Manuscript accepted on : 25-07-2022
Published online on: 26-07-2022
Plagiarism Check: Yes
Reviewed by: Dr. Jurdas SEZIRAHIGA
Second Review by: Dr. Daya Shankar Gautam
Final Approval by: Dr. Fernando José Cebola Lidon
Overview of Molecular Quantification of the BCR-ABL Oncogene in CML Patients
Ali Hazazi1* , Mohammed Albayedh1
, Mohammed Albayedh1 , Fawaz Albloui1
, Fawaz Albloui1 , Sarh Abu Dahish1
, Sarh Abu Dahish1 and Mishal Alsulami2
and Mishal Alsulami2
1Department of Pathology and Laboratory Medicine, Security Forces Hospital Program, Riyadh, Kingdom of Saudi Arabia
2Clinical Laboratory Department, Cytogenetic, and Molecular Genetics Laboratory, Prince Sultan Military Medical City, Riyadh, Saudi Arabia.
Corresponding Author E-mail: ahazazi@sfh.med.sa
DOI : http://dx.doi.org/10.13005/bbra/3021
ABSTRACT:
Chronic myeloid leukemia (CML) is considered a common blood cancers and accounts for approximately 15–20% of the total cases of leukemia. Recent studies indicated that above 95% of patients suffering of CML have been found with a distinctive Philadelphia chromosome that originates from a mutual translocation between both arms of chromosomes 9 and 22. During this mutation the translocation of the ABL gene located on chromosome 9 get transferred to the breakpoint cluster region (BCR) of chromosome 22 as an effect of a joined BCR-ABL gene. Furthermore, BCR-ABL oncogene is characteristically found in CML, causing cells to divide uncontrollably and inducing severe consequences among CML patients. In line with this, applying quantification technique of the BCR-ABL gene using molecular approaches is crucial for patient controlling, initiation of the proper treatment, measurement of response to therapy, and prediction of relapse. Of greater significance, molecular assay and monitoring of the BCR-ABL gene in CML using quantitative RT-PCR provides physicians with essential diagnostic and prognostic information.
KEYWORDS: BCR-ABL; CML; leukemia; Genetics
Download this article as:| Copy the following to cite this article: Hazazi A, Albayedh M, Albloui F, Dahish S. A, Alsulami M. Overview of Molecular Quantification of the BCR-ABL Oncogene in CML Patients. Biosci Biotech Res Asia 2022;19(3). |
| Copy the following to cite this URL: Hazazi A, Albayedh M, Albloui F, Dahish S. A, Alsulami M. Overview of Molecular Quantification of the BCR-ABL Oncogene in CML Patients. Biosci Biotech Res Asia 2022;19(3). Available from: https://bit.ly/3z2Pdf0 |
Leukemia
Leukemia is a type of cancer that takes place when the bone marrow start to release an enormous number of atypical white blood cells that accumulate in the blood and in different organs. Two methods of leukemia classification are used: the first is by its speed of progression (acute or chronic), and the second is by the type of white blood cells involved (lymphoid or myeloid) 1. Myeloid leukemias start in neutrophils and monocytes, which are bacterial and fungal fighters, while the progression of lymphocytic leukemias occurs in natural killer cells, B lymphocytes, and T lymphocytes, which are important in the production of antibodies that fight infections 2.
The main types of leukemia are chronic myeloid leukemia (CML), acute lymphocytic leukemia, chronic lymphocytic leukemia and acute myeloid leukemia. Common leukemia symptoms include fever, weakness, frequent infections, and enlarged liver, spleen, and lymph nodes 2. Leukemia is usually diagnosed by physical examination followed by blood count and bone marrow biopsies in the early stages. Recently, Molecular analysis is playing a significant role where RT-PCR competent to identify leukemia-specific gene or mutation 3.
There are no clear causes for the development of different types of leukemia; however, most cases are caused by genetic alterations in the DNA that trigger oncogenes or disable the function of tumor suppressor genes 4. In addition, some genetic disorders and chromosomal abnormalities, such as Down Syndrome, can be considered risk factors. Other environmental factors include radiation, chemicals, and viruses 5.
