How to Cite | Publication History | PlumX Article Matrix
A Review of Naturally Occuring Food Allergens, and Their Impact on Health
Renu Indhikkattu Chittoor and Harikumaran Thampi Balakrishnan Saraswathi*
Department of Life Sciences, University of Calicut, Kerala (State), India.
Corresponding Author E-mail: bsharik111@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/2965
ABSTRACT:
Allergic reactions to foods influence a remarkable amount of population and are related with significant wellbeing results. It is one of the most significant issues that have expanding consideration. Current comprehension of the turn of events and utilization of allergenic capability of certain types of food proteins is restricted. In spite of the fact that there is a selection of in vivo models for assessing hypersensitivity, none of the current models has been approved, is prescient, or generally acknowledged with respect to their allergen explicit inhibitors. Hence, there is a proceeded with enthusiasm on the knowledge recovery based on food allergy so as to give more enlightening way to the current research field. In this paper, the current status of purification, characterization, and types of food allergens and their impacts is thoroughly reviewed. The present available methods for the allergen assessment (in view of animal, cell and clinical methodologies) are emphasized.
KEYWORDS: Characterization; Food Allergens; in vivo; in vitro; Mechanism
Download this article as:| Copy the following to cite this article: Chittoor R. I, Saraswathi H. T. B. A Review of Naturally Occuring Food Allergens, and Their Impact on Health. Biosci Biotech Res Asia 2022;19(1). |
| Copy the following to cite this URL: Chittoor R. I, Saraswathi H. T. B. A Review of Naturally Occuring Food Allergens, and Their Impact on Health. Biosci Biotech Res Asia 2022;19(1).Available from: https://bit.ly/3uiJH7e |
Introduction
Food sensitivity is a most common medical issue of both developed and developing countries causes critical anaphylactic reactions consequently leads to notable morbidity. It affects personal satisfaction and makes a substantial economic burden1. The pace of food allergy differs by age, local diet, chemical nature of food and numerous other factors2, and different quantities of clinical highlights impacts the common history of food hypersensitivity3. Studies conducted in the western countries based on nut hypersensitivity, recommended that the prevalence rates have expanded, virtually duplicating, and outperform 1 % in children those who are school aged4. The report from Centres for Disease Control and Prevention indicated that, youth food sensitivity raised around 18 % with the direct influence of 3.9 % of youngsters are happens from 1997 to 20075. In westernized nations, harmful safe reactions to food items influence around 5 % of young children and in case of grown-ups is around 3 to 4 %, seem to have more predominance2.
India is the second most crowded nation, having rate of population nearly 1.3 billion. Recent reports revealed that there are an expected 37.5 million instances of asthma and a rise in predominance of hypersensitive rhinitis 6. A food specific sensitization study conducted on children aged 7 – 10 years indicated that food allergy appears to be less common when compared with developed nations 7.
A survey conducted by EuroPrevall – INCO in Indian kids revealed, food hypersensitivity to be 0.14 % among youngsters with rate of sensitization was high (around 19.1 %) to food allergens. Considering the case of nut hypersensitivity, it was discovered to be low (around 0.03%) in Indian kids, although the sensitization rate was high at 6.3%7. Therefore, further examination is expected to explore why predominance of clinically applicable food sensitivity is low in India in spite of high sensitisation rate.
Table 1: List of known food allergens and their classification
| SL No: | Food source | Identified allergens | Allergen family | Molecular weight (kDa) |
| 1 | Peanut 8 | Ara h 1
Ara h 2 Ara h 3 Ara h 4 Ara h 5 Ara h 6 Ara h 7 Ara h 8 Ara h 9 Ara h 10 Ara h 11
|
Cupin superfamily
Prolamin superfamily Cupin superfamily Cupin superfamily Profilin family Prolamin superfamily Prolamin superfamily Bct v 1-related protein family Prolamin superfamily Olcosin family Olcosin family |
64
17 60.37 60 15 15 15 17 11 16 14 |
| 2 | Soybean 9 | Gly m 5
Gly m 6 Gly m Bd 28K Gly m Bd 30K Gly m T1 |
Cupin superfamily
Cupin superfamily Cupin superfamily Papaya proteinase superfamily Kunitz soybean trypsin inhibitor family |
140~180
320~360 26 30~34 20 |
| 3 | Egg 10 | Gal d 1
Gal d 2
Gal d 3 Gal d 4
|
Kazal-type serine protease inhibitor family
Serpin serine protease inhibitor family Transferrin family Bet v 1-related protein family |
28
44.5
76 14.3 |
| 4 | Crustacea11 | Pen a 1 | Tropomyosin family | 36 |
| 5 | Milk 12 | Bos d 4
Bos d 5 Bos d 8 |
C-type lysozyme/alpha-lactalbumin family
Lipocalin family
|
14.2
18.3 20~30 |
| 6 | Nut 13,14 | Jug r 1
Jug r 2 Jug r 3 Jug n 1 Jug n 2 Ana o 1 Ana o 2 Ana o 3 2S albumins NsLTP Vicilins legumins Oleosins |
Prolamin superfamily
Cupin superfamily Prolamin superfamily Prolamin superfamily Cupin superfamily Cupin superfamily Cupin superfamily Prolamin superfamily prolamin superfamily prolamin superfamily cupin superfamily cupin superfamily oleosin superfamily |
15~16
44 9 19 56 50 55 14 15 6.038~9.922 150–190 150–190 ∼15–26 |
| 7 | Wheat 15 | Tri a gliadin
Tri a 26 Tri a 36 |
Prolamin superfamily
Prolamin superfamily Prolamin superfamily |
30~80
80~120 30~75 |
| 8 | Fish 16 | Parvalbumin | EF hand domain family | 12 |
(Abbreviation used: Ara h – Arachis hypogaea, Gly m – Glycine max, Gal d – Gallus domesticus, Pen a – Penaeus aztecus, Bos d – Bos domesticus , Jug r – Juglans regia, Jug n – Juglans nigra, Ana o – Anacardium occidentale, NsLTP – Non-specific lipid transfer proteins, Tri a – Triticum aestivum)
The function of food related proteins
Hypersensitive responses to milk, egg, fish, peanut, shellfish, wheat, tree nuts and soy represent most important food sensitivities in the worldwide17. Majority food allergens having atomic weight varying from 10 to 70 kDa and are water dissolvable glycoproteins moderately stable to acid, proteases, and temperature.
Furthermore, food preparation, and manner of food ingestion also affect allergenicity. The studies conducted on peanut indicate that Millard reaction due to high temperature roasting (180 0c) and emulsification due to adjuvant effect are the example which describes how the food preparation methods affect the food allergenicity4,18. The Meta – analysis study conducted on fruits and vegetables accounts that allergic reactions shifted from 0.1 – 1.4 for tree nuts, fruits about 0.1 – 4.3 % and for vegetables under 1 %. Food initiated hypersensitivity are normally connected with severe reactions. Certain foods like nuts and seafood activates anaphylactic reactions among children and adults. For example: In the case of young children, cow milk and egg are very common culprits19.
Common food allergies
Food allergy affects over 200 million people in worldwide. The prevalence rate is mainly raised in developed nations20. An investigation of government schools in Australia (>550,000 students) taking a gander at those in danger of hypersensitivity mentioned around 41 % expansion from 2009 to 2014 (0.98 – 1.38 %)21. The US Centres for Disease Control and Prevention, utilizing information from one inquiry in the US National Health Interview Survey, disclosed that the commonness of food sensitivities expanded among kids from 3.4 % in 1997 to 1999 and 5.1 % in 2009 to 201122. AUS overview depending on parental report of youngster nut hypersensitivity utilizing comparable approach over time exhibit a rate of 0.4 % in 1997 expanding to 1.4 % in 2008 2. As per National Institute of Allergy and Infectious Diseases, sensitivity of the food item is characterized as “an antagonistic wellbeing impact emerging from a particular resistant response that happens reproducibly on introduction to given nourishment”. This reaction incorporates essentially a wide range of safe intervened responses23.
The term sensitivity was named by the Austrian paediatrician Clemens von Pirquetin 1906, who explained instances of serum affliction in kids treated with immunizer arrangements 24. The common hypersensitivities are fluctuates relying upon the nation. There are many types of allergies. We have given a brief overview of some of the most common food allergies.
Milk allergy
Milk allergy is common among paediatric population. Carlos & Hania summarizes the dairy animals’ milk sensitivity for its better analysis and risk management25. The occurrence of cow’s milk sensitivity in kids living in the created world is roughly 2 to 3 %, and lower predominance rate is only observed in breastfed new – born infants (0.5 %)26,27.
The significant allergens seen in bovines milk are belongs to casein fraction of proteins and to whey proteins 28. The two fundamental systems which clarify unfavourably susceptible reaction of cow’s milk hypersensitivity just as other food sources are: IgE intervened and non – IgE interceded.
IgE mediated manifestation includes acute urticaria and angioedema. Non IgE mediated mechanism involves indications on epidermis and the digestive tract. At the level of digestive tract incorporates: bovine’s milk – prompted enterocolitis, bovine’s milk – incited enteropathy and bovine’s milk – initiated proctitis and proctocolitis29. The indications may make before multi month mature enough, and as frequently as conceivable inside multi week after the associate of dairy creatures’ milk proteins with their eating routine26.
In case of non – IgE mediated response; symptoms are delayed and typically happen in no time and up to 2 h of ingestion. The epidermis is normally included and followed by gastrointestinal plot and, marginally the respiratory and additionally cardiovascular frameworks. The seriousness related with the responses may fluctuate from mild to moderate, and sometimes leads to critical anaphylaxis 30,31. The diagnosis of cow’s milk allergy includes certain routine work-up like skin prick tests (SPTs), direct IgE (sIgE) estimations, and feeding tests29,31,32,33,34.
Egg allergy
Egg allergy most commonly IgE – mediated, and is seen in childhood. It has been reported that prevalence rate is of 1.3 to 10.1 %35. This may develops in the main year of life creating, and it is the second most basic reason for food sensitivities in kids36,37.
Egg white contains in excess of 20 distinct glycoproteins and proteins. The significant allergens seen in hen’s egg are Ovomucoid (Gal d 1), ovalbumin (Gal d 2), conalbumin (Gal d 3), and lysozyme (Gal d 4). The principle allergen found in egg yolk is Alpha – livetin (Gal d 5) 38. Clinical responses are basically IgE and non – IgE intervened or a combination of the two kinds.
