-1 and 2930 cm-1 is associated with the aliphatic hydrocarbons group in polysaccharide, proteins or polyphenols of water molecule bounds in Ag surface respectively 37-40. The observed vibration bands below 600 cm-1 for AgO surface 41,42.The obtained results are consistent with previous publications confirming Ziziphus nummularia‘s efficacy as a reducing agent in the synthesis of AgNPs and polymer functionalized AgNPs 43,44.
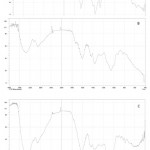 |
Figure 3: FT IR Spectrum of [A] Ziziphus nummularia leaf extract [B] Ag NPs [C] PVP functionalized Ag NPs |
X-RAY diffraction analysis
The XRD pattern of polymer-functionalized AgNPs (as shown in Fig.4) revealed a well-crystallized sample with the principal diffraction peaks located at 2 theta values of 27.12°, 32.39°,46.33° and77.4° which corresponds to the plane(100), (111), (200), (311) respectively. The phase change modification caused by PVP might be attributed to the bio conjugate formed between the polymer component and the produced polymer-capped AgNPs. The mean particle size of PVP AgNPs was determined using the Debye-Scherer formula which is given as D = 0.9k/b cos, where D is the crystalline size (nm), k is the X-ray wavelength (0.1541 nm), b represents the angular line full width at half maximum (FWHM) of the peak (in radians), and his is the Braggs angle (in radians)[45].Calculations indicate that the PVP AgNPs have an average particle size of 9.20 nm, which is in good agreement with the HR-TEM average particle size of 10 nm.
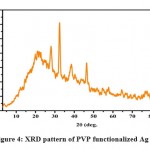 |
Figure 4: XRD pattern of PVP functionalized Ag NPs. |
HR TEM analysis
The H-7500 model was used for high-resolution transmission electron microscopy (HR-TEM). HR-TEM was used to investigate size and shape morphology, as illustrated in figure 5. The polymer functionalized AgNPs with a spherical morphology and uniform size, with an average particle size of 10 nm. The crystallinity of the biosynthesized polymer functionalized AgNPs was demonstrated using a selected area electron diffraction (SAED) pattern with bright circular spots.
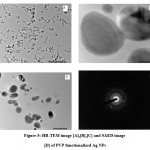 |
Figure 5: HR-TEM image [A],[B],[C] and SAED image [D] of PVP functionalized Ag NPs |
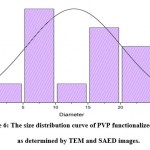 |
Figure 6: The size distribution curve of PVP functionalized AgNPs as determined by TEM and SAED images. |
Antibacterial activity of AgNPs and Polymer functionalized AgNPs
The antibacterial activity of AgNPs was examined by measuring the Inhibition zone of plant extract, silver nanoparticles and polymer functionalized nanoparticles was summarized in table 1. The PVP AgNPs inhibit the growth of Escherichia coli, Staphylococcus aureus , shown in figure 7 It also shows good activities then the all the organisms in comparison with standard drug27. Due to their activity at lower concentrations, PVP AgNPs have a higher chance of competing with conventional antibacterial medicines.
Table 1: Antibacterial activity of Ag NPs and PVP Ag NPs
| SR.NO. | Organism
|
Zone of Inhibition (In mm) | |||
| AgNO3
(10 mM) |
AgNPS | PVP AgNPS | Ampicillin
(1 mg/ml) |
||
| 1 | Escherichia coli | 15 | 19 | 20 | 15 |
| 2 | Staphylococcus Aureus | 15 | 18 | 19 | 17 |
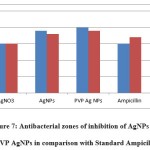 |
Figure 7: Antibacterial zones of inhibition of AgNPs and PVP AgNPs in comparison with Standard Ampicilin. |
Antioxidant activity of AgNPs and Polymer capped AgNPs
DPPH is a stable molecule that may be reduced by absorbing hydrogen or electrons, and it is often used to evaluate antioxidant activity. AgNPs displayed strong antioxidant activity, with their ability to scavenge free radicals increasing with concentration. The figure displays AgNPs’ antioxidant activity, which is around 48.83 percent. The proportion of PVP-AgNPs was around 51.15 percent. Polymer-capped AgNPs had better antioxidant activity than AgNPs, according to the findings. AgNPs have antioxidant properties due to plant component absorption on silver nanoparticles 46.