CML is styled by abnormal growth and increase of myeloid cells in the blood. It is a class of myeloproliferative neoplasm linked to chromosomal translocation identified as the “Philadelphia chromosome” 6, which involves chromosomes 9 and 22 switching places, and leads to fusion between part of the breakpoint cluster region (BCR) of chromosome 22 and the ABL gene on chromosome 9 7. This increases the speed of cell division and activates the proteins responsible for the regulation of the cell cycle 8. In addition, BCR-ABL fusion affects DNA repair and decreases genome stability, which results in other genomic abnormalities that speed up cell division 9.
Current WHO criteria for the diagnosis and classification of CML
World health organization classified the CML into three main significantly prognostic clinical phases before tyrosine kinase inhibitor (TKI) treatment. The main and essential pathological characteristics of Philadelphia chromosome (+) CML in the WHO 2016 classification are defined as the following three phases 10.
Chronic phase CML
Chronic phase CML defined as a myelo-proliferative neoplasm which is characterized by chromosomal translocation (t9;22) (q34.1;q11.2), that results in the formation of BCR-ABL fusion gene 11. This gene fusion causes the formation of the abnormal chromosome (Philadelphia chromosome), that results in an increase in peripheral blood granulocyte cells and bone marrow myeloid precursors 12.
Accelerated CML phase AP
In this phase, CML cells number is fast growing and symptomatic, including fever, fatigue, splenomegaly, and weight loss 13. The typical bone marrow histopathology for the accelerated CML phase was explained before the TKI-treatment era. After the TKI treatment era, the criteria were adjusted considering the therapy 14. CML cases that show respond to the treatment by TKI-era are characterized by normalization finding of the bone marrow cellular composition after treatment 15.
This phase expresses the presence of BCR-ABL or t(9;22) (q34.1;q11.2) by molecular or chromosomal analysis (karyotyping), together with TKI resistance or genomic-cytogenetic evolution 16. Increasing abnormal white blood cell (WBC) counts occur despite TKI treatment (WBC count more than 10×109/L), thrombocytosis (platelet count more than 1000×109/L), or thrombocytopenia (platelet count less than 100×109/L) unrelated to therapy (between 10 to 19 % blast cells and around 20 % or more basophils) 17.
Blastic phase CML (BP-CML)
The CML blastic phase involves the presence of significantly increase of blastic infiltration, accordance with the typical CML blastic phase clinical presentation 18. Further, the blastic phase shows the presence of BCR-ABL gene or t(9;22) (q34.1;q11.2) by molecular or chromosomal analysis (karyotyping), with genomic-cytogenetic progress or resistance of TKI treatment 19. This involves at least 20 % blast cells presence in the peripheral blood or bone marrow with or without the presence of extra medullary-blastic infiltration in organ or tissue 20.
CML diagnosis
Many patients with CML are asymptomatic when they diagnosed first time. The most common finding of CML is leukocytosis (abnormal high WBC count). Often discovered during routine blood tests (complete blood count (CBC)) or checkups for an unrelated health problem 21. Usually, to discover and diagnose new CML cases, physicians request a variety of routine laboratory tests to examine blood cells and bone marrow 22. A hematopathologist (who specializes in identifying hematologic disorders) reviewing white blood cells morphology and count using a microscope to evaluate the bone marrow and peripheral blood smear 23.
Laboratory tests used to diagnose CML
Complete Blood Count (CBC) with differential
The CBC is a routine hematology test used to measure the number of red blood cells, total white blood cell count, and platelets. Also, CBC measures the amount of hemoglobin in the red blood cells (MCH) and the percentage of red blood cells to the whole blood (HCT) 24. The CBC test includes a differential white cell count to measure the different types and count of white blood cells in peripheral blood sample. Figure 1, demonstrate the presence of blast cells in CML peripheral blood smear. CML patients often have a leukocytosis (greatly increased WBC count), decreased red blood cell count (anemia), and an increase or decrease in platelet count (thrombocytopenia) based on the severity of the disease 25.