The diagnosis of egg sensitivity mainly depending on history taking, in vitro allergen-explicit blood IgE tests, skin prick test, histamine discharge test, and feeding tests39. Among this, SPT shows a decent affectability yet a helpless particularity. Currently, oral immunotherapy (OIT) provides better results to egg allergy. It had mild to severe side effects. Additionally, there is a need to develop safer OIT for novel modified food antigens40.
Peanut allergy
Peanut allergy affects 1 to 4.5 % of children, with severe reactions41. Chiara & Heimo reviewed about certain peanut allergens 42. Bicupin seed storage proteins contain Ara h 1 and Ara h 343. Ara h 2, Ara h 6, and Ara h 7 are seed stockpiling proteins, which are included in the prolamin superfamily. Ara h 5 is said to be an individual from the profilin family8.
Diagnostic investigations of nut allergens incorporate skin prick tests, nut explicit IgE (sIgE), and absolute IgE44. Thermal processing like boiling, frying, or roasting appears to have impact on event of nut hypersensitivity. Among these cooking methods, boiling of peanuts has lesser prevalence45. Recently, researchers have mainly focussed on the deep immunological characterization of nut allergens and the utilization of decontaminated allergens symptomatic field. Kelly Bruton & his colleagues were discussed about the current state of oral immune therapy in the field of peanut allergy 46.
Tree nut hypersensitivity
Tree nut hypersensitivity predominantly creates by age of 2 years, and the sensitization to allergen exposure naturally increases with age47. Most of proteins engaged with tree nut hypersensitivity have a place with protein groups of vicilins, nsLTPs, 2S albumins, and legumins. Of these, dust related tree nut hypersensitivity mediated by Bet v 1 – homologues and profilins. Moreover, oleosins and thaumatin – like proteins were demonstrated to be noteworthy allergens14,48. Andrzej Kuźmiński & his colleague’s reviewed about the different nut allergies 49. Individual tree nut components and their allergic factors are given in table 2.
The analysis of tree nut sensitivity is performed with detailed clinical history, skin prick testing, serum – explicit IgE, and oral food challenges (OFCs) 50,51,52. Dietary management is the only prevention method recommended for tree nut allergy.
Table 2: Individual tree nuts and their allergic components 13
| Tree nut | Allergic component | Protein family | Protein type |
| Hazelnut | Corylus avellana
Cor a 1 Cor a 2 Cor a 8 Cor a 9 Cor a 11 Cor a 14 |
Pan-allergens
Storage proteins |
PR-10 Profilin LTP 11S globulin 7S globulin 2S albumin |
| Cashew | Anacardium occidentale
Ana o 1 Ana o 2 Ana o 3 |
Storage proteins |
7S globulin 11S globulin 2S albumin |
| Pistachio | Pistacia vera
Pis v 1 Pis v 2 Pis v 3 Pis v 5 |
Storage proteins |
2S albumin 11S globulin 7S globulin 11S globulin |
| Walnut | Juglans regia
Jug r 1 Jug r 2 Jug r 4 Jug r 3 Jug r 5 |
Storage proteins
Pan-allergens |
2S albumin 7S globulin 11S globulin LTP Profilin |
| Pecan | Carya illinoinensis
Car i 1 Car i 2 Car i 4 |
Storage proteins |
2S albumin 7S globulin 11S globulin |
| Almond | Prunus dulcis
Pru du 6 Pru du 3 Pru du 4 |
Storage proteins Pan-allergens |
11S globulin LTP Profilin |
| Pine nut | Pinus pinea
Pin p 1 |
Storage proteins |
2S albumin |
| Brazil nut | Bertholletia excelsa
Ber e 1 Ber e 2 |
Storage proteins
|
2S albumin 11S globulin |
Abbreviation used: LTP, lipid transfer protein.
Soy allergy
Soy allergy typically happens in the beginning of early stage; with announced pinnacle rate of soy sharpening occur in the age of 2 years53. Generally, soy sensitivity is not common as other food hypersensitivities.
International Union of Immunological Societies Allergen Sub – Committee acknowledged birch dust – related proteins, soybean hull proteins (Gly m 1 and Gly m 2), a profiling (Gly m 3), and a PR-10 protein (Gly m 4), are the significant allergenic proteins found in soy (http://www.allergen.org?Allergen.aspx). Additionally, certain storage proteins b -conglycinin and glycinin (Gly m 5 and Gly m 6), the thiol protease (Gly m Bd 30 k), the 2S albumin soy protein, and the soybean Kunitz trypsin inhibitor are also have been described54,55,56,57,58.
Patients suffering from soy sensitivity present a scope of clinical disorder like IgE – interceded and non – IgE – intervened. Kattan et al., discussed briefly about the clinical manifestation behind soy allergy59. The finding of soy sensitivity is mainly involves by taking the clinical history and allergy testing. Now a days, oral food challenge and oral immunotherapy are the methods used in the field of management of soy allergy.
Wheat allergy
Wheat allergy is very common in paediatric population. The prevalence rate of wheat allergy differs based on age and region 60. Wheat allergens are belonging to the grass family Poaceae and contain numerous allergenic proteins, which are partitioned in four classes dependent on the extraction solvents. American chemist T. B. Osborne standardized this classification61. Important wheat allergens responsible for wheat allergy are given below (Table: 3):
Table 3: Important wheat allergens 62
| SL no: | Allergen component | Common name |
| 1 | Tri a 12 | Profilin |
| 2 | Tri a 14 | Lipid Transfer Proteins |
| 3 | Tri a 15 | alpha-Amylase Inhibitors |
| 4 | Tri a 18 | Agglutinins |
| 5 | Tri a 19 | ω-5 gliadins |
| 6 | Tri a 20 | γ-gliadins |
| 7 | Tri a 21 | α-β-gliadins |
| 8 | Tri a 25 | Thioredoxin |
| 9 | Tri a 26 | Glutenins |
| 10 | Tri a 27 | ThiolReductase |
| 11 | Tri a 28 | α -amylase Inhibitor |
| 12 | Tri a 29 | α -amylase Inhibitor |
| 13 | Tri a 30 | α -amylase Inhibitor |
| 14 | Tri a 31 | TriosephosphateIsomerases |
| 15 | Tri a 32 | Peroxiredoxines |
| 16 | Tri a 33 | Trypsin Inhibitors |
| 17 | Tri a 34 | Glyceraldehyde-3-phosphate dehydrogenases |
| 18 | Tri a 35 | Dehydrins |
| 19 | Tri a 36 | Glutenins |
| 20 | Tri a 37 | Thionins |
(Abbreviation used: Tri a – Triticum aestivum)
The clinical sign of wheat hypersensitivity relies upon the course of allergen introduction. Usually, it is liable for IgE – intervened responses, with the event of urticaria, angioedema, bronchial impediment, queasiness, stomach torment, or fundamental hypersensitivity63. Non – IgE – intervened indication incorporates food protein-incited enterocolitis condition. The prognosis involves SPT and serum IgE tests. The current management of patients suffering from wheat sensitivity is dietary shirking. Recently, feeding tests has been announced for wheat hypersensitivity with positive outcomes, regardless of whether further investigations are requires for building up the best convention so as to advance resistance in wheat – allergic youngsters64,65,66,67,68.
Meat allergy
Meat allergy is varied according to region basis, and is accompanied with local dietary habits and environmental factors. This is mainly occurs during the primary long stretches of life, therefore it is uncommon in grown – ups69. The major allergens found in meat are serum albumins and immunoglobulin. Important allergens seen in meat are listed below (Table 4).
Table 4: common allergens found in meat69,70
| Species | Allergen component | Molecular weight (kDa) |
| Domestic cattle | Bos d 6
Bos d 7 |
67
160 |
| Chicken | Gal d 5 | 69 |
| Dog | Can f 3 | 69 |
| Guinea pig | Cav p 4 | 66 |
| Domestic horse | Equ c 3 | 67 |
| Cat | Fel d 2 | 69 |
| Domestic pig | Sus s 1 | 60 |
Several studies shown that activity of this antigenic peptide can be destructed by domestic cooking and some other technological treatments. Certain treatments like warming can adjust or inactivate the epitopic spaces liable for the IgE holding by changing the conformation of the epitopes; or by the denaturation of the three dimensional structure of a protein can create new epitopes and improve the availability of the allergenic determinant to explicit antibodies. Likewise, freeze – drying, homogenization and mechanical cycles are generally employed to meat during the creation of infant nourishments, can altogether diminish the allergenic capability of beef 71.
Cooking methods frequently destroys heat- sensitive allergens. Conversely, Fiocchi et al., shown that a couple of patients will regardless react to cooked nourishments72. Apart from this, researchers reported that allergenic potential of the antigen can be tested through cross reactions, and this can be used in the diagnostic field73.
Fruit allergy
Literature reports confirmed that the prevalence rate of fruit allergy was ~5-8 % children and 2 – 3 % adults. Mostly 12 – 15 fruits are associated with fruit allergy, commonly market available fruits and vegetables. Fruit allergic reactions are in relation with oral allergy syndrome (OAS) together with pollen – fruit-vegetable syndrome74,75.
Table 5: List of allergens found in fruits
| Sl No: | Fruits | Allergen | Molecular weight
(kDa) |
| 1 | Apple
(Malus domestica) |
TLP (Mal d 2)
Bet v 1 homolog (Mal d 1) Profilin (Mal d 4) LTP (Mal d 3)
|
23.0
17.5 14.0 9.0
|
| 2 | Peach
(Prunus persica) |
TLP (Pru p 2)
Bet v 1 homolog (Pru p 1) Profilin (Pru p 4) LTP (Pru p 3)
|
23.0
17.5 14.0 9.0
|
| 3 | Musk melon
(Cucumis melo) |
serine protease (Cuc m 1.01)
serine protease (Cuc m 1.02) serine protease (Cuc m 1.03) PR-1 protein (Cuc m 3) Profilin (Cuc m 2)
|
67.0
54.0 36.0 16.0 14.0
|
| 4 | Gold kiwi, green kiwi
(Actini diachinensis, A. deliciosa) |
Chitinase (Act d 3)
Actinidin (Act d 1) Kiwellin (Act d 5) TLP (Act d 2) Cystatin (Act d 4)
|
43.0
30.0 28.0 23.0 11.0
|
| 5 | Sweet cherry
(Prunus avium) |
TLP (Pruav 2)
Bet v 1 homolog (Pruav 1) Profilin (Pruav 4) LTP (Pruav 3)
|
23.0
17.5 14.0 9.0
|
| 6 | Grape
(Vitis vinifera) |
Chitinase, hevein-like (Vit v 5)
TLP (Vit v TLP) Bet v 1 homolog (Vit v 8) Profilin (Vit v 4) LTP (Vit v 1)
|
30.0
23.0 17.5 14.0 9.0
|
| 7 | Strawberry
(Fragaria ananassa) |
Bet v 6 homolog (isoflavonereductase)
Bet v 1 homolog (Fra a 1) Profilin (Fra a 4) LTP (Fra a 3)
|
35.0
17.5 14.0 9.0
|
| 8 | Banana
(Musa acuminata) |
β-1,3-glucanase (Mus a 5)
Class I chitinase (Mus a 2) TLP (Mus a 4)
|
33.0
31.0 21.0
|
| 9 | Custard apple
(Annona cherimola) |
Class I chitinase(Ann c Chitinase)
|
45.0
|
| 10 | Mango
(Anacardium occidentale) |
Bet v 1-like (Man i 14kD)
Class I chitinase (Man i Chitinase)
|
14.0
30-45
|
| 11 | Pomegranate
(Punica granatum) |
PR-4 protein (Barwin family)
PR-4 protein PR-4 protein LTP (Pun g 1)
|
28.0
17.0 16.0 9.0
|
Abbreviations used: LTP, lipid transfer protein; PR-, pathogenesis-related; TLP, thaumatin-like protein.
