How to Cite | Publication History | PlumX Article Matrix
Nagamuthu Vinothkumar  and Pachaiappan Pugalendhi *
and Pachaiappan Pugalendhi *
Department of Biochemistry and Biotechnology, Faculty of Science, Annamalai University, Annamalai Nagar, Chidambaram - 608002, Tamil Nadu, India
Corresponding Author E-mail: pugalendhibiotech@gmail.com
DOI : http://dx.doi.org/10.13005/bbra/3069
ABSTRACT: Sea anemone-associated bacteria were considered promising candidates for the synthesis of many novel bioactive compounds. Thus culturable symbiotic bacteria that exist in the sea anemones met much attention when compared to other benthic marine dwellers. In this study, an attempt was made to explore the anticancer potentials of symbiotic bacteria isolated from a sea anemone, Heteractis species. Nine symbiotic bacteria were isolated, pure cultured and screened for their anticancer potential using two breast cancer cell lines. Among the strains, SAGM 3 showed appreciable growth inhibition activity of 43.1% and 47.1% against the studied cell lines, viz. MCF7 and MDA-MB-231 and this strain was selected for further studies. Based on the 16S rRNA molecular profiling, the SAGM 3 isolate was noted as Paenibacillus lentimorbus and the sequence of SAGM 3 was deposited in GeneBank with the accession number MW737456.1. During the growth kinetics profiling, maximum bacterial growth rate and anticancer activities were recorded from 60 to 96 hrs of incubation. The present investigation provides baseline data understanding the pharmaceutical significance of a symbiotic marine bacterium procured from the sea anemone, Heteractis species.
KEYWORDS: Anticancer; Heteractis species; Paenibacillus lentimorbus; Sea anemone; Symbiotic bacteria
Download this article as:| Copy the following to cite this article: Vinothkumar N, Pugalendhi P. Isolation and Characterization of Anticancer Compound Producing Marine Paenibacillus lentimorbus SAGM 3 Collected from a Sea Anemone, Heteractis species. Biosci Biotech Res Asia 2023;20(1). |
| Copy the following to cite this URL: Vinothkumar N, Pugalendhi P. Isolation and Characterization of Anticancer Compound Producing Marine Paenibacillus lentimorbus SAGM 3 Collected from a Sea Anemone, Heteractis species. Biosci Biotech Res Asia 2023;20(1). Available from: https://bit.ly/409Ccgg |
Introduction
Living organisms synthesise several molecules which stimulate metabolism and supports certain function in the body and they are named bioactive molecules. These molecules have extensive therapeutical values and provide the greatest solution for human diseases and disorders by regulating numerous biological activities in the living system1,2. Thus, exploring these kinds of promising bioactive compounds from living organisms has reached much attention among researchers. Among various diverse living beings, marine organisms are noted as a promising source for exploring many biotechnologically important products including bioactive molecules3. Numerous naturally occurring substances from marine invertebrates share striking structural similarities with recognised metabolites with microbial origins, indicating that microorganisms including bacteria, fungi and microalgae are either directly responsible for their biosynthesis or are the true sources of this particular metabolites4.
Our ocean comprises the biggest unaccounted ecological, chemical, and biological variety and makes up 70% of the earth’s atmosphere. Marine-dwelling organisms including bacteria, fungi, actinomycetes, cyanobacteria, sponges, bryozoans, tunicates, molluscs, microalgae and seaweeds were reported to produce bioactive compounds to treat various diseases in living beings5 and from marine species, 16,000 products from marine habitat have been procured and remaining yet to be explored6. Microbiota associated with other organisms from marine ecosystems considered to be an excellent endless base to develop novel drugs against various deadly pathogens and multiple drug-resistant pathogenic strains4, 5.