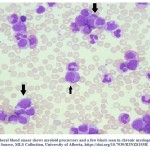 |
Figure 1: Peripheral blood smear shows myeloid precursors and a few blasts seen in chronic myelogenous leukemia. Source, MLS Collection, University of Alberta |
Bone marrow aspiration and biopsy
Bone marrow examinations is a hematopathologic test used to identify abnormalities in bone marrow cells. The bone marrow aspiration and biopsy are taken by the hematologist from the hip bone. Figure 2, shows bone marrow aspiration and the presence of blast cells in CML patient. Both sample aspiration and biopsy are examined by hematopathologist under a microscope to look for abnormal cells (such as blast cells) and indicate chromosomal abnormality and other blood cell changes 26.
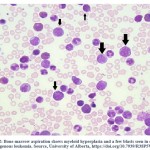 |
Figure 2: Bone marrow aspiration shows myeloid hyperplasia and a few blasts seen in chronic myelogenous leukemia. Source, University of Alberta. |
Cytogenetic analysis
The cytogenetic analysis test is an effective laboratory test that used to involves the study of chromosomal abnormalities in patient using bone marrow aspiration sample 27. Figure 1, illustrate the gene mutation in CML (Philadelphia chromosome). A bone marrow aspiration sample is examined by cytogenetic using a microscope for Philadelphia chromosome detection and any chromosomal changes or abnormalities 27.
 |
Figure 3: Gene mutations in CML case include the fused gene called BCR-ABL and the abnormal Philadelphia chromosome. |
Fluorescence in situ hybridization (FISH)
FISH is a cytogenetic lab test that is used to examine chromosomes and genes in patient cells 28. FISH test is more sensitive and accurate method for detecting CML by identifying the presence of the BCR-ABL gene than standard cytogenetic tests that identify the Philadelphia chromosome 29.
Quantitative polymerase chain reaction (qPCR)
qPCR is considered as an effective technique and actual sensitive method for distinguishing and measuring the quantity of BCR-ABL gene 30. Using bone marrow or blood sample, More further, qPCR technique is able to detect a low amounts of the BCR-ABL gene particularly when Philadelphia chromosome cannot be identified in blood or bone marrow cells in cytogenetic testing 31. The BCR-ABLE qPCR assay is a fundamental tool for aiding in CML diagnosis from the genetic side 32. Likewise, physicians practice BCR-ABLE to assess the ongoing patient situation and subsequently prescribe appropriate medication and the ideal dose 33. Consistently, pre-analysis and analysis for such genetic testing are very sensitive, and not complying with the correct techniques could affect the final outcomes 34.
Processing the sample for qPCR BCR-ABL testing requires extraordinary parameters, such as using an EDTA tube for blood collection in which the anticoagulant substance provides the ideal preservation of cellular components and the blood cells morphology 35. In addition, it is crucial to perform the processing step as soon as the specimens are received in order to avoid any changes to the WBCs that subsequently affect the final yield of extracted DNA 36. Approaching the highest quality standards in terms of processing samples will reflect the eventual result, which will be elaborated on in the patient’s treatment plan 37. Furthermore, the BCR-ABLE assay needs to be carried out by skilled personnel as a vital part of quality 38. Programming the optimal time for blood collection is a vital part of the quality standard, which assists in testing BCR-ABL with no postponement and avoiding subsequent freezing of the patient’s specimens 39. Considering these parameters will eventually support accomplishing the perfect end result, which will lead to prescribing precise medication 40.
Conclusion
Molecular inspection for BCR-ABLE gene is being a crucial method to evaluate patients with CML illness in early phase to avoid inadequate diagnosis. More in depth, The BCR-ABL molecular results provide valuable guidance for starting medication. In addition, BCR-ABL results should be engaged into consideration before proceeding any type of clinical decisions. Likewise, quantitative BCR-ABL testing affords the advantage of detecting small quantity of BCR-ABL oncogene that act to reflect tendencies in tumor burden.
Acknowledgment
We highly acknowledge Dr. Abdulmalik Alsheikh entitle the director of pathology & laboratory medicine for his usual support particularly in term of clinical and scientific research. Further, we appreciate the efforts of health education department for their assistance in providing all needs for our study.