Classification, mechanism of pathogenesis, and clinical features of food allergy
The epidemic of food sensitivity has expanded over the previous decade. Despite the fact that the innermost processes involved in food related sensitive responses are cleared, yet the ideal molecular events food allergen – instigated hypersensitive showings are not definitely perceived76. Recently, researchers evaluated the food allergens mainly through bioinformatics examination, and clinical studies like model tests, cell model creature tests, serological examination, and mimicked gastric assimilation. The studies revealed that using of cell models is more convenient and flexible method instead of using animal models. Yet, in vivo test is the most immediate and instructive strategy to assess the possible sensitivity of the food item. Sampson et al. provide a detailed review of mechanism of food allergy. They described the factors which effects tissue and immune responses to food antigens, the recent findings with respect to the advancement of invulnerable resilience, the role microbiota in the GI tract and the immunologic response of desensitization system 77.
Food hypersensitivity is generally IgE – mediated type I hypersensitivity, non – IgE-mediated, and mixed type.
IgE mediated
When the food allergens initially enter the body, the initiated cells were emitted interleukin (IL)- 4,5,13, and different cytokines which prompt antibody (IgE) creation in B cells. The communication between IgE and the objective cells (granulocyte cells, basophil, and mast cells) leads the body overly sensitive. Again a similar allergen enters the body once more, it communicates with the IgE molecules on the objective cells mediates the spanning response, activating the underlying pathway, brings about the degranulation of target cells, and deliveries the mediators like 5-hydroxytryptamine, histamine, and leukotriene. This leads to unfavorably susceptible response. This enhances the permeability of intestinal epithelial cells to the allergenic protein, and accordingly influencing the pathway of specific cytokines, and creating a Th2 – type provocative reaction. This is the mechanism behind IgE – mediated food allergy 73.
Mixed type food allergies
This class of food hypersensitivity is intervened by both IgE – dependent and IgE – free pathways. This is delayed type, and the allergy – associated atopic dermatitis seen after 6– 48 hours. This is caused by T helper 2 cells. Apart from this eosinophilic gastrointestinal disorders like eosinophilico esophagitis (EoE), brought about by the eosinophilic penetration of tissues78,79,80,81. Further examination is required in this sort of food sensitivity.
Non – IgE – mediated food sensitivity
Mostly Non – IgE-interceded hypersensitivities principally affects digestive tract, as opposed to the skin and respiratory system 82,83. At this moment, the framework behind the food sensitivity is very clear and we show it in the figure 2. Major features associated with both type of mechanism are given in the table 6.
Table 6: Highlights of IgE and non – IgE – mediated hypersensitivity 25
| Factors | IgE mediated | Non – IgE mediated |
| Time of exposure to allergen | Minutes to 2 hr | Several hours to days |
| Intensity | Mild to anaphylaxis | Mild to moderate |
| Time span | May continue beyond 1 year of age | Usually resolved by 1 year |
| Detection | Specific serum IgE, skin prick test (SPT) | Oral challenge |
Coombs and Gells proposed four types of allergy induction pathways by considering the system of pathogenesis24.
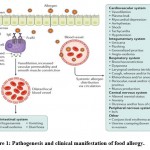 |
Figure 1: Pathogenesis and clinical manifestation of food allergy. |
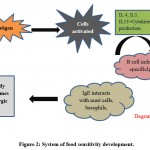 |
Figure 2: System of food sensitivity development. |
It isn’t surely known to what extent the mechanism behind food allergy; hence there is a need to discuss about the extraction and purification of food allergens from various food sources for the detection and diagnosis of its allergenicity. In this communication, the current status of purification and characterization of food allergens and evaluation of food hypersensitivity is briefly reviewed, and the present available methods for the allergen assessment (in light of creature, cell and clinical methodologies) are listed. In addition to this, we presented some related examination on food allergen specific inhibitors, and want to have some motivation in the field of food hypersensitivity.
Table 7: Disorders associated with food allergy84
| Types | Gastrointestinal | Cutaneous | Respiratory |
| IgE mediated | Oral allergy syndrome, gastrointestinal anaphylaxis | Urticaria, angioedema, morbilliform rashes and
flushing |
Acute rhinoconjunctivitis, bronchospasm
(wheezing) |
| Mixed IgE and cell mediated | Allergic eosinophilic esophagitis, allergic
eosinophilic gastroenteritis |
Atopic dermatitis | Asthma |
| Cell mediated | Food protein induced enterocolitis, food Protein induced proctocolitis, food Protein induced enteropathy syndromes, celiac disease | Contact dermatitis, dermatitis herpetiformis | Food-induced pulmonary hemosiderosis
(Heiner syndrome) |
Plant and animal food allergens
Normally, all food allergens are proteins, yet not all food proteins are allergens85. There are in excess of 170 nourishments have been accounted to had hypersensitive responses. Of these, the most widely recognized foods with investigated food sensitivities are peanuts, soyabeans, wheat, fish, eggs, crustacea, tree nuts, and dairy animals’ milk 86. To assess the relative allergenicity of novel proteins, it is fundamental to utilize the regular food allergens as reference proteins like cereals containing gluten, crustaceans, eggs, peanut, soya, milk etc., 3.
The main reason behind the advancement of new food hypersensitivities is to the introduction of new proteins into the eating regimen. Hence, it is needed to elucidate the development and allergenic capability of existing and new food proteins (plants as well as animal sources) for evaluating their safety assessment on clinical studies. Current comprehension of this is deficient and has set number of prescient strategies dependent on in vivo examination of food allergens, and in vitro techniques. Thus, there is a proceeded with enthusiasm for the improvement of appropriate cell models as well as animal models that give an essential way to deal with the appraisal of hypersensitive capability of proteins87,88.
Extraction, purification, and characterization of food allergens
Dietary inclinations for the noteworthy people in an area accept a critical part in the improvement of food hypersensitivity. That is, increased utilization of certain foods may prompt increased sensitization to it, for e.g., sensitivity to soy in Japan, nut hypersensitivity in the France, UK, and North America and sesame sensitivity in Israel89. Evidences of IgE-mediated hypersensitive responses incited by legumes in Asian and Mediterranean nations have been accounted as legumes are the major source of dietary protein in these nations90.
Astwood et al. described different types of extraction methods for allergen proteins from various plant sources91. Phosphate buffer of different salt concentrations at pH 7.0 were used for the extraction. With the improvement of molecular biology, genomics, and immunology, numerous allergens and allergenic sources are presently revealed from various sources92. Ramkrashan Kasera et al. were purified the kidney bean allergen using anion exchanger Q sepharose column. Its characterization was done with immunochemical methods, and the results showed that the molecular weight of the purified protein was found to be 31 kDa93. Pastorello & Trambaioli reviewed concerning to the field of isolation of allergens from various food sources94.
Table 8: Different types of extraction methods used in common food sources.
| SL no: | Food source | Method of extraction | References |
| 1 | Fruits & vegetable sources | 10 mmol/l potassium phosphate buffer (pH 7.0) + 2% polyvinylpolypyrrolidone + 2 mmol/l EDTA + 10 mmol/1sodium diethyldithiocarbamate (DIECA) + 3 mmoI/1 sodium azide (NaN3) | Bjorksten et al. 95,
Vassilopoulou et al.96 |
| 2 | Cereals | 01 M Ammonium Hydrogen Carbonate(AHC) (pH 7-65)
|
Shewry et al.97,
Qi et al.98, Sandiford et al.99 |
| 3 | Raw &cooked fish | Phosphate buffered saline (PBS) (pH 8) | Crespo et al.100,
Pastorello&Trambaioli94 |
| 4 | Egg | Physiological saline solution | Langeland101
Pastorello&Trambaioli94 |
| 5 | Milk | Precipitation at pH 4.65 + titration with 1 M HCl + precipitated fraction is to be dissolved in water at pH 7 by slow addition of 1 M NH4OH. | Makinen&Sorva102,
Pastorello&Trambaioli94 |
Usually food allergens have been distinguished by immune tests, utilizing serum tests from unfavorably susceptible patients. Enzyme-linked immunosorbent assay (ELISA) was the basic strategy utilized for their detection. Currently, there are mainly three methods accessible for the detection and measurement of food allergen, namely: DNA based techniques, protein based methods and cell based methods. In addition, these methods can likewise be utilized in food sensitivity diagnostics103,104,105.
DNA based strategy: Methods for estimating allergen coding genes (e.g.: PCR).
Protein based strategy: Methods for measuring allergenic protein levels (e.g.: ELISA, Protein microarray, Mass spectrometric methods).
Cell based strategy (otherwise called basophil activation test (BAT)): Methods for measuring effector cell activation levels.
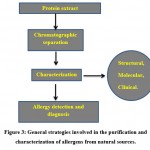 |
Figure 3: General strategies involved in the purification and characterization of allergens from natural sources. |
Literature reports underlined that there is a correlation between quantitative and subjective advancement of allergen extraction and the protein recovery. Hocine et al. constructed a D – optimal plan to advance allergen extraction proficiency at the same time from non – roasted, roasted, non – defatted and defatted peanut, almond, pistachio flours and hazel nut utilizing three denaturing aqueous buffers at different states of, buffer – to – protein ratio, ionic strength, extraction time duration and temperature. Statistical examination demonstrated that the protein recovery varied by depending on the extraction condition with respect to the samples 9.