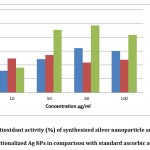 |
Figure 8: Antioxidant activity (%) of synthesized silver nanoparticle and polymer functionalized Ag NPs in comparison with standard ascorbic acid. |
Conclusion
Using an extract of Ziziphus nummularia leaves and AgNO3 salt solution silver nanoparticles were successfully synthesized. To improve biocompatibility, the AgNPs were further functionalized with PVP without the addition of any hazardous or poisonous chemicals. At the preliminary level, the colour change of solution was used to confirm the synthesis of AgNPs and polymer capped AgNPs. Various characterization procedures were employed to confirm the comparison of AgNPs and polymer capped AgNPs. UV visible confirmed the formation of AgNPs trolls by changing the visible colour to dark brown after 2 hours of peak at 431 nm. The FTIR spectrum identified the several functional groups present in the Ziziphus nummularia extract that are involved in the synthesis of AgNPs and polymer-formed AgNPs.XRD examination confirmed the crystallinity and an average particle size of 9.20 nm of AgNPs and polymer capped AgNPs. HR-TEM imaging microscopy revealed that the particles were spherical in shape and ranged in size from 2 to 25 nm. The antimicrobial activity of AgNPs and polymer-capped AgNPs was evaluated against gram-positive and gram-negative bacteria with a significant zone of inhibition. Also, the good antioxidant activity of synthesized silver nanoparticles and polymer capped silver nanoparticles. This study proposed a biological technique for synthesis polymer-capped nanoparticles with antibacterial and antioxidant activity that is both eco-friendly and cost-effective.
Acknowledgement
The Authors highly acknowledge the Department of Chemistry and Department of Biotechnology for providing the research facilities.
Conflict of interest
The authors confirm that the content of this manuscript has no conflict of interest.
Funding Sources
There is no Funding sources for this paper.
References
- Jeyaraj M, Sathishkumar G, Sivanandhan G, MubarakAli D, Rajesh M, Arun R, Kapildev G, Manickavasagam M, Thajuddin N, Premkumar K, Ganapathi A. Biogenic silver nanoparticles for cancer treatment: an experimental report. Colloids and surfaces B: Biointerfaces. 2013 Jun 1;106:86-92.https://doi.org/10.1016/j.colsurfb.2013.01.027
CrossRef - Pal D, Sahu CK, Haldar A. Bhasma: the ancient Indian nanomedicine. Journal of advanced pharmaceutical technology & research. 2014 Jan;5(1):4.https://dx.doi.org/10.4103%2F2231-4040.126980
CrossRef - Sarkar PK, Das S, Prajapati PK. Ancient concept of metal pharmacology based on Ayurvedic literature. Ancient Science of Life. 2010 Apr;29(4):1.
- Thuesombat P, Hannongbua S, Akasit S, Chadchawan S. Effect of silver nanoparticles on rice (Oryzasativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicology and environmental safety. 2014 Jun 1;104:302-9.https://doi.org/10.1016/j.ecoenv.2014.03.022
CrossRef - Larue C, Castillo-Michel H, Sobanska S, Cécillon L, Bureau S, Barthès V, Ouerdane L, Carrière M, Sarret G. Foliar exposure of the crop Lactucasativa to silver nanoparticles: evidence for internalization and changes in Ag speciation. Journal of hazardous materials. 2014 Jan 15;264:98-106.https://doi.org/10.1016/j.jhazmat.2013.10.053
CrossRef - Bindhu MR, Umadevi M. Antibacterial and catalytic activities of green synthesized silver nanoparticles. Spectrochimicaacta part A: molecular and biomolecular spectroscopy. 2015 Jan 25;135:373-8.https://doi.org/10.1016/j.saa.2014.07.045
CrossRef - Kotcherlakota R, Das S, Patra CR. Therapeutic applications of green-synthesized silver nanoparticles. InGreen synthesis, characterization and applications of nanoparticles 2019 Jan 1 (pp. 389-428). Elsevier.https://doi.org/10.1016/B978-0-08-102579-6.00017-4
CrossRef - Kulkarni N, Muddapur U. Biosynthesis of metal nanoparticles: a review. Journal of Nanotechnology. 2014 Jan 1;2014.https://doi.org/10.1155/2014/510246
CrossRef - Ventola CL. The nanomedicine revolution: part 2: current and future clinical applications. Pharmacy and Therapeutics. 2012 Oct;37(10):582.