Conflict of Interest
The authors have no conflicts of interest. We declare no conflict of interest, including any financial, personal, or other relationships with other people or organizations, within three years of beginning the submitted work that could inappropriately influence, or be perceived to influence, our work.
Funding Sources
This project has not been funded by any institute, so I declare no funding.
References
- Clarke, R.T., A. Van den Bruel, C. Bankhead, C.D. Mitchell, B. Phillips, and M.J. Thompson, Clinical presentation of childhood leukaemia: a systematic review and meta-analysis. Archives of Disease in Childhood, 2016. 101(10): p. 894-901.
CrossRef - Janssen, J., Schuurhuis, G., Terwijn, M. and Ossenkoppele, G., 2009. Towards Cure of CML: Why We Need to Know More About CML Stem Cells?. Current Stem Cell Research & Therapy, 4(3), pp.224-236.
CrossRef - Döhner, H., E.H. Estey, S. Amadori, F.R. Appelbaum, T. Büchner, A.K. Burnett, H. Dombret, P. Fenaux, D. Grimwade, R.A. Larson, F. Lo-Coco, T. Naoe, D. Niederwieser, G.J. Ossenkoppele, M.A. Sanz, J. Sierra, M.S. Tallman, B. Löwenberg, and C.D. Bloomfield, Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood, 2010. 115(3): p. 453-474.
CrossRef - Hutter, J.J., Childhood Leukemia. Pediatrics in Review, 2010. 31(6): p. 234-241.
CrossRef - Renneville, A., C. Roumier, V. Biggio, O. Nibourel, N. Boissel, P. Fenaux, and C. Preudhomme, Cooperating gene mutations in acute myeloid leukemia: a review of the literature. Leukemia, 2008. 22(5): p. 915-31.
CrossRef - Cheng, J., L. Qu, J. Wang, L. Cheng, and Y. Wang, High expression of FLT3 is a risk factor in leukemia. Molecular medicine reports, 2018. 17(2): p. 2885-2892.
CrossRef - Ley, T.J., L. Ding, M.J. Walter, M.D. McLellan, T. Lamprecht, D.E. Larson, C. Kandoth, J.E. Payton, J. Baty, J. Welch, C.C. Harris, C.F. Lichti, R.R. Townsend, R.S. Fulton, D.J. Dooling, D.C. Koboldt, H. Schmidt, Q. Zhang, J.R. Osborne, L. Lin, M. O’Laughlin, J.F. McMichael, K.D. Delehaunty, S.D. McGrath, L.A. Fulton, V.J. Magrini, T.L. Vickery, J. Hundal, L.L. Cook, J.J. Conyers, G.W. Swift, J.P. Reed, P.A. Alldredge, T. Wylie, J. Walker, J. Kalicki, M.A. Watson, S. Heath, W.D. Shannon, N. Varghese, R. Nagarajan, P. Westervelt, M.H. Tomasson, D.C. Link, T.A. Graubert, J.F. DiPersio, E.R. Mardis, and R.K. Wilson, DNMT3A mutations in acute myeloid leukemia. The New England journal of medicine, 2010. 363(25): p. 2424-2433.
CrossRef - Kiyoi, H., T. Naoe, Y. Nakano, S. Yokota, S. Minami, S. Miyawaki, N. Asou, K. Kuriyama, I. Jinnai, C. Shimazaki, H. Akiyama, K. Saito, H. Oh, T. Motoji, E. Omoto, H. Saito, R. Ohno, and R. Ueda, Prognostic Implication of <em>FLT3</em> and N-<em>RAS</em> Gene Mutations in Acute Myeloid Leukemia. Blood, 1999. 93(9): p. 3074-3080.
- Hunger, S.P. and C.G. Mullighan, Acute Lymphoblastic Leukemia in Children. N Engl J Med, 2015. 373(16): p. 1541-52.