Researchers who are interested in exploring marine animals as sources of natural commodities have been interested in the biology behind the relationship between bacteria and their hosts7. Associated bacteria create possible physiologically active substances that the host might find useful8. Numerous bioactive compounds including antimicrobial and anticancer chemicals have been identified in bacteria associated with marine hosts9, recorded possible microbial symbionts against the host’s defence. In rare cases, it has also been demonstrated that the host’s related bacteria are the source of these chemicals. Because of their high value, several experimental studies involving marine microorganisms connected to plants and animals have been done, but these studies are still in the early stages.
Sea anemones create a variety of biologically active compounds, including biomacromolecules like cytolysins and phospholipases as well as small-molecule natural products10. Alkaloids, sterols, terpenes, and other structural types are just a few examples of the diverse structural types found in sea anemone small-molecule natural products11. In addition, sea anemone-derived bacteria were promising candidates for the production of new antimicrobial metabolites12. However, sea anemone-associated bacteria are currently less studied than other marine invertebrates including sponges, corals, and ascidians13. Thus, the present study aimed to isolate and identify bacterial communities associated with sea anemones and to investigate their efficiency against the anticancer activity.
Materials and Methods
Collection and sample processing
With the assistance of a local fisherman, a sea anemone (scientific name: Heteractis species; common name: Club anemone) was procured from the Gulf of Mannar, South India. The freshly acquired sample was brought to the lab right away in a food-grade plastic container with enough natural seawater, and the container was properly battery aerated throughout the entire journey. Before being processed for the separation of symbiotic bacteria, the specimen of the sea anemone was slightly splashed twice with pre-sterilized sea water with 34 ppt and pH 8.4 and seawater was made using synthetic sea salt (Catalog no: M1344, HiMedia, India) and sufficient tentacles were dissected with a wet weight of one gram that was homogenised using a mortar and pestle. To serially dilute the homogenised material, synthetic seawater was freshly created and pre-sterilized.
Isolation and purification of sea anemone-associated symbiotic bacteria
Individual spread plating of the serially diluted samples was done on freshly made Zobell marine agar (Catalog No:M384, HiMedia, India) plates. Using the quadrant strike plate technique, morphologically different colonies of bacteria were isolated after two days of incubation and cultured in freshly manufactured Zobell marine agar plates. Using Gram’s staining under microscopic observation and colony shape under visual observation, the purity of the bacterial strains was established.
Screening of anticancer compounds
Extracellular or intracellular production
Except for agar, all of the axenic strains were individually grown in screw-cap tubes with 10 ml working volumes of Zobell marine broth. After 48 hours of incubation, each cultured broth was spun at 3000 rpm for 15 minutes, and the cell pellet and cell-free supernatant were separately collected. The cell pellet was dissolved in 50 cc of phosphate buffer and ultrasonically processed for 45 seconds at 20 KHz using an ultrasonicator (Hielscher, USA). The cell debris generated during the sonication process was removed using the same applied centrifugal conditions as previously specified, while the cell-free supernatant from the produced broth was immediately applied for the evaluation of anticancer efficacy. The current study provides evidence that the test bacterial strains produced anticancer chemicals either intracellularly or extracellularly or both.
Cancer cell lines
The cells were purchased from the National Centre for Cell Sciences (NCCS), Pune, India, and they were maintained in Dulbecco’s Modified Eagle Medium (DMEM) (Catalog No: AL186, HiMedia, India). The cell line was cultured in DMEM with sodium bicarbonate, 10% FBS, and an antibiotic solution including streptomycin (100g/ml) and penicillin (100U/ml) as well as other growth factors. A tissue culture flask measuring 25 cm2 was filled with this combination. The grown cell line was kept at 37°C in a humid incubator with 5% CO2. A two-day-old confluent monolayer of cells was separated using trypsinization; the separated cells were then suspended in 10% growth media and seeded in 96-well tissue culture plates at a density of 5×104 cells/well. After that, the cells were incubated for 24 hours at 37°C in a humid atmosphere with 5% CO2.