Currently, there is also an improved procedure for the extraction of allergenic proteins in fish was developed 106,107. The reports revealed that parvalbumin was the major allergen found in various fish species, cause 95 % of patients allergic to fish. Now day, at least 7 different allergens from shellfish have been identified, mostly from crustaceans108.
The method of purification and characterization of food allergen is an important criterion in diagnostic field. Commonly, chromatographic techniques are used for their purification like gel – filtration, ion – exchange, and affinity chromatography. Barbara Cases et al. explained with respect to the chromatographic columns, packing materials, buffers, and methods for extract preparation103. Clare Mills et al. clarified the analytical tools used in food hypersensitivity for the identification and management of numerous food allergens in the food sources. This approach mainly focussed on the quantitative MS – based method 109.
Immunoelectrophoresis was the major technique used for the identification of individual allergens from the sample extracts. This can be used to analyze single protein from a complex mixture. The basic disadvantage of this technique is the dependence on rabbit antisera as reagents110. Tandem mass spectrometry can also be used for the identification of purified allergen93.
Natalia Gasilova and Hubert H Girault summarized the recent advances in food hypersensitivity determination, allergen identification from food items as well as disclosure of the latest allergenic particles via in vitro methods. The review pointed on the new symptomatic strategies under lab advancement includes various aptamer and immune based tests. Furthermore, combination of allergenomic techniques with peptide sequencing by the Edman degradation is the powerful apparatuses for the revelation and study of new allergens111.
In vitro studies
Cell models are mainly defined to explain the molecular basis of an illness and to describe the cell pathways included. These can be taken as a tool for recognizable proof of medication targets, and for the screening of inherited, environmental or pharmacological threat factors related with the infections. In line with this, there are various cell models have been introduced to explain the allergenicity and sensitization processes, including human basophil granulocyte models, mast cell models, and so forth.
Literature reviews revealed that the most ordinarily utilized cell line assigned as RBL – 2H3, is commonly considered as mast cell line. Particularly, these cell lines are mainly used for the investigations on binding of IgE to receptors (i.e., Fc€RI) and further underlying events112,113. Mast cells and basophils are the two distinct cell types that assume vital roles in inception and advancement of type I hypersensitivity responses. Granulocyte basophils represent less than 1 % of the WBC population, while developed mast cells can be seen in tissues and specifically situated at interfaces with the outer environment like skin, lungs, and mucosal surfaces114.
Eccleston and co – workers were found Rat basophilic cells in 1973, when one of the rats was treated with strong cancer- causing agent (2-(a-chlor-b- isopropylamine)ethylnaphthalene), an uncommon type of granulocytic leukemia marked by a significant peripheral blood basophilia115. It is the usually utilized histamine – releasing cell line in immunological exploration, inflammation, and hypersensitivity. It has been extensively reviewed that the behavior of cells in regard of their reaction to immunological boosts.
RBL-2H3 cells, similar to basophils and mast cells, react with degranulation by cross linking of their IgE – bound Fc€RI by multivalent allergens, with the appearance of a preformed and newly synthesized mediators that brings a powerful immune allergic responses116. EglePassante & Neil Frankish were also reviewed the provenance and suitability of RBL – 2H3 cells113. Subsequently, it has been broadly utilized as the mast cell model in in vitro studies, due to their large amount of growth in culture, and its responsiveness to FcεRI- mediated triggers117. Bing-Hung Chen et al. were studied the anti – hypersensitive activity of grape seed extract on RBL – 2H3 cells. They investigated the effect of grape seed extract on the activation and degranulation of these cells118.
The broadly utilized cell model for molecular and practical examination of intestinal epithelium is Human colon adeno carcinoma cell line (Caco-2 cells). These are profoundly spellbound with a well – shaped brush border and tight intersection119. Eun-Ju Lee et al. researched the inhibitory impacts of quercetin and kaempferol on the concealment of hypersensitive reactions mediated by IgE in RBL – 2H3 and Caco-2 cells lines. The outcomes were showed that certain flavonols have anti – oxidant and anti – inflammatory activities, hindered the secretion of sensitive mediators in RBL-2H3 cells and terminated the expression of CD23 mRNA and mitogen – activated protein kinase (p38 MAPK) enactment in IL – 4 stimulated Caco – 2 cells 120. Obviously, human basophil cells, mast cells, and Caco – 2 cells, RBL – 2H3 cells were commonly used in food sensitivity assessment.
In vivo studies
Animal models are also utilized as useful asset in food sensitivities to clear out the chance of troublesome, life- threatening activities and ethical impediments in clinical trials. This has been useful in permitting more fast and broad examinations to the mechanism behind the hypersensitive pathway, which helps to answer some of the troublesome inquiries still associating with the food sensitivity pandemic88.
Laure Castan et al. explained the In vivo and ex vivo methodological endpoints utilized in murine food sensitivity models. The methods relied on the measurement of activity, anaphylaxis, physiological as well as skin response. But still, there is a need to set up validated and effective methods to assist the researchers working with animal model of food allergy121. Similarly, Babu Gonipeta and colleagues concluded the mechanism, sensitization and elicitation reactions of food allergens in both humans and mice is partially understood and therefore further research is mandatory 122.
Animal models are mainly categorised into two: small animal models and large animal models. Murine species (like BALB/ c, C3H/HeJ, DBA/2, and C57/BL6), rodents (with the Brown Norway (BN) strain, Hooded Lister, Wistar, and Piebald Virol Glaxo (PVG)) rats, and guinea pig were considered as small animal models, while dogs, pigs and sheep are the significant instances of enormous animal models that have been reported in food sensitivity 88,123.
Commonly, mice are the important laboratory animal used to examine the advancement of numerous sicknesses, because of their breeding cycle, size, and sensible housekeeping when compared to larger models124,125. Literature reports suggest that different murine species have the ability to deliver IgE and additionally IgG1 anaphylactic antibodies126. One of the challenges involved in developing murine models of food sensitivity is the propensity of the immune system to form oral tolerance to ingested antigens127,128. As an option in contrast to mice Brown Norway (BN) had moderate size, and strong IgE antibody responses, therefore, the screening of serum specific antibody reactions inside individual animals can be done effectively. Compared with mice, another advantage of this model is that test antigen without adjuvant can be conveyed day by day over a time of weeks129.
Alternatively dogs are spontaneously susceptible to sensitivities, when compared with murine models, and it is a decent animal variety for assessing food hypersensitivity. In any case, it is difficult to utilize dogs as model for safety assessment. Moreover, their maintenance is expensive, predetermined number of strains, more noteworthy inter – animal concerning rodents, and lack of availability of immunological reagents. Similarly, this requires long dosing time for sensitization130,129.
Published reports suggest that there is a momentous advancement with the use of animal models has been made a better comprehension of the essential mechanisms of food hypersensitivity and the allergen refinement. Considering the current state of food allergy animal model, a single model cannot be satisfactory for all research purposes. The objectives, experiment design, data interpretation are the factors which determine the selection of animal model for experimental purposes. For example, murine models are acceptable for determining the food allergy mechanism, alternatively, swine or dog is acceptable for the studies with a focus on clinical symptoms of allergic disease88.
Food allergy assessment based on clinical trials
Now days, food allergy assessment have mainly pointed on the better diagnosis and treatment of reactions131. Stefano Luccioli reviewed the guidelines for food allergy assessment and its treatment based on clinical aspects. His review suggested that, it is necessary to use a better tool for the management of food allergenicity in order to reduce the risk in human beings132.
Similarlly, F.Estelle. R. et al. summarize the key points, which are underlying in the anaphylactic management of food allergy. Based on research articles, they validated the clinical strategies involved in food induced anaphylaxis. The proposed review documented that, there is a need to develop a method for allergen specific inhibitors, which will help to reduce the clinical risk of food allergenicity, and hence its management can be done easily133.
ThaKarrin Hoffman et al. studied the applications of molecular tools in food allergy. They validated allergen specific tests associated with food allergy, which will enable easy diagnosis.
Skin prick testing (SPK)
Serum IgE testing
Basophil activation test
Atopy patch testing (APT)
Immunotherapy
Skin prick test (SPK)
Skin prick testing (SPK) widely used in plant food allergens (fresh fruits, vegetables, and nuts). In line with, several studies have been performed on the plant sources. S. Bolhar et al. carried out these test with apple extracts, and the results showed that, this method is highly reproducible and reliable for assessing food allergy134. Likewise, Garcia et al. carried out SPKs in peach extracts135. Peeters and his coworker’s isolated natural allergens from pea nut are Ara h 1, Ara h 2, Ara h 3, and Ara h 6, and are applied in SPK. Recently, Kollman et al. practiced a purified recombinant Bet v 1, Mal d 1 (birch dust allergen) and birch pollen extract (BPE) in skin prick testing136. Literature reviews reported that SPK is an easy and reproducible method for the assessment of plant food allergens.
Serum IgE testing
Serum IgE testing is the currently used, extract based strategy, and this is an in vitro testing for specific IgE antibodies. Research articles shows that this may be a predictive method due to the advanced knowledge and increased applications in molecular biology 137.
Basophil activation tests (BATs)
This test utilizes entire blood to recognize the capacity of allergens to activate basophils. To distinguish the expression of basophil activation markers (CD63, CD203c) after stimulation with allergens, flow cytometry are used. This reflects cell degranulation, which confirms that CD63 is a key biomarker for hypersensitive responses. Furthermore, this test can also be used in immunotherapy to reflect the desensitized condition of the person138.
Others
Likewise, cell tests are accessible to get out the presence of patients with antigen specific IgE sera, and their ability to enact basophils. The test is mainly centered on the measurement of cell activation accompanied with determination of histamine or sulfidoleukotriene release139. Atopy patch testing is rarely used in clinical studies140.
Ru‑Xin Foong and co – workers led a pilot study to assess the clinical identification of sensitivity in atopic children using a microarray technique in addition with SPK and serum IgE testing. Their findings showed that serum specific IgE testing produces more positive results rather than others141. Consequently, the risk of allergic reactions cannot be effectively managed without understanding the associated risk factors. This can be reduced through the confined diets.
Keet et al. proposed another theory that how to forestall food hypersensitivity in most ideal manner. They explored the advances in food sensitivity that was distributed in 2017 and beyond. The review highlights the published standardized treatment for food hypersensitivity. Finally, concluding that there was an increased attention to non – IgE-mediated food sensitivities142.