CrossRef - Vardiman, J.W., J. Thiele, D.A. Arber, R.D. Brunning, M.J. Borowitz, A. Porwit, N.L. Harris, M.M. Le Beau, E. Hellström-Lindberg, A. Tefferi, and C.D. Bloomfield, The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood, 2009. 114(5): p. 937-951.
CrossRef - Faderl, S., M. Talpaz, Z. Estrov, and H.M. Kantarjian, Chronic Myelogenous Leukemia: Biology and Therapy. Annals of Internal Medicine, 1999. 131(3): p. 207-219.
CrossRef - Hehlmann, R., A. Hochhaus, and M. Baccarani, Chronic myeloid leukaemia. The Lancet, 2007. 370(9584): p. 342-350.
CrossRef - Vogelstein, B., D. Lane, and A.J. Levine, Surfing the p53 network. Nature, 2000. 408(6810): p. 307-310.
CrossRef - Amin, N.A. and S.N. Malek, Gene mutations in chronic lymphocytic leukemia. Seminars in Oncology, 2016. 43(2): p. 215-221.
CrossRef - Arber DA, Orazi A, Hasserjian R, Thiele J, Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M, Vardiman JW. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood. 2016;127:2391–2405.
CrossRef - Nicolini FM, Etienne G, Huguet F, Guerci-Bresler A, Charbonnier A, Escoffre-Barbe M, Dubruille V, Johnson-Ansah H, Legros L, Coiteux V, Cony-Makhoul P, Lenain P, Roy L, Rousselot P, Guyotat D, Lanotto JC, Gardembas M, Larosa F, Caillot D, Turlure P, Courby S, Quittet P, Hermet E, Ame S, Lapusan S, Verane Schwiertz, Pharm D, Morisset S, Etienne M, Rea D, Dulucq S, Mahon FX. Nilotinib Versus Nilotinib Combined to Pegylated-Interferon Alfa 2a in First-Line Chronic Phase Chronic Myelogenous Leukemia Patients. Interim Analysis of a Phase III Trial. Blood. 2017;130(Suppl 1):899.
CrossRef - Saglio G, Jabbour E. First-line therapy for chronic phase CML: selecting the optimal BCR-ABL1-targeted TKI. Leuk Lymphoma. 2018;59:1523–1538.
CrossRef - White DL, Dang P, Engler J, et al. Functional Activity of the OCT-1 Protein Is Predictive of Long-Term Outcome in Patients With Chronic-Phase Chronic Myeloid Leukemia Treated With Imatinib. Journal of Clinical Oncology. 2010;28(16):2761–2767.
CrossRef - Giannoudis A, Davies A, Lucas CM, Harris RJ, Pirmohamed M, Clark RE. Effective dasatinib uptake may occur without human organic cation transporter 1 (hOCT1): implications for the treatment of imatinib-resistant chronic myeloid leukemia. Blood. 2008;112(8):3348–3354.
CrossRef - Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa Writing Committee for the Collaborative CML Prognostic Factors Project Group. Journal of the National Cancer Institute. 1998;90(11):850–858.
CrossRef - Inaba, H., M. Greaves, and C.G. Mullighan, Acute lymphoblastic leukaemia. Lancet (London, England), 2013. 381(9881): p. 1943-1955.
CrossRef - Harper, J., Wells, D. and Simpson, J., 2016. Current controversies in prenatal diagnosis 4: preimplantation genetic screening should be routinely offered to all preimplantation genetic diagnosis cases. Prenatal Diagnosis, 36(1), pp.25-28.
CrossRef - Huang, F., Guang, P., Li, F., Liu, X., Zhang, W. and Huang, W., 2020. AML, ALL, and CML classification and diagnosis based on bone marrow cell morphology combined with convolutional neural network. Medicine, 99(45), p.e23154.
CrossRef - Laksman, Z., Dulay, D., Gollob, M., Skanes, A. and Krahn, A., 2013. Evolution of a genetic diagnosis. Clinical Genetics, 86(6), pp.580-584.
CrossRef - Eşkazan, A., 2021. A clinical appraisal of chronic myeloid leukaemia (CML)‐related death and CML‐specific death—Are they synonymous?. International Journal of Clinical Practice, 75(6).