Test sample
To prepare 5% DMEM, 2ml of the sample was added to 4ml of tissue culture-grade water, which was well mixed using a cyclomixer. After that, a 0.22 m Millipore syringe filter was used to guarantee sterility on the sample that included 5% DMEM. In a nutshell, the growth medium was taken out after 24 hours, and freshly produced samples containing 5% DMEM were put in triplicate to the appropriate wells and cultured for 24 hours in the incubator at 37°C and 5% CO2 humidified. The MTT assay method was used to determine the vitality of the cells, and then the cells were directly observed by an inverted phase contrast microscope.
In vitro anticancer assay
For the MTT (3-(4, 5-dimethyl thiazol-2yl)-2, 5-diphenyl tetrazolium bromide) experiment, care should be taken while transferring the medium from the wells after incubation. Following two or three rounds of washing with 5% DMEM (without) FCS, 200 l of MTT (5 mg/ml) was applied to each well. For 6 hours, the plate was placed in an incubator with 5% CO2 to test for cytotoxicity. DMSO (1 ml) was added to every well after incubation, stirred, and allowed to sit for 45 seconds. Cells which are viable in the medium produced crystals that were dissolved by the addition of the solubilizing reagent dimethyl sulphoxide (DMSO), which produced a purple colour. By using DMSO as a reference and measuring the suspension’s absorbance spectrophotometrically at 540 nm14 and the growth percentage of inhibition was calculated.
Microscopic observation
Using calcein AM and ethidium homodimer III, adherent cells on well plates were stained for fluorescence microscopy (ZEISS LSM 880, Germany). Fluorescence was recorded using a 490 nm excitation filter and a 520 nm emission filter for Calcein AM, which denotes viable cells, and a 545 nm excitation filter and a 620 nm emission filter for Ethidium homodimer III, which denotes dead cells. A 495 nm excitation filter and above the range of 520 nm emission filter were used for combined staining (Calcein AM and Ethidium homodimer III). The microscopic image confirms the toxicity of the test samples.
Molecular identification of the potential anticancer compounds producing marine symbiotic bacterium
DNA extraction was performed using 2 ml cultured broth which was collected at the mid-exponential growth phase using the Roche Kit (Germany). Universal set of the Eubac primers 27F – 5′- AGAG TTTG ATCM TGGC TCAG -3′ and 1492R – 5′- TACG GYTA CCTT GTTA CGAC TT -3′ were used in this study for the molecular identification15. The PCR was performed on a thermal cycler (Eppendorf) followed by Qiagen PCR purification kit was used to purify the product before it was sequenced on an ABI Prism 377 automated sequencer (Applied Biosystems, CA, USA). The Neighbour-Joining method was used for evolutionary distances computation16 and the software MEGA 7 was used for evolutionary analysis17 phylogenetic trees were inferred using the neighbour-joining method, and the tree topologies were assessed using bootstrap analysis with 1,000 replicates.
Growth kinetics profile as a function of time on anticancer compound production
A 250 ml conical flask with a 100 ml working volume of yeast glucose broth18 that contained yeast extract (10 g/L) and glucose (20 g/L) with a pH of 7.5. A 37 °C temperature, 150 rpm of agitation, and a 1 ml inoculum were also required. The exponential growth phase of the potential strain was used to create the inoculum under the same basic fermentation medium conditions. Following that, the inoculum’s OD 620 nm was changed to 0.1 following the McFarland turbidity 0.5 standards, which is equal to a concentration of 1 108 CFU/ml of bacteria. Additionally, using the MTT assay described earlier14, the inhibitory activity of the anticancer drug against the two cancer cell lines MCF-7 and MDA-MB-231 was used to quantify the amount of the anticancer chemical produced. To find the optimal time for the maximum production of anticancer chemicals in connection to the development of cell biomass, the experiment was also carried out at regular intervals of 12 hours from 0 to 144 hours. A portion of the cultured broth was used to track evaluations, and centrifugation at 3000 rpm for 15 min was used to separate the cell biomass from the cell-free supernatant. Using the inhibitory activities as described above, the production of anticancer compounds was directly estimated from the cell-free supernatant, and bacterial cell growth was estimated using the dry weight of cell biomass obtained from centrifuged cell pellets that had been hot air oven dried at 50 °C for 30 min.