Impacts of food preparing techniques on food allergens
Currently, food exposed to various kinds of processing treatments like conventional methods to enhance the shelf life of the food, by inactivating the toxins. It has been mentioned that the processing of foods can also alter the stability of allergenic compounds. This is mainly due to the alteration of amino acids or small linear stretch that occurs in the epitopes. Now days, the clinical symptoms associated with the nature of allergenic epitopes and its severity is not well clear. By understanding these features, the targeted allergen can easily easily be eliminated143.
Food processing affects physical, chemical, and biochemical changes of food, which leads to the alteration of different parts including protein and allergenicity of the particular protein epitope. During this process, new epitopes are formed, referred to as ‘neoallergens’144.
Conventional food processing methods are classified mainly into two types: thermal and non – thermal (figure: 4)145. Sathe ,et al., and vanga et al., explained the foood processing methods and the effects of allergens during these processes.
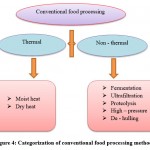 |
Figure 4: Categorization of conventional food processing methods. |
Evidently, food processing aids to inactivate or removes the epitopes present on allergens. Based on the quality and acceptability of food products, enzymatic hydrolysis results in unwanted changes in the food structure. Consequently, to develop robust, dependable, sensitive, and exact allergen identification techniques is the crucial thing in the field of food sensitivity.
Modern trends in food allergy
Modern trends in food hypersensitivity are primarily connected with the utilization of new strategies in analysis and detection of food allergens. Rita c. alves et al. reviewed the latest detection method for food allergens. They mainly focussed on the use of biosensors as tool for food allergen detection. It has been mentioned that immunological tests and the DNA – based measures, mass spectrometry are the classical available methods used for the confirmation of food allergens. Compared to those tools, biosensors have innovative, sensitive, specific, natural friendly, less expensive and quick methods (particularly when computerized as well as on-going investigation). Therefore, this method easily replaces the earlier ones 146.
Various sorts of biosensors are created, and they set for various objectives in food safety assessment. Literature reviews suggest that for the purpose of food allergen detection, three major groups of biosensors are used, namely: optical, electrochemical, and piezoelectric biosensors147. Immunosensors are the identification element used here. The allergenic proteins are immobilized on the outside of these gadgets, and the coupling action between the particles can be estimated by utilizing transducers. General schematic diagram of biosensors is given in the figure 5146.
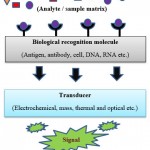 |
Figure 5: Working plan of a biosensor. |
Stefanie and colleagues developed the sensitive and specific primers used for the identification of soy allergens (Glycine max) with Loop – mediated isothermal amplification (LAMP). This is simple and rapid detection involved in the DNA – based assays, mainly applicable in the manufacturing of foods. Notably in field – like applications and on – site screening. The study suggests that ORF160b gene was exceptionally explicit for the soybean. Furthermore, LAMP joined with LFD (Lateral Flow Dipstick) -like detection facilitates a simple, highly selective and sensitive recognition of the soybean without the requirement for costly scientific equipment 148.
Overall, additional method validations like to resolve the limit of detection and the performance testing versus established immunoassays for different kind of food matrices are needed for the better detection of allergens. As a next step, the effect of food handling strategies on recognition of allergens and the enhancement of this protocol is need to be validated.
Biomarkers in food allergy
Food allergy is a complex and diverse disease. Therefore it is essential to develop specific and sensitive markers for its proper management. However, many progresses have been made in this way, but further ways are needed for the better comprehension of the reason and system of food sensitivity, giving a remedial methodology focused to the patient.
Currently, biomarkers are used in food allergy, especially on investigations related with allergen immunotherapies (AITs) including epicutaneous immunotherapy (EPIT), sublingual immunotherapy (SLIT), and oral immunotherapy (OIT). The studies associated with reliable biomarkers to foresee treatment results are extremely low in number. Therapeutic benefit of AIT (Investigational allergen immunotherapies) includes different cell types like basophils, T cells, mast cells and B cells. LaKeya C. Hardy and colleagues reviewed biomarkers that are at presently being researched for AIT138.
Numerous endeavours have been created to describe reliable biomarkers in order to recognize specific endotypes and phenotypes in food hypersensitivity. Eventually, sIgE/total IgE ratios, T cell tests, and Specific IgE (sIgE) tests are only a few that have been examined. Muraro & Arasi summarizes the currently using biomarkers in food hypersensitivity149.
Various in vitro biomarkers have been assessed to evaluate clinical viability of allergen immunotherapy. Recognizing singular patient responders and nonresponders are the primary issue behind with this concept. Several intermediate steps like the degree, dose, the circumstance of allergen introduction corresponding to manifestations, and the utilization of rescue medication, all of which may jumble the connection between IgE – Fab and clinical indications and reaction to immunotherapy150. However many progress has been made in the finding and management of food hypersensitivity, the future strategy seeks the underlying immunologic mechanism and tolerance.
Conclusion
This review summarizes the basic key objectives regarding to food hypersensitivity. It is an anomalous invulnerable reaction to food. The indications of the unfavorably susceptible response may extend from gentle to extreme, and is regularly happens inside minutes to a few hours of presentation. By understanding about particular methods of extraction and optimisation of those allergens from common food source improves the diagnosis of its allergenicity. The extraction, purification, and characterization of food allergens from various sources are simultaneously reviewed. The improvement of animal models to test different food hypersensitivities has been gainful for more quick and broad examination about the component behind the unfavorably susceptible pathway. Researchers had continued interest on experimental models (animal as well as plant), in order to explain the mechanism of food induced allergy. Therefore, currently available and existing models of food allergy are briefly discussed. Now days, allergen specific inhibition is a great challenge faced by researchers. It is a key problem involved in food allergy. So, there is a need to look at the current techniques for the evaluation of clinical finding of food hypersensitivity. The effect of food handling techniques on the recognition of allergens is likewise significantly affected. Optimisation of this objective is the key element regarding to the future aspect of food allergen detection. The clarification of these issues will help the researchers to design the experiments with various models (both in vivo and in vitro), so as to assist further findings of food allergy.
Acknowledgement
The authors are would like to thank to the Faculties, Researchers and Students of the Department of Life Sciences, University of Calicut.
Conflict of Interest
There is no conflict of interest.
Funding sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Faisal M, Vasiljevic T, Donkor ON. A review on methodologies for extraction , identification and quantification of allergenic proteins in prawns. Food Res Int. 2019;121(March):307-318. doi:10.1016/j.foodres.2019.03.040
CrossRef - Sicherer SH, Sampson HA. Food allergy. J Allergy Clin Immunol. 2010;125(2 SUPPL. 2):S116-S125. doi:10.1016/j.jaci.2009.08.028
CrossRef - Savage J, Sicherer S, Wood R. The Natural History of Food Allergy. J Allergy Clin Immunol Pract. 2016;4(2):196-203. doi:10.1016/j.jaip.2015.11.024
CrossRef - Sicherer SH, Sampson HA. Peanut allergy: Emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120(3):491-503. doi:10.1016/j.jaci.2007.07.015
CrossRef - Branum AM, Lukacs SL. Food allergy among United States children: Trends in prevalence and hospitalizations. Food Allergy Overv Child Heal Issues. 2010;(10):31-39.
- Krishna MT, Mahesh PA, Vedanthan P, Moitra S, Mehta V, Christopher DJ. An appraisal of allergic disorders in India and an urgent call for action. World Allergy Organ J. 2020;13(7):100446. doi:10.1016/j.waojou.2020.100446
CrossRef - Li J, Ogorodova LM, Mahesh PA, et al. Comparative Study of Food Allergies in Children from China, India, and Russia: The EuroPrevall-INCO Surveys. J Allergy Clin Immunol Pract. 2020;8(4):1349-1358.e16. doi:10.1016/j.jaip.2019.11.042
CrossRef - Sicherer SH, Wood RA. Advances in diagnosing peanut allergy. J Allergy Clin Immunol Pract. 2013;1(1):1-13. doi:10.1016/j.jaip.2012.10.004
CrossRef - L’Hocine L, Pitre M. Quantitative and qualitative optimization of allergen extraction from peanut and selected tree nuts. Part 1. Screening of optimal extraction conditions using a D-optimal experimental design. Food Chem. 2016;194:780-786. doi:10.1016/j.foodchem.2015.08.031
CrossRef - Benhamou AH, Caubet JC, Eigenmann PA, et al. State of the art and new horizons in the diagnosis and management of egg allergy. Allergy Eur J Allergy Clin Immunol. 2010;65(3):283-289. doi:10.1111/j.1398-9995.2009.02251.x
CrossRef - Taylor SL. Molluscan Shellfish Allergy. Adv Food Nutr Res. 2008;54(07):139-177. doi:10.1016/S1043-4526(07)00004-6
CrossRef - Verhoeckx KCM, Vissers YM, Baumert JL, et al. Food processing and allergenicity. Food Chem Toxicol. 2015;80:223-240. doi:10.1016/j.fct.2015.03.005
CrossRef - Weinberger T, Sicherer S. Current perspectives on tree nut allergy: A review. J Asthma Allergy. 2018;11:41-51. doi:10.2147/JAA.S141636
CrossRef - Geiselhart S, Hoffmann-Sommergruber K, Bublin M. Tree nut allergens. Mol Immunol. 2018;100(March):71-81. doi:10.1016/j.molimm.2018.03.011
CrossRef - Matsuo H, Dahlström J, Tanaka A, et al. Sensitivity and specificity of recombinant ω-5 gliadin-specific IgE measurement for the diagnosis of wheat-dependent exercise-induced anaphylaxis. Allergy Eur J Allergy Clin Immunol. 2008;63(2):233-236. doi:10.1111/j.1398-9995.2007.01504.x
CrossRef - Morgan JE, O’Neil CE, Daul CB, Lehrer SB. Species-specific shrimp allergens: RAST and RAST-inhibition studies. J Allergy Clin Immunol. 1989;83(6):1112-1117. doi:10.1016/0091-6749(89)90454-5
CrossRef - Editors C, Chapman JA, Bernstein IL, et al. Food allergy: A practice parameter. Ann Allergy, Asthma Immunol. 2006;96(3 SUPPL. 2):S1-S68. doi:10.1016/S1081-1206(10)60926-X
CrossRef - Maleki SJ, Viquez O, Jacks T, et al. The major peanut allergen, Ara h 2, functions as a trypsin inhibitor, and roasting enhances this function. J Allergy Clin Immunol. 2003;112(1):190-195. doi:10.1067/mai.2003.1551
CrossRef - Niederberger V, Reisinger J, Valent P, et al. Vaccination with genetically modified birch pollen allergens: Immune and clinical effects on oral allergy syndrome. J Allergy Clin Immunol. 2007;119(4):1013-1016. doi:10.1016/j.jaci.2006.12.661
CrossRef - Hoi AY, Ross L, Day J, Buchanan RRC. Immunotherapeutic strategies in antiphospholipid syndrome. Intern Med J. 2017;47(3):250-256. doi:10.1111/imj.13245
CrossRef - Loke P, Koplin J, Beck C, et al. Statewide prevalence of school children at risk of anaphylaxis and rate of adrenaline autoinjector activation in Victorian government schools, Australia. J Allergy Clin Immunol. 2016;138(2):529-535. doi:10.1016/j.jaci.2016.02.014
CrossRef - Jackson KD, Howie LJD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997-2011. NCHS Data Brief. 2013;(121):1-8.