- Mukherjee S, Chowdhury D, Kotcherlakota R, Patra S. Potential theranostics application of bio-synthesized silver nanoparticles (4-in-1 system). Theranostics. 2014;4(3):316.https://dx.doi.org/10.7150%2Fthno.7819
CrossRef - Ahmad F, Ashraf N, Ashraf T, Zhou RB, Yin DC. Biological synthesis of metallic nanoparticles (MNPs) by plants and microbes: their cellular uptake, biocompatibility, and biomedical applications. Applied microbiology and biotechnology. 2019 Apr;103(7):2913-35.https://doi.org/10.1007/s00253-019-09675-5
CrossRef - Jadhav J, Shah R. Synthesis of Biogenic Silver Nanoparticles From Medicinal Plant And It’s Antibacterial Activity.https://doi.org/10.9790/5736-0908012933
CrossRef - Vaghela H, Shah R, Parmar K. Biogenic synthesis of silver nanoparticles using Bauhinia variegata bark extract and its antibacterial efficacy. International Journal of Nanomaterials and Chemistry. 2017;3(2):45-9.http://dx.doi.org/10.18576/ijnc/030205
CrossRef - Kanipandian N, Kannan S, Ramesh R, Subramanian P, Thirumurugan R. Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthuscollinus extract as surface modifier. Materials Research Bulletin. 2014 Jan 1;49:494-502.https://doi.org/10.1016/j.materresbull.2013.09.016
CrossRef - Jeyaraj M, Rajesh M, Arun R, MubarakAli D, Sathishkumar G, Sivanandhan G, Dev GK, Manickavasagam M, Premkumar K, Thajuddin N, Ganapathi A. An investigation on the cytotoxicity and caspase-mediated apoptotic effect of biologically synthesized silver nanoparticles using Podophyllumhexandrum on human cervical carcinoma cells. Colloids and Surfaces B: Biointerfaces. 2013 Feb 1;102:708-17.https://doi.org/10.1016/j.colsurfb.2012.09.042
CrossRef - Hiral V, Rahul S, Shailesh V, Amanullakhan P. Biosynthesized Silver Nanoparticles Using an Aqueous Root Extract of Iris germanica as a Reducing Agent and Its Antibacterial Efficacy. European Journal of Medicinal Plants. 2020 Apr 21:1-0.https://doi.org/10.9734/ejmp/2020/v31i730248
CrossRef - Bhakya S, Muthukrishnan S, Sukumaran M, Grijalva M, Cumbal L, Benjamin JF, Kumar TS, Rao MV. Antimicrobial, antioxidant and anticancer activity of biogenic silver nanoparticles-an experimental report. RSC advances. 2016;6(84):81436-46.https://doi.org/10.1039/C6RA17569D
CrossRef - Abhimannue AP, Menon A. GREEN SYNTHESIS OF SILVER NANOPARTICLES USING SAUROPUS ANDROGYNOUS LEAF EXTRACT WITH POTENTIAL BIOLOGICAL PROPERTIES. http://dx.doi.org/10.13040/IJPSR.0975-8232.12(8).4267-74
CrossRef - Willian N, Syukri S, Zulhadjri Z, Arief S. Marine plant mediated green synthesis of silver nanoparticles using mangrove Rhizophorastylosa: Effect of variable process and their antibacterial activity. F1000Research. 2021 Aug 6;10(768):768.