Results and Discussion
Isolation of sea anemone associated bacteria
A large portion of the biota, particularly the sea anemone, has several defence mechanisms that are either unique to it or derived from the organs that it is linked with to gain a competitive edge. Many multicellular creatures have been found to host ecto and endo symbiotic microbes and sea anemones are well known to produce various harmful and harmless compounds, however, a symbiotic association of bacteria in sea anemones was less established. In the present investigation, bacterial association with Heteractis sp was studied and a sea anemone was collected from the Gulf of Mannar in Mandapam, Ramanathapuram District, Tamil Nadu (Fig. 1). Bacterial isolates were cultured using a spread plate of the serially diluted samples on Zobell marine agar plates. After the incubation period, 9 distinct bacterial cultures were isolated and they were labelled as SAGM 1 to SAGM 9 for further reference. The pure culture was noted using a distinctive colony shape which was observed under visual examination and the Gram’s staining procedure, and axenic strains were kept for further examination. Prakash Williams19 reported that the associated bacterial counts were maximum in tentacle tissues than in body tissue and the present study also attempted to isolate bacteria from tentacle tissue. He also pointed out Stichodactyla haddoni derived tissue and associated bacterial extracts showed antimicrobial activity19.
 |
Figure 1: Club anemone (Scientific name: Heteractis species) collected from Gulf of Mannar, Ramanathapuram |
Primary screening of anticancer activity
Xie et al.20 highlighted the promising application of bacteria isolated from Sea anemones with cytotoxic activity along with antimicrobial activity21. Thus, in this study, preliminary screening for anticancer potential towards cell lines MCF7 and MDA-MB-231 was done for all 9 marine bacterial isolates. During the primary screening, among 9 bacterial isolates, SAGM 3 showed maximum inhibition of the growth percentage of both the selected breast cancer cell lines. Nearly 43.1±1.5% growth inhibition was observed in the MCF7 cell line similarly in MDA-MB-231 cell line showed 47.1±1.7% growth inhibition and notable inhibition of the MDA-MB-231 cell line was also observed in bacterial isolate SAGM 8 (Table 1). Anticancer activity of lysed cell supernatant of axenic marine bacteria was tested using selected cell lines. Growth inhibition was absent in all the tested bacterial lysed cell supernatant (Table 1). Further marine bacterial isolate SAGM 3 was selected for further analysis (Fig. 2) and culture of axenic bacteria was also maintained in Zobell marine broth for further investigation (Fig. 3). This study proved that extracellular compound present in the bacterial isolates showed more anticancer activity than the intracellular compound. Similar to the present investigation, Thomas et al.22 tested both extracellular and intracellular extracts of thirty-six bacterial metabolites.
Table 1: Screening of anticancer compounds from the lysed cell and cell free supernatant of axenic marine bacteria, all the experimental values are expressed as mean ± standard deviation, (n=3).
|
Marine bacteria |
Growth inhibition percentage of breast cancer cell lines |
|||
|
MCF7 |
MDA-MB-231 |
|||
|
Cell free supernatant |
Lysed cell supernatant |
Cell free supernatant |
Lysed cell supernatant |
|
|
SAGM 1 |
– |
– |
– |
– |
|
SAGM 2 |
– |
– |
– |
– |
|
SAGM 3 |
43.1±1.5 |
– |
47.1±1.7 |
– |
|
SAGM 4 |
– |
– |
– |
– |
|
SAGM 5 |
– |
– |
– |
– |
|
SAGM 6 |
– |
– |
– |
– |
|
SAGM 7 |
– |
– |
– |
– |
|
SAGM 8 |
– |
– |
22.9±1.9 |
– |
|
SAGM 9 |
– |
– |
– |
– |
 |
Figure 2: Axenic cultured promising anticancer compound producing marine symbiotic bacterium, P. lentimorbus SAGM 3 in the Zobell marine agar. |
 |
Figure 3: Culture of axenic bacteria using Zobell marine broth for the anticancer studies. |
Molecular profiling of associated bacteria
The majority of the time, 16S rRNA gene sequencing has been employed to research the dispersion of microorganisms. Similarly in the present study, molecular characterization of SAGM 3 was analysed using the 16s rRNA gene sequencing method. The molecular characterization study of the selected microbial strain was identified as Paenibacillus lentimorbus SAGM 3 and the sequence of this bacterial strain was deposited with the accession number MW737456.1. The neighbour-joining method was done to establish evolutionary history among the isolates of the maximum first 12 similar sequences as obtained in the Blastn homology search (Fig. 4) and the optimal tree with the sum of branch length is equal to 11.440. The resulting sequence was analysed with a total of 1450 positions in the final dataset. Kamke et al.23 studied the active profiles of marine sponge-associated bacteria using 16S rRNA vs 16S rRNA gene comparisons.