- Hendrix, Abernethy, Sloane, Misuraca & M. 基因的改变NIH Public Access. Bone. 2013;23(1):1-7. doi:10.1038/jid.2014.371
CrossRef - Valenta R, Hochwallner H, Linhart B, Pahr S. Food allergies: The basics. Gastroenterology. 2015;148(6):1120-1131.e4. doi:10.1053/j.gastro.2015.02.006
CrossRef - Lifschitz C, Szajewska H. Cow’s milk allergy: evidence-based diagnosis and management for the practitioner. Eur J Pediatr. 2015;174(2):141-150. doi:10.1007/s00431-014-2422-3
CrossRef - Høst A. Frequency of cow’s milk allergy in childhood. Ann Allergy, Asthma Immunol. 2002;89(6 SUPPL. 1):33-37. doi:10.1016/S1081-1206(10)62120-5
CrossRef - Sicherer SH. Epidemiology of food allergy. J Allergy Clin Immunol. 2011;127(3):594-602. doi:10.1016/j.jaci.2010.11.044
CrossRef - Wal JM. Bovine milk allergenicity. Ann Allergy, Asthma Immunol. 2004;93(5 SUPPL.):S2-S11. doi:10.1016/S1081-1206(10)61726-7
CrossRef - Fiocchi A, Brozek J, Schünemann H, et al. World allergy organization (WAO) diagnosis and rationale for action against cow’s milk allergy (DRACMA) guidelines. Pediatr Allergy Immunol. 2010;21(SUPPL. 21):1-125. doi:10.1111/j.1399-3038.2010.01068.x
CrossRef - Luyt D, Ball H, Makwana N, et al. BSACI guideline for the diagnosis and management of cow’s milk allergy. Clin Exp Allergy. 2014;44(5):642-672. doi:10.1111/cea.12302
CrossRef - Sicherer SH, Sampson HA. Food allergy: Epidemiology, pathogenesis, diagnosis, and treatment. J Allergy Clin Immunol. 2014;133(2):291-307.e5. doi:10.1016/j.jaci.2013.11.020
CrossRef - Boyce JA. Guidelines for the diagnosis and management of food allergy in the United States: Report of the NIAID-sponsored expert panel. J Allergy Clin Immunol. 2010;126(6 SUPPL.):301-402. doi:10.1016/j.jaci.2010.10.007
CrossRef - Koletzko S, Niggemann B, Arato A, et al. Diagnostic approach and management of cow’s-milk protein allergy in infants and children: Espghan gi committee practical guidelines. J Pediatr Gastroenterol Nutr. 2012;55(2):221-229. doi:10.1097/MPG.0b013e31825c9482
CrossRef - Abrams EM, Sicherer SH. Cow’s milk allergy prevention. Ann Allergy, Asthma Immunol. 2021;127(1):36-41. doi:10.1016/j.anai.2021.01.007
CrossRef - Peters RL, Allen KJ, Dharmage SC, et al. Skin prick test responses and allergen-specific IgE levels as predictors of peanut, egg, and sesame allergy in infants. J Allergy Clin Immunol. 2013;132(4):874-880. doi:10.1016/j.jaci.2013.05.038
CrossRef - Urisu A, Kondo Y, Tsuge I. Hen’s egg allergy. Chem Immunol Allergy. 2015;101:124-130. doi:10.1159/000375416
CrossRef - Anagnostou A. Optimizing Patient Care in Egg Allergy Diagnosis and Treatment. Published online 2021:621-628.
CrossRef - Mine Y, Yang M. Recent advances in the understanding of egg allergens: Basic, industrial, and clinical perspectives. J Agric Food Chem. 2008;56(13):4874-4900. doi:10.1021/jf8001153
CrossRef - Sato S, Tachimoto H, Shukuya A, et al. Utility of the peripheral blood basophil histamine release test in the diagnosis of hen’s egg, cow’s milk, and wheat allergy in children. Int Arch Allergy Immunol. 2011;155(SUPPL. 1):96-103. doi:10.1159/000327490
CrossRef - Urisu A, Tanaka K, Ogura K, et al. New approach for improving the safety of oral immunotherapy for food allergy. Clin Exp Allergy Rev. 2012;12(SUPPL.N2):25-28. doi:10.1111/j.1472-9733.2012.01167.x
CrossRef - Shaker M, Greenhawt M. Peanut allergy: Burden of illness. Allergy Asthma Proc. 2019;40(5):290-294. doi:10.2500/aap.2019.40.4240
CrossRef - Palladino C, Breiteneder H. Peanut allergens. Mol Immunol. 2018;100(March):58-70. doi:10.1016/j.molimm.2018.04.005
CrossRef - Dunwell JM, Purvis A, Khuri S. Cupins: The most functionally diverse protein superfamily? Phytochemistry. 2004;65(1):7-17. doi:10.1016/j.phytochem.2003.08.016
CrossRef - Leickly FE, Kloepfer KM, Slaven JE, Vitalpur G. Peanut Allergy: An Epidemiologic Analysis of a Large Database. J Pediatr. 2018;192:223-228.e1. doi:10.1016/j.jpeds.2017.09.026
CrossRef - Beyer K, Morrow E, Li XM, et al. Effects of cooking methods on peanut allergenicity. J Allergy Clin Immunol. 2001;107(6):1077-1081. doi:10.1067/mai.2001.115480
CrossRef - Bruton K, Spill P, Chu DK, Waserman S, Jordana M. Peanut allergy: Beyond the oral immunotherapy plateau. Clin Transl Allergy. 2021;11(6):2-7. doi:10.1002/clt2.12046
CrossRef - Clark AT, Ewan PW. The development and progression of allergy to multiple nuts at different ages. Pediatr Allergy Immunol. 2005;16(6):507-511. doi:10.1111/j.1399-3038.2005.00310.x
CrossRef - Bezerra M, Ribeiro M, Igrejas G. An Updated Overview of Almond Allergens. 2021.
CrossRef - Kuźmiński A, Przybyszewski M, Przybyszewska J, Ukleja-Sokołowska N, Pałgan K, Bartuzi Z. Tree nut allergy. Postep Dermatologii i Alergol. 2021;38(4):544-549. doi:10.5114/ada.2021.108894
CrossRef - Price A, Ramachandran S, Smith GP, Stevenson ML, Pomeranz MK, Cohen DE. Oral allergy syndrome (Pollen-food allergy syndrome). Dermatitis. 2015;26(2):78-88. doi:10.1097/DER.0000000000000087
CrossRef - Burks AW, Jones SM, Boyce JA, et al. NIAID-sponsored 2010 guidelines for managing food allergy: Applications in the pediatric population. Pediatrics. 2011;128(5):955-965. doi:10.1542/peds.2011-0539
CrossRef - Sampsopiin HA, Aceves S, Bock SA, et al. Food allergy: A practice parameter update – 2014. J Allergy Clin Immunol. 2014;134(5):1016-1025.e43. doi:10.1016/j.jaci.2014.05.013
CrossRef - El Mecherfi KE, Saidi D, Kheroua O, et al. Food allergy. Part 1: immunopathogenesis and clinical disorders. Eur food Res Technol. 2011;233(5):717-728. doi:10.1016/j.smallrumres.2006.09.016
CrossRef - Ogawa T, Bando N, Tuji H, Nishikawa K, Kitamura K. α-Subunit of β-Conglycinin, an Allergenic Protein Recognized by IgE Antibodies of Soybean-sensitive Patients with Atopic Dermatitis. Biosci Biotechnol Biochem. 1995;59(5):831-833. doi:10.1271/bbb.59.831
CrossRef - Beardslee TA, Zeece MG, Sarath G, Markwell JP. Soybean glycinin G1 acidic chain shares IgE epitopes with peanut allergen Ara h 3. Int Arch Allergy Immunol. 2000;123(4):299-307. doi:10.1159/000053642
CrossRef - Helm RM, Cockrell G, Connaughton C, et al. A Soybean G2 Glycinin Allergen. Int Arch Allergy Immunol. 2000;123(3):213-219. doi:10.1159/000024446
CrossRef - Helm RM, Cockrell G, Herman E, Burks AW, Sampson HA, Bannon GA. Cellular and molecular characterization of a major soybean allergen. Int Arch Allergy Immunol. 1998;117(1):29-37. doi:10.1159/000023987
CrossRef - Paper O. nIdentification of IgE-Binding Proteins i. 2001;0919:218-225.
CrossRef - Kattan JD, Cocco RR, Järvinen KM. Milk and Soy Allergy. Pediatr Clin North Am. 2011;58(2):407-426. doi:10.1016/j.pcl.2011.02.005
CrossRef - Mäkelä MJ, Eriksson C, Kotaniemi-Syrjänen A, et al. Wheat allergy in children – new tools for diagnostics. Clin Exp Allergy. 2014;44(11):1420-1430. doi:10.1111/cea.12393
CrossRef - Thomas B Osborne. The vegetable proteins. In: The Vegetable Proteins. UK: Longmans, Green and Co.; 1924:154.