CrossRef - Nguyen VP, Le Trung H, Nguyen TH, Hoang D, Tran TH. Synthesis of Biogenic Silver Nanoparticles with Eco-Friendly Processes Using Ganodermalucidum Extract and Evaluation of Their Theranostic Applications. Journal of Nanomaterials. 2021 Aug 4;2021.https://doi.org/10.1155/2021/6135920
CrossRef - Vaghela H, Shah R, Parmar KA. Plant mixture mediated biogenic copper nanoparticles: Antibacterial assay. Current Nanomaterials. 2018 Aug 1;3(2):86-94.https://doi.org/10.2174/2405461503666180803152152
CrossRef - Vaghela H, Shah R, Pathan A. Palladium nanoparticles mediated through bauhinia variegata: Potent in vitro anticancer activity against mcf-7 cell lines and antimicrobial assay. Current Nanomaterials. 2018 Dec 1;3(3):168-77.https://doi.org/10.2174/2405461504666190131142303
CrossRef - Shah R, Pathan A, Vaghela H, Ameta SC, Parmar K. Green synthesis and characterization of copper nanoparticles using mixture (Zingiberofficinale, Piper nigrum and Piper longum) extract and its antimicrobial activity. Chemical Science. 2019;8(1):63-9.https://doi.org/7598/cst2019.1517
CrossRef - Vaghela HM, Pathan AA, Shah RH. The biogenic synthesis of Au, Pd and Pt nanoparticles and its medicinal applications: A review.
- Padalia H, Chanda S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artificial cells, nanomedicine, and biotechnology. 2017 Nov 17;45(8):1751-61.https://doi.org/10.1080/21691401.2017.1282868
- Dureja AG, Dhiman K. Free radical scavenging potential and total phenolic and flavonoid content of Ziziphusmauritiana and Ziziphus nummularia fruit extracts. International Journal of Green Pharmacy (IJGP). 2012;6(3).http://dx.doi.org/10.22377/ijgp.v6i3.259
CrossRef - Mistry H, Thakor R, Patil C, Trivedi J, Bariya H. Biogenically proficient synthesis and characterization of silver nanoparticles employing marine procured fungi Aspergillusbrunneoviolaceus along with their antibacterial and antioxidative potency. Biotechnology Letters. 2021 Jan;43(1):307-16.https://doi.org/10.1007/s10529-020-03008-7
CrossRef - Wang Y, Ou JZ, Chrimes AF, Carey BJ, Daeneke T, Alsaif MM, Mortazavi M, Zhuiykov S, Medhekar N, Bhaskaran M, Friend JR. Plasmon resonances of highly doped two-dimensional MoS2. Nano letters. 2015 Feb 11;15(2):883-90.https://doi.org/10.1021/nl503563g
CrossRef - Khatoon N, Mishra A, Alam H, Manzoor N, Sardar M. Biosynthesis, characterization, and antifungal activity of the silver nanoparticles against pathogenic Candida species. BioNanoScience. 2015 Jun 1;5(2):65-74.https://doi.org/10.1007/s12668-015-0163-z
CrossRef - Wu T, Lu F, Wen Q, Yu K, Lu B, Rong B, Dai F, Lan G. Novel strategy for obtaining uniformly dispersed silver nanoparticles on soluble cotton wound dressing through carboxymethylation and in-situ reduction: antimicrobial activity and histological assessment in animal model. Cellulose. 2018 Sep;25(9):5361-76.https://doi.org/10.1007/s10570-018-1907-z
CrossRef - Behravan M, Panahi AH, Naghizadeh A, Ziaee M, Mahdavi R, Mirzapour A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. International journal of biological macromolecules. 2019 Mar 1;124:148-54.https://doi.org/10.1016/j.ijbiomac.2018.11.101
CrossRef - Jacob SJ, Finub JS, Narayanan A. Synthesis of silver nanoparticles using Piper longum leaf extracts and its cytotoxic activity against Hep-2 cell line. Colloids and Surfaces B: Biointerfaces. 2012 Mar 1;91:212-4.https://doi.org/10.1016/j.colsurfb.2011.11.001
CrossRef - Singh PK, Bhardwaj K, Dubey P, Prabhune A. UV-assisted size sampling and antibacterial screening of Lantana camara leaf extract synthesized silver nanoparticles. RSC advances. 2015;5(31):24513-20.https://doi.org/10.1039/C4RA17233G
CrossRef - Oberdörster G, Oberdörster E, Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental health perspectives. 2005 Jul;113(7):823-39.