 |
Figure 4: The evolutionary history inferred using the Neighbor-Joining method |
In vitro anticancer assay
Studies on breast cancer disease related to associated bacteria were limited and MCF 7 and MDA-MB-231 are aggressive mesenchymal cancer cell lines24. Thus, in the present study sea anemones associated with bacteria were tested against breast cancer cell lines. Fig. 5 & 6 shows cytotoxicity analysis of cell-free supernatant from the marine symbiotic bacteria SAGM 3 and SAGM 8 against MCF7 and MDA-MB-231 breast cell line culture under fluorescence microscopy using two dyes, Calcein AM (fluorescent green colour denotes viable cells) and Ethidium homodimer III (fluorescent red colour denotes dead cells).
The presence of maximum viable cancer cells was observed at the control without any dead cells and in the MCF7 cell line, 43.1% growth inhibition was observed in SAGM 3 cell-free supernatant. In the MDA-MB-231 cell line, SAGM 3 showed 47.1% growth inhibition and SAGM 8 showed 22.9% growth inhibition. Piscibacillus sp. isolated from soda-like showed promising anticancer activity against breast cancer MDA-MB-231 cells. Sea anemone and its associated bacteria produce various toxins, bacteriocins, enzymes, and peptides and they can fight against various cancer25,26,27. This could be the reason for the promising result of anticancer activity using sea anemones-associated bacteria in this study. Previous researchers remarkably highlighted microbial community associated with other living organisms showed effective antiproliferative activity against various carcinoma cell lines.
 |
Figure 5: Growth inhibition activity of the cell free supernatant from the marine symbiotic bacterium, SAGM 3 against the MCF7 breast cancer cells |
 |
Figure 6: Growth inhibition activity of cell free supernatant from the marine symbiotic bacteria, SAGM 3 and SAGM 8 against the MDA-MB-231 breast cancer cells |
Growth kinetics profiling
Growth kinetic profiling of P. lentimorbus SAGM 3 growth was observed along with anticancer activity against MCF7 and MDA-MB-231 (Fig. 7). The study was done for different time intervals from 0 to 144hrs of incubation and results showed that maximum bacterial growth of 4g/L was initiated at 60 to 96 hrs of incubation. Similarly, anticancer activity was also high during the same incubation period. Compare to both the cell line, anticancer activity was high against MDA-MB-231 than MCF7. From the start of the lag phase to the end of the decrease growth phase, which was the focus of our study, anticancer activity and growth rate were demonstrated. The bioactive molecule’s production pattern provided proof that it is a growth associated metabolite synthesis. Marinispora NPS12745, which was isolated from sediment samples collected from the marine coast in California, provided evidence in support of this investigation by producing new bioactive secondary metabolites that were effective against bacteria28. According to Bhatnagar and Kim29, diverse microorganisms created the majority of the bioactive compounds as secondary metabolites, hence the metabolite of this study is unique.