- Ricci G, Andreozzi L, Cipriani F, Giannetti A, Gallucci M, Caffarelli C. Wheat allergy in children: A comprehensive update. Med. 2019;55(7):1-11. doi:10.3390/medicina55070400
CrossRef - Inomata N. Wheat allergy. Curr Opin Allergy Clin Immunol. 2009;9(3):238-243. doi:10.1097/ACI.0b013e32832aa5bc
CrossRef - Nowak-Węgrzyn A, Wood RA, Nadeau KC, et al. Multicenter, randomized, double-blind, placebo-controlled clinical trial of vital wheat gluten oral immunotherapy. J Allergy Clin Immunol. 2019;143(2):651-661.e9. doi:10.1016/j.jaci.2018.08.041
CrossRef - Rodríguez Del Río P, Díaz-Perales A, Sanchez-García S, et al. Oral immunotherapy in children with IgE-mediated wheat allergy: Outcome and molecular changes. J Investig Allergol Clin Immunol. 2014;24(4):240-248
- Sato S, Utsunomiya T, Imai T, et al. Wheat oral immunotherapy for wheat-induced anaphylaxis. J Allergy Clin Immunol. 2015;136(4):1131-1133.e7. doi:10.1016/j.jaci.2015.07.019
CrossRef - Khayatzadeh A, Gharaghozlou M, Ebisawa M, Shoormasti RS, Movahedi M. A safe and effective method for wheat oral immunotherapy. Iran J Allergy, Asthma Immunol. 2016;15(6):525-535.
- Rekabi M. 2017-Evaluation of a new protocol for wheat desensitization. 2017;9:637-645.
CrossRef - Restani P, Ballabio C, Tripodi S, Fiocchi A. Meat allergy. Curr Opin Allergy Clin Immunol. 2009;9(3):265-269. doi:10.1097/ACI.0b013e32832aef3d
CrossRef - Wilson JM, Platts-Mills TAE. Meat allergy and allergens. Mol Immunol. 2018;100(March):107-112. doi:10.1016/j.molimm.2018.03.018
CrossRef - Fiocchi A, Restani P, Riva E, et al. Meat allergy: Ii – effects of food processing and enzymatic digestion on the allergenicity of bovine and ovine meats. J Am Coll Nutr. 1995;14(3):245-250. doi:10.1080/07315724.1995.10718503
CrossRef - Fiocchi A, Bouygue GR, Sarratud T, Terracciano L, Martelli A, Restani P. Clinical tolerance of processed foods. Ann Allergy, Asthma Immunol. 2004;93(5 SUPPL.):S38-S46. doi:10.1016/S1081-1206(10)61731-0
CrossRef - Çelebioğlu E, Akarsu A, Şahiner ÜM. Ige-mediated food allergy throughout life. Turkish J Med Sci. 2021;51(1):49-60. doi:10.3906/sag-2006-95
CrossRef - Hassan AKG, Venkatesh YP. An overview of fruit allergy and the causative allergens. Eur Ann Allergy Clin Immunol. 2015;47(6):180-187.
- Popescu F-D. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015;5(2):31. doi:10.5662/wjm.v5.i2.31
CrossRef - Kim JW, Lee JH, Hwang BY, et al. Morin inhibits Fyn kinase in mast cells and IgE-mediated type I hypersensitivity response in vivo. Biochem Pharmacol. 2009;77(9):1506-1512. doi:10.1016/j.bcp.2009.01.019
CrossRef - Sampson HA, O’Mahony L, Burks AW, Plaut M, Lack G, Akdis CA. Mechanisms of food allergy. J Allergy Clin Immunol. 2018;141(1):11-19. doi:10.1016/j.jaci.2017.11.005
CrossRef - Teng P, Bateman NW, Darcy KM, et al. Guidelines of Care for the Management of Atopic Dermatitis Part 4: Prevention of Disease Flares and Use of Adjunctive Therapies and Approaches. Gynecol Oncol. 2015;136(3):554-561. doi:10.1016/ j.ygyno.2014. 12.035.Pharmacologic
CrossRef - Spergel JM. Nonimmunoglobulin E-mediated immune reactions to foods. Allergy, Asthma Clin Immunol. 2006;2(2):78-85. doi:10.2310/7480.2006.00009
CrossRef - Zuo L, Rothenberg ME. Gastrointestinal Eosinophilia. Immunol Allergy Clin North Am. 2007;27(3):443-455. doi:10.1016/j.iac.2007.06.002
CrossRef - Simon D, Cianferoni A, Spergel JM, et al. Eosinophilic esophagitis is characterized by a non-IgE-mediated food hypersensitivity. Allergy Eur J Allergy Clin Immunol. 2016;71(5):611-620. doi:10.1111/all.12846
CrossRef - Das C Hansen KC and Tyler JK LMS. 乳鼠心肌提取 HHS Public Access. Physiol Behav. 2017;176(3):139-148. doi:10.1016/j.physbeh.2017.03.040
CrossRef - Cianferoni A. Non–IgE-mediated anaphylaxis. J Allergy Clin Immunol. 2021;147(4):1123-1131. doi:10.1016/j.jaci.2021.02.012
CrossRef - Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113(5):805-819. doi:10.1016/j.jaci.2004.03.014
CrossRef - Bannon GA. What makes a food protein an allergen? Curr Allergy Asthma Rep. 2004;4(1):43-46. doi:10.1007/s11882-004-0042-0
CrossRef - Hefle SL, Nordlee JA, Taylor SL. Allergenic foods. Crit Rev Food Sci Nutr. 1996;36(SUPPL.):37-41. doi:10.1080/10408399609527760
CrossRef - Muraro A, Werfel T, Hoffmann-Sommergruber K, et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and management of food allergy. Allergy Eur J Allergy Clin Immunol. 2014;69(8):1008-1025. doi:10.1111/all.12429
CrossRef - McClain S, Bannon GA. Animal models of food allergy: Opportunities and barriers. Curr Allergy Asthma Rep. 2006;6(2):141-144. doi:10.1007/s11882-006-0052-1
CrossRef - Dalal I, Binson I, Reifen R, et al. Food allergy is a matter of geography after all: Sesame as a major cause of severe IgE-mediated food allergic reactions among infants and young children in Israel. Allergy Eur J Allergy Clin Immunol. 2002;57(4):362-365. doi:10.1034/j.1398-9995.2002.1s3412.x
CrossRef - Sánchez-Monge R, Pascual CY, Díaz-Perales A, Fernández-Crespo J, Martín-Esteban M, Salcedo G. Isolation and characterization of relevant allergens from boiled lentils. J Allergy Clin Immunol. 2000;106(5):955-961. doi:10.1067/mai.2000.109912
CrossRef - Astwood JD, Leach JN, Fuchs RL. Stability of Food Allergens to Digestion in Vitro. Nat Biotechnol. 1996;14(10):1269-1273. doi:10.1038/nbt1096-1269
CrossRef - Harrer A, Egger M, Gadermaier G, et al. Characterization of plant food allergens: An overview on physicochemical and immunological techniques. Mol Nutr Food Res. 2010;54(1):93-112. doi:10.1002/mnfr.200900096
CrossRef - Kasera R, Singh AB, Lavasa S, Nagendra K, Arora N. Purification and Immunobiochemical Characterization of a 31 kDa Cross-Reactive Allergen from Phaseolus vulgaris (Kidney Bean). PLoS One. 2013;8(5). doi:10.1371/journal.pone.0063063
CrossRef - Pastorello EA, Trambaioli C. Isolation of food allergens. J Chromatogr B Biomed Sci Appl. 2001;756(1-2):71-84. doi:10.1016/S0378-4347(01)00072-X
CrossRef - Björkstén F, Halmepuro L, Hannuksela M, Lahti A. Extraction and Properties of Apple Allergens. Allergy. 1980;35(8):671-677. doi:10.1111/j.1398-9995.1980.tb02020.x
CrossRef - Vassilopoulou E V., Zuidmeer L, Akkerdaas J, et al. Optimized techniques for the extraction of grape allergens appropriate for in vivo and in vitro testing and diagnosis. Mol Nutr Food Res. 2007;51(3):360-366. doi:10.1002/mnfr.200600194
CrossRef - Shewry PR, Napier JA, Tatham AS. Seed storage proteins: Structures and biosynthesis. Plant Cell. 1995;7(7):945-956. doi:10.1105/tpc.7.7.945
CrossRef - Qi PF, Wei YM, Yue YW, Yan ZH, Zheng YL. Biochemical and molecular characterization of gliadins. Mol Biol. 2006;40(5):713-723. doi:10.1134/S0026893306050050
CrossRef - Sandiford CP, Nieuwenhuijsen MJ, Tee RD, Newman Taylor AJ. Measurement of airborne proteins involved in Bakers’ asthma. Clin Exp Allergy. 1994;24(5):450-456. doi:10.1111/j.1365-2222.1994.tb00933.x
CrossRef - Crespo JF, Pascual C, Helm R, et al. Cross‐reactivity of IgE‐binding components between boiled Atlantic shrimp and German cockroach. Allergy. 1995;50(11):918-924. doi:10.1111/j.1398-9995.1995.tb02499.x
CrossRef - LANGELAND T. A clinical and immunological study of allergy to hen’s egg white: I. A clinical study of egg allergy. Clin Exp Allergy. 1983;13(4):371-382. doi:10.1111/j.1365-2222.1983.tb02611.x
CrossRef - Makinen-Kiljunen S, Sorva R. Bovine β-lactoglobulin levels in hydrolysed protein formulas for infant feeding. Clin Exp Allergy. 1993;23(4):287-291. doi:10.1111/j.1365-2222.1993.tb00324.x
CrossRef - EUFIC Academy. Food Allergens: Methods and Protocols. EUFIC Rev. 2017;1592:1-299. doi:10.1007/978-1-4939-6925-8
CrossRef - Besler M. Determination of allergens in foods. TrAC – Trends Anal Chem. 2001;20(11):662-672. doi:10.1016/S0165-9936(01)00119-4
CrossRef - Peters RL, Krawiec M, Koplin JJ, Santos AF. Update on food allergy. 2021;(December 2020):647-657. doi:10.1111/pai.13443
CrossRef - Ma J, Pavase TR, Li ZX, Lin H. Optimisation of an extraction technique of fish allergens suitable for detection and diagnosis. Czech J Food Sci. 2017;35(1):24-31. doi:10.17221/578/2015-CJFS
CrossRef - Bugajska-Schretter A, Rumpold H, Spitzauer S, et al. Purification, biochemical, and immunological characterisation of a major food allergen: Different immunoglobulin E recognition of the apo- and calcium-bound forms of carp parvalbumin. Gut. 2000;46(5):661-669. doi:10.1136/gut.46.5.661
CrossRef - Sun S, Lopata AL. The role of shellfish proteases in allergic diseases and inflammation. Curr Allergy Clin Immunol. 2010;23(4):174-179.