CrossRef - Soylu EM, Soylu S, Kurt S. Antimicrobial activities of the essential oils of various plants against tomato late blight disease agent Phytophthorainfestans. Mycopathologia. 2006 Feb 1;161(2):119-28.https://doi.org/10.1007/s11046-005-0206-z
CrossRef - Li Q, Zhang Z, Haque SS, Zhang M, Xia L. Localized surface plasmon resonance effects by naturally occurring Chinese yam particles. Journal of Applied Physics. 2010 Dec 15;108(12):123502.https://doi.org/10.1063/1.3520667
CrossRef - Rivera-Rangel RD, González-Muñoz MP, Avila-Rodriguez M, Razo-Lazcano TA, Solans C. Green synthesis of silver nanoparticles in oil-in-water microemulsion and nano-emulsion using geranium leaf aqueous extract as a reducing agent. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 2018 Jan 5;536:60-7.https://doi.org/10.1016/j.colsurfa.2017.07.051
CrossRef - de Oliveira Silva BS, Seabra AB. Characterization of iron nanoparticles produced with green tea extract: a promising material for nitric oxide delivery. Biointerface Research in Applied Chemistry. 2016 May 1;6(3).
- Ahluwalia V, Elumalai S, Kumar V, Kumar S, Sangwan RS. Nano silver particle synthesis using Swertiapaniculata herbal extract and its antimicrobial activity. Microbial pathogenesis. 2018 Jan 1;114:402-8.https://doi.org/10.1016/j.micpath.2017.11.052
CrossRef - Song JY, Jang HK, Kim BS. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Process Biochemistry. 2009 Oct 1;44(10):1133-8.https://doi.org/10.1016/j.procbio.2009.06.005
CrossRef - Kombaiah K, Vijaya JJ, Kennedy LJ, Bououdina M, Ramalingam RJ, Al-Lohedan HA. Okra extract-assisted green synthesis of CoFe2O4 nanoparticles and their optical, magnetic, and antimicrobial properties. Materials Chemistry and Physics. 2018 Jan 15;204:410-9.https://doi.org/10.1016/j.matchemphys.2017.10.077
CrossRef - Ladole CA. Preparation and characterization of spinel zinc ferrite ZnFe2O4. Int J Chem Sci. 2012;10(3):1230-4.
CrossRef - Rolim WR, Pelegrino MT, de Araújo Lima B, Ferraz LS, Costa FN, Bernardes JS, Rodigues T, Brocchi M, Seabra AB. Green tea extract mediated biogenic synthesis of silver nanoparticles: Characterization, cytotoxicity evaluation and antibacterial activity. Applied Surface Science. 2019 Jan 1;463:66-74.https://doi.org/10.1016/j.apsusc.2018.08.203
CrossRef - Kumar D, Kumar G, Agrawal V. Green synthesis of silver nanoparticles using Holarrhenaantidysenterica (L.) Wall. bark extract and their larvicidal activity against dengue and filariasis vectors. Parasitology research. 2018 Feb;117(2):377-89.https://doi.org/10.1007/s00436-017-5711-8
CrossRef - Guo R. Analysis of cation-treated clay microstructure using zeta potential and x-ray diffraction (Doctoral dissertation, University of Alaska Fairbanks).
- Keshari AK, Srivastava A, Chowdhury S, Srivastava R. Green synthesis of silver nanoparticles using Catharanthusroseus: Its antioxidant and antibacterial properties. Nanomedicine Research Journal. 2021 Jan 1;6(1):17-27.https://dx.doi.org/10.22034/nmrj.2021.01.003

This work is licensed under a Creative Commons Attribution 4.0 International License.