CrossRef - Gunes, A., Millot, F., Kalwak, K., Lausen, B., Sedlacek, P., Versluijs, B., Dworzak, M., De Moerloose, B. and Suttorp, M., 2018. Features and Outcome of Chronic Myeloid Leukemia (CML) at Very Young Age: Data from the International Pediatric CML Registry (I-CML-Ped Study). Blood, 132(Supplement 1), pp.1748-1748.
CrossRef - Moya, T. and Morales, F., 2020. CML-245: Central Nervous System Restricted Lymphoid Blast Phase Transformation of CML. Clinical Lymphoma Myeloma and Leukemia, 20, p.S238.
CrossRef - Saydam, G., 2019. Molecular monitorization of CML under TKI therapy. Leukemia Research, 85, pp.S3-S4.
CrossRef - Atallah, E., Sadek, I., Maegawa, R., Cao, X., Latremouille-Viau, D., Pivneva, I., Rossi, C., Guerin, A. and Kota, V., 2020. CML-085: Discontinuation of Tyrosine Kinase Inhibitor (TKI) Treatment in Patients with Chronic Myeloid Leukemia in Chronic Phase (CML-CP) in US Clinical Practice Following Updates to NCCN CML Practice Guidelines. Clinical Lymphoma Myeloma and Leukemia, 20, p.S231.
CrossRef - Damlaj, M., Monczak, Y. and Assouline, S., 2013. Impact Of Dedicated CML Clinic On The Outcome Of Patients With Newly Diagnosed CML: Experience Of a Single Canadian Center. Blood, 122(21), pp.5186-5186.
CrossRef - Shah, P., Cossor, F., Murarka, S. and Dixit, H., 2017. Comparison of digital PCR with real-time PCR calibrated to the International Scale for BCR-ABL monitoring. Journal of Clinical Oncology, 35(15_suppl), pp.e18545-e18545.
CrossRef - Oehler, V., Helton, B., Kalebic, T. and Radich, J., 2009. Quantitative PCR Using a Nanofluidic Platform to Quantify Bcr-Abl mRNA in Patients Who Are Negative by Routine Quantitative PCR Testing. Blood, 114(22), pp.3300-3300.
CrossRef - Salih, G., 2016. Detection of a High Frequency of e1a3/BCR-ABL in Chronic Myeloid Leukemia Patients of Sulaimania Province by RT-PCR. Journal of Leukemia, 04(04).
CrossRef - Matte-Martone, C., Venkatesan, S., Tan, H., Athanasiadis, I., Chang, J., Pavisic, J. and Shlomchik, W., 2011. Graft-versus-Leukemia (GVL) against Mouse Blast-Crisis Chronic Myelogenous Leukemia (BC-CML) and Chronic-Phase Chronic Myelogenous Leukemia (CP-CML): Shared Mechanisms of T Cell Killing, but Programmed Death Ligands Render CP-CML and Not BC-CML GVL Resistant. The Journal of Immunology, 187(4), pp.1653-1663.
CrossRef - Virgili, A. and Nacheva, E., 2010. Genomic amplification of BCR/ABL1 and a region downstream of ABL1 in chronic myeloid leukaemia: a FISH mapping study of CML patients and cell lines. Molecular Cytogenetics, 3(1), p.15.
CrossRef - Saydam, G., 2019. Molecular monitorization of CML under TKI therapy. Leukemia Research, 85, pp.S3-S4.
CrossRef - Nacheva, E., 2007. I13 Recent advances in molecular cytogenetics of CML. Blood Reviews, 21, pp.S48-S51.
CrossRef - Baccarani, M. and Soverini, S., 2014. Molecular response in CML: where is the bar?. Blood, 124(4), pp.469-471.
CrossRef - Wolf, D. and Sopper, S., 2018. Correction: Molecular response prediction in CML: novel ideas?. Oncotarget, 9(88), pp.35871-35871.
CrossRef - Dib, E., 2004. Chronic Lymphocytic Leukemia: Molecular Genetics, Biology, Diagnosis, and Management. Leukemia Research, 28(9), p.995.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