- Clare Mills EN, Adel-Patient K, Bernard H, et al. Detection and quantification of allergens in foods and minimum eliciting doses in food-Allergic individuals (ThRAll). J AOAC Int. 2019;102(5):1346-1353. doi:10.5740/jaoacint.19-0063
CrossRef - Stefura WP, Graham C, Lotoski L, Hayglass KT. Chapter 7 Improved Methods for Quantifying Human Chemokine and Meso Scale Electrochemiluminescence Assays. 2020:91-114. doi:10.1007/978-1-4939-9591-2
CrossRef - Gasilova N, Girault HH. Bioanalytical methods for food allergy diagnosis, allergen detection and new allergen discovery. Bioanalysis. 2015;7(9):1175-1190. doi:10.4155/bio.15.49
CrossRef - Maeyama K, Hohman RJ, Metzger H, Beaven MA. Quantitative relationships between aggregation of IgE receptors, generation of intracellular signals, and histamine secretion in rat basophilic leukemia (2H3) cells. Enhanced responses with heavy water. J Biol Chem. 1986;261(6):2583-2592.
CrossRef - Passante E, Frankish N. The RBL-2H3 cell line: Its provenance and suitability as a model for the mast cell. Inflamm Res. 2009;58(11):737-745. doi:10.1007/s00011-009-0074-y
CrossRef - Gibbs BF. Basophils as Key Regulators of Allergic Inflammation and Th2-type Immunity. World Allergy Organ J. 2008;1(7):123-128. doi:10.1097/wox.0b013e31817a76fb
CrossRef - Cells L, Kulczycki BYA, Isersky C, Metzger H. ( From the Section on Chemical Immunology , Arthritis and Rheumatism Branch , National Institute of Arthritis , Metabolism and Digestive Diseases , National Institutes of Health , Bethesda , Maryland 20014 ). 1974:600-616.
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6(3):218-230. doi:10.1038/nri1782
CrossRef - Falcone FH, Wan D, Barwary N, Sagi-Eisenberg R. RBL cells as models for in vitro studies of mast cells and basophils. Immunol Rev. 2018;282(1):47-57. doi:10.1111/imr.12628
CrossRef - Chen BH, Hung MH, Chen JYF, et al. Anti-allergic activity of grapeseed extract (GSE) on RBL-2H3 mast cells. Food Chem. 2012;132(2):968-974. doi:10.1016/j.foodchem.2011.11.079
CrossRef - Bischoff SC, Wedemeyer J, Herrmann A, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28(1):1-13. doi:10.1046/j.1365-2559.1996.262309.x
CrossRef - Lee EJ, Ji GE, Sung MK. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm Res. 2010;59(10):847-854. doi:10.1007/s00011-010-0196-2
CrossRef - Castan L, Bøgh KL, Maryniak NZ, et al. Overview of in vivo and ex vivo endpoints in murine food allergy models: Suitable for evaluation of the sensitizing capacity of novel proteins? Allergy Eur J Allergy Clin Immunol. 2020;75(2):289-301. doi:10.1111/all.13943
CrossRef - Gonipeta B, Kim E, Gangur V. Mouse Models of Food Allergy: How Well do They Simulate the Human Disorder? Crit Rev Food Sci Nutr. 2015;55(3):437-452. doi:10.1080/10408398.2012.657807
CrossRef - Leeman WR, Van Den Berg KJ, Houben GF. Transfer of chemicals from feed to animal products: The use of transfer factors in risk assessment. Food Addit Contam. 2007;24(1):1-13. doi:10.1080/02652030600815512
CrossRef - Aldemir H, Bars R, Herouet-Guicheney C. Murine models for evaluating the allergenicity of novel proteins and foods. Regul Toxicol Pharmacol. 2009;54(3 SUPPL.):S52-S57. doi:10.1016/j.yrtph.2008.11.004
CrossRef - Meeusen EN, Snibson KJ, Hirst SJ, Bischof RJ. Sheep as a model species for the study and treatment of human asthma and other respiratory diseases. Drug Discov Today Dis Model. 2009;6(4):101-106. doi:10.1016/j.ddmod.2009.12.002
CrossRef - Helm RM. Food allergy animal models: an overview. Ann N Y Acad Sci. 2002;964:139-150. doi:10.1111/j.1749-6632.2002.tb04139.x
CrossRef - Thang CL, Baurhoo B, Boye JI, Simpson BK, Zhao X. Effects of Lactobacillus rhamnosus GG supplementation on cow’s milk allergy in a mouse model. Allergy, Asthma Clin Immunol. 2011;7(1):1-9. doi:10.1186/1710-1492-7-20
CrossRef - Bailón E, Cueto-Sola M, Utrilla P, et al. A shorter and more specific oral sensitization-based experimental model of food allergy in mice. J Immunol Methods. 2012;381(1-2):41-49. doi:10.1016/j.jim.2012.04.007
CrossRef - Dearman RJ, Kimber I. Animal models of protein allergenicity: Potential benefits, pitfalls and challenges. Clin Exp Allergy. 2009;39(4):458-468. doi:10.1111/j.1365-2222.2008.03194.x
CrossRef - Ladics GS, Knippels LMJ, Penninks AH, Bannon GA, Goodman RE, Herouet-Guicheney C. Review of animal models designed to predict the potential allergenicity of novel proteins in genetically modified crops. Regul Toxicol Pharmacol. 2010;56(2):212-224. doi:10.1016/j.yrtph.2009.09.018
CrossRef - Oriel RC, Wang J. Diagnosis and Management of Food Allergy. Pediatr Clin North Am. 2019;66(5):941-954. doi:10.1016/j.pcl.2019.06.002
CrossRef - Luccioli S. Food allergy guidelines and assessing allergic reaction risks: A regulatory perspective. Curr Opin Allergy Clin Immunol. 2012;12(3):323-330. doi:10.1097/ACI.0b013e3283535aaf
CrossRef - Simons FER, Ardusso LRF, Bilò MB, et al. 2012 Update: World Allergy Organization Guidelines for the assessment and management of anaphylaxis. Curr Opin Allergy Clin Immunol. 2012;12(4):389-399. doi:10.1097/ACI.0b013e328355b7e4
CrossRef - Bolhaar STHP, Van De Weg WE, Van Ree R, et al. In vivo assessment with prick-to-prick testing and double-blind, placebo-controlled food challenge of allergenicity of apple cultivars. J Allergy Clin Immunol. 2005;116(5):1080-1086. doi:10.1016/j.jaci.2005.07.004
CrossRef - García BE, González-Mancebo E, Barber D, et al. Sublingual immunotherapy in peach allergy: Monitoring molecular sensitizations and reactivity to apple fruit and Platanus pollen. J Investig Allergol Clin Immunol. 2010;20(6):514-520.
- Kollmann D, Geroldinger-Simic M, Kinaciyan T, et al. Recombinant Mal d 1 is a reliable diagnostic tool for birch pollen allergen-associated apple allergy. J Allergy Clin Immunol. 2013;132(4):1008-1010. doi:10.1016/j.jaci.2013.05.030
CrossRef - Hoffmann-Sommergruber K, Pfeifer S, Bublin M. Applications of Molecular Diagnostic Testing in Food Allergy. Curr Allergy Asthma Rep. 2015;15(9). doi:10.1007/s11882-015-0557-6
CrossRef - Hardy LKC, Smeekens JM, Kulis MD. Biomarkers in Food Allergy Immunotherapy. Curr Allergy Asthma Rep. 2019;19(12):1-7. doi:10.1007/s11882-019-0894-y
CrossRef - Erdmann SM, Sachs B, Schmidt A, et al. In vitro analysis of birch-pollen-associated food allergy by use of recombinant allergens in the basophil activation test. Int Arch Allergy Immunol. 2005;136(3):230-238. doi:10.1159/000083949
CrossRef - Newberry SJ, Riedl MA, Bravata DM, et al. CLINICIAN ’ S CORNER. 2010;303(18).
- Foong RX, Roberts G, Fox AT, Toit G. Pilot study: Assessing the clinical diagnosis of allergy in atopic children using a microarray assay in addition to skin prick testing and serum specific IgE. Clin Mol Allergy. 2016;14(1):1-7. doi:10.1186/s12948-016-0046-z
CrossRef - Keet CA, Allen KJ. Advances in food allergy in 2017. J Allergy Clin Immunol. 2018;142(6):1719-1729. doi:10.1016/j.jaci.2018.10.020
CrossRef - Sathe SK, Teuber SS, Roux KH. Effects of food processing on the stability of food allergens. Biotechnol Adv. 2005;23(6):423-429. doi:10.1016/j.biotechadv.2005.05.008
CrossRef - Thomas K, Herouet-Guicheney C, Ladics G, et al. Evaluating the effect of food processing on the potential human allergenicity of novel proteins: International workshop report. Food Chem Toxicol. 2007;45(7):1116-1122. doi:10.1016/j.fct.2006.12.016
CrossRef - Vanga SK, Singh A, Raghavan V. Review of conventional and novel food processing methods on food allergens. Crit Rev Food Sci Nutr. 2017;57(10):2077-2094. doi:10.1080/10408398.2015.1045965
CrossRef - Alves RC, Barroso MF, González-García MB, Oliveira MBPP, Delerue-Matos C. New Trends in Food Allergens Detection: Toward Biosensing Strategies. Crit Rev Food Sci Nutr. 2016;56(14):2304-2319. doi:10.1080/10408398.2013.831026
CrossRef - Viswanathan S, Radecka H, Radecki J. Electrochemical biosensors for food analysis. Monatshefte fur Chemie. 2009;140(8):891-899. doi:10.1007/s00706-009-0143-5
CrossRef - Allgöwer SM, Hartmann CA, Holzhauser T. The development of highly specific and sensitive primers for the detection of potentially allergenic soybean (Glycine max) using loop-mediated isothermal amplification combined with lateral flow dipstick (LAMP-LFD). Foods. 2020;9(4). doi:10.3390/foods9040423
CrossRef - Muraro A, Arasi S. Biomarkers in Food Allergy. Curr Allergy Asthma Rep. 2018;18(11). doi:10.1007/s11882-018-0816-4
CrossRef - Shamji MH, James LK, Durham SR. Serum Immunologic Markers for Monitoring Allergen-Specific Immunotherapy. Immunol Allergy Clin North Am. 2011;31(2):311-323. doi:10.1016/j.iac.2011.03.005
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