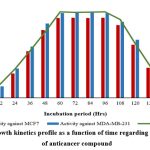 |
Figure 7: Growth kinetics profile as a function of time regarding the production of anticancer compound |
Conclusion
This investigation overcomes the paucity of research work in the examination of sea anemone-associated bacteria-producing anticancer compounds. In this study, a symbiotic marine bacterium, Paenibacillus lentimorbus SAGM 3 was isolated which revealed an appreciable anticancer compound production against two breast cancer cell lines viz., MCF7 and MDA-MB-231. Further, the potential strain evidenced growth-dependent production of the anticancer compound which constitutes a rare phenomenon. Based on this observation, this study stood as proof that sea anemone-associated bacteria are important resources of new bioactive compounds with many pharmaceutical potentials.
Acknowledgment
The authors gratefully acknowledge Department of Biotechnology, Faculty of Science, Annamalai University, Annamalai Nagar, Chidambaram – 608002, Tamil Nadu, India for providing lab facilities and supporting our research.
Conflict of interest
The authors declare that they have no conflict of interest on publication of this article.
Funding Sources
There is no funding source to disclose.
References
- Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., Bi Y. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020; 395: 565-574.
CrossRef - Atanasov A.G., Zotchev S.B., Dirsch V.M., Supuran C.T. Natural products in drug discovery: advances and opportunities. Rev. Drug Discov. 2021; 20(3): 200-216.
CrossRef - Karthikeyan K., Karthikeyan P., Baskonus H.M., Venkatachalam K., Chu Y.M. Almost sectorial operators on Ψ‐Hilfer derivative fractional impulsive integro‐differential equations. Methods Appl. Sci. 2022; 45(13): 8045-8059.
CrossRef - Proksch P., Edrada R., Ebel R. Drugs from the seas–current status and microbiological implications. Microbiol. Biotechnol. 2002; 59(2): 125-134.
CrossRef - Barzkar N., Jahromi S.T., Poorsaheli H.B., Vianello F. Metabolites from marine microorganisms, micro, and macroalgae: Immense scope for pharmacology. Drugs. 2019; 17(8): 464.
CrossRef - Bhakuni D.S., Rawat D.S. Bioactive metabolites of marine algae, fungi and bacteria. Bioactive Mar. Nat. Pro. 2005; 1-25.
- Piel J., Hui D., Wen G., Butzke D., Platzer M., Fusetani N., Matsunaga S. Antitumor polyketide biosynthesis by an uncultivated bacterial symbiont of the marine sponge Theonella swinhoei. Natl. Acad. Sci. 2004; 101(46): 16222-16227.
CrossRef - Hentschel U., Schmid M., Wagner M., Fieseler L., Gernert C., Hacker J. Isolation and phylogenetic analysis of bacteria with antimicrobial activities from the Mediterranean sponges Aplysina aerophoba and Aplysina cavernicola. FEMS Microbiol. Ecol. 2001; 35(3): 305-312.
CrossRef - Haygood M.G., Schmidt E.W., Davidson S.K., Faulkner D.J. Microbial symbionts of marine invertebrates: opportunities for microbial biotechnology. Mol Microbiol Biotechnol. 1999; 1(1): 33-43.
- Guillen P.O., Jaramillo K.B., Genta-Jouve G., Thomas O.P. Marine natural products from zoantharians: bioactivity, biosynthesis, systematics, and ecological roles. Prod. Rep. 2020; 37(4): 515-540.
CrossRef - Zhang B., Zhang Y.H., Wang X., Zhang H.X., Lin Q. The mitochondrial genome of a sea anemone Bolocera exhibits novel genetic structures potentially involved in adaptation to the deep‐sea environment. Ecol Evol. 2017; 7(13): 4951-4962.
CrossRef - Qin X.Y., Yang K.L., Li J., Wang C.Y., Shao C.L. Phylogenetic diversity and antibacterial activity of culturable fungi derived from the zoanthid Palythoa haddoni in the South China Sea. Biotechnol.2015; 17(1): 99-109.
CrossRef - Calabon M.S., Sadaba R.B., Campos W.L. Fungal diversity of mangrove-associated sponges from New Washington, Aklan, Philippines. Mycol. 2019; 10(1): 6-21.
CrossRef - Wilson A.P. Cytotoxicity and viability assays. Animal cell culture: a practical approach. 3rd ed. Oxford: Oxford University Press; 2000; pp 175-219.
- Gürtler V., Stanisich V.A. New approaches to typing and identification of bacteria using the 16S–23S rDNA spacer region. Microbiol. 1996; 142: 3–16.
CrossRef - Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Bio. Evol. 1987; 4:406-425.
- Kumar S., Stecher G., Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Biol. Evol. 2016; 33 (7): 1870-1874.
CrossRef - Atlas R.M. Handbook of Microbiological Media, 4th ed. CRC Press: BocaRaton FL; 2010; pp 1933.
CrossRef - Williams G.P., Babu S., Ravikumar S., Kathiresan K., Prathap S.A., Chinnapparaj S., Marian M.P., Alikhan S.L. Antimicrobial activity of tissue and associated bacteria from benthic sea anemone Stichodactyla haddoni against microbial pathogens. Environ. Biol. 2007; 28(4): 789–793
- Xie X. Screening of cytotoxic activity of marine animal symbiotic and epiphyte microorganisms against B16 tumor cell. Chinese J Mar. Drugs (in Chinese). 2006; 25(6): 26–30
- Zheng Z., Chen L., Huang Y., Chen P., Su W. Antimicrobial activity of symbiotic and epiphyte microorganisms on marine organisms in intertidal zone of Xiamen. Oceanogr. Taiwan Strait. 1998;17:438-43.
- Thomas A.T., Rao J.V., Subrahmanyam V.M., Chandrashekhar H.R., Maliyakkal N., Kisan T.K., Joseph A., Udupa N. In vitro anticancer activity of microbial isolates from diverse habitats. J. Pharm. Sci.2011; 47: 279-287.
CrossRef - Kamke J., Taylor M.W., Schmitt S. Activity profiles for marine sponge-associated bacteria obtained by 16S rRNA vs 16S rRNA gene comparisons. ISME J. 2010; 4(4): 498-508.
CrossRef - Sheridan C., Kishimoto H., Fuchs R.K., Mehrotra S., Bhat-Nakshatri P., Turner C.H., Goulet R., Badve S., Nakshatri H. CD44+/CD24-breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 2006; 8(5): 1-13.
CrossRef - Yaghoubi N., Soltani A., Ghazvini K., Hassanian S.M., Hashemy S.I. PD-1/PD-L1 blockade as a novel treatment for colorectal cancer. Biomed Pharmacother. 2019; 110: 312-318.
CrossRef - Moreels L., Peigneur S., Galan D.T., De Pauw E., Béress L., Waelkens E., Pardo L.A., Quinton L., Tytgat J. APETx4, a Novel Sea Anemone Toxin and a Modulator of the Cancer-Relevant Potassium Channel KV1. Mar. drugs. 2017; 15(9): 287.
CrossRef - Kalina R.S., Kasheverov I.E., Koshelev S.G., Sintsova O.V., Peigneur S., Pinheiro-Junior E.L., Popov R.S., Chausova V.E., Monastyrnaya M.M., Dmitrenok P.S., Isaeva M.P., Tytgat J., Kozlov S.A., Kozlovskaya E.P., Leychenko E.V., Gladkikh I.N. Nicotinic Acetylcholine Receptors Are Novel Targets of APETx-like Toxins from the Sea Anemone Heteractis magnifica. Toxins. 2022; 14(10): 697.
CrossRef - McArthur K.A., Mitchell S.S., Tsueng G., Rheingold A., White D.J., Grodberg J., Lam K.S., Potts B.C. Lynamicins A-E, chlorinated bisindole pyrrole antibiotics from a novel marine actinomycete. Natl. Prod. 2008; 71:1732.
CrossRef - Bhatnagar I., Kim S.K. Marine microbial bioactive compounds. Drugs 2010; 8:2673-701.
CrossRef

This work is licensed under a Creative Commons Attribution 4.0 International License.





